ABSTRACT
In the present study, we investigated the antifungal and antibacterial activity of a novel membrane-active peptide, K04, the derivative of KSL. K04 inhibited the growth of various microorganisms, including Candida albicans, Staphylococcus aureus, Streptococcus mutans, Escherichia coli. In this study, we also investigated a membrane fusion inhibitor JX0020, a modified SARS-CoV-2-HR2P peptide, which inhibited the infection of a spike containing SARS-CoV-2 pseudovirus with IC50 value of 0.049 mg/ mL. More interesting, though K04 showed mild in vitro activity against SARS-CoV-2 infectivity with IC50 value of 0.43 mg/mL, it showed a strong synergism with JX0020 and significantly reduced the effective concentration of JX0020. Besides, K04 had no cytotoxicity activity to various mammalian cells. These results suggest that K04 has great advantages in the development of multi-functional antimicrobial agent.
Keywords: Antimicrobial Agent; SARS-CoV-2; Peptide
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), put significant strains on healthy system and economies around the world, there are widespread negative social, psychological and economic effects. Up to now, millions of people had died of SARS-CoV-2 infection and new variants are still emerging [1]. Delta and Omicron variants currently pose a “double threat” to the global outbreak. And as the outbreak continues, new strains may also emerge during a pandemic. Although the vaccines including whole-virus (live-attenuated virus, inactivated virus, and viral-vector) vaccines [2], subunit vaccines [3,4], and nucleic acid vaccines (pDNA and mRNA) [5] had been designed to enable broad-spectrum protection against coronavirus pathogens, the emergence of SARS-CoV-2 variants that harbor mutations in the spike glycoprotein receptor binding domain(RBD) may resist neutralization by antibodies, comprising vaccine efficacy [6,7]. The continued emergence of SARS-CoV-2 mutations poses a major challenge to the development of antiviral drugs and vaccines.
It has been reported that the tropism of SARS-CoV-2 was determined by the S protein which binds to the membrane receptors, angiontensin-converting enzyme 2 (ACE2) and/or CD147 [8,9]. These membrane receptors on the host cells mediated the viral and cellular membrane fusion and contributed to SARS-CoV-2 invasion for host cells. S protein is divided into two subunits, S1 and S2. The RBD of S1 is responsible for recognizing ACE2 on the surface of human cells and completing the binding of virus to cells. Then S2 subunit is responsible for the fusion of virus envelope and human cell membrane to complete the invasion process. Two important conserved repeating amino acid sequences, heptad repeat 1 (HR1) and 2(HR2), are present in the S2 subunit of SARS-CoV-2. The two domains combine to form a spiral structure called six-helix bundle core fusion structure (6-HB) which could bring the viral envelop closed to the cellular membranes [10]. Recent studies have shown that this 6-HB is the key to complete SARS-CoV-2 envelope and cell membrane fusion, which is also shown to be stronger and more efficient in entering cells than SARS virus [11]. Several peptides such as AP-9, EK1 et al were identified to potentially block the infection of SARS-CoV-2 [12,13], As a broad-spectrum anti-Human Coronaviruses (HCoV) therapeutic and prophylactic agent, it has broad development prospects in the treatment and prevention of COVID-19 and other emerging HCoV diseases.
KSL can directly act on microbial lipid membranes and has amphiphilic α helix and Net positive charge, so it has strong irreversible inhibitory activity against both bacteria and fungi. What’s more, KSL has no hemolytic activity [14], so it has great potential to be developed as a new antibacterial agent. In this study, we generated a novel decapeptide, K04 (RRVVFHVKFK) derived from KSL which killed microorganisms through the action on the membrane as its primary target [15]. Our results demonstrated that K04 had higher activity against bacteria and fungi without cytotoxicity. More interesting, K04 had a strong synergism with JX0020 peptide, a membrane-fusion inhibitor of SARS-CoV-2 and significantly reduced the effective inhibition concentration of JX0020. The specific mechanism needs further study.
Materials and Methods
Cells and Peptide
Human embryonic kidney cell line 293T, Human hepatocyte cell line LO2 and human fetal lung fibroblast cell MRC-5 were purchased from Cell Bank/Stem Cell bank (Shanghai institute of Biochemistry and Cell Biology). Human epidermal keratinocyte cell line HaCaT was obtained from Biomedical Cell Resource Center (BMCR). LO2 and 293T cells were cultured in DMEM medium plus 10% FBS, MRC-5 and HaCaT cells were cultured in MEM medium plus 10% FBS and 1% NEAA in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Peptides were synthesized with Fmoc-strategy solid phase methodology by Harbin Jixianglong Biotech Co. Ltd.
Growth Inhibition Assay
This test was performed with the strains Candida albicans CMCC( B) 98001, Staphylococcus aureus CMCC(B) 26003, Streptococcus mutans UA159, Escherichia coli CMCC(B) 44102. The reference drugs used were chlorhexidine and bisabolol. Aliquots of microbial suspension in indicated culture medium were mixed with peptides or reference drugs respectively and incubated at 37°C for different time (0, 4, 8, 12, 16, 20, 24 h). Then, the viable cell count was done by diluting the suspension sample and plating aliquots of the dilutions onto an appropriate culture medium.
Inhibition of Pseudotyped SARS-CoV-2 Infection
Plasmid pLVX-puro, psPAX2, pcDNA3.1-Spike (SARS-CoV-2), pGL-Basic were purchased from Miaoling Plasmid Sharing Platform. To generate pLVX-luciferase-puro plasmid, Luciferase gene was amplified using pGL-Basic plasmid as template and cloned into pLVX-puro plasmid. The pseudo viruses were produced using methods as previously described. Briefly, 293T cells were inoculated into the 6-well cell plate, and the cell density was controlled at 70%-90% confluence before transfected with psPAX2 (3μg), pLVX-luciferase-puro (4 μg), pcDNA3.1-spike (SARS-CoV-2) plasmid DNA mix using Lipofectamine 2000. Six hours later, the medium was changed, and the transfected cells were cultured with fresh medium containing DMEM medium plus 10% FBS for 48 h. Then, SARS-CoV-2 pseudo viruses containing culture supernatants were harvested, filtered and stored at -80°C in aliquots. To detect the inhibitory activity of a peptide on infection of SARS-CoV-2 pseudo virus, target cells (293T/ACE2) were seeded at a density of 1.5 × 104 cells per well in a 96-well plate one day prior to infection. Pseudo virus was mixed with an equal volume of a peptide which was series diluted with PBS at 37°C for 1 h. EK1 peptide was used as positive control. The mixture was transferred to the target cells and incubated for 72 h. Then, cell supernatant was collected, and luciferase activity was analyzed by the Luciferase assay system (Promega).
Cytotoxicity Assay
This test was performed with the cell lines LO2, HEK293T, MRC- 5, HaCaT using CCK-8 assay as previously described with some modifications. Cells (5 × 103 or 1.5 × 104 per well) were seeded in 96-well cell plate and cultured at 37°C in a humidified atmosphere with 5% CO2 for 12 h. Then, cell culture medium was changed with fresh medium containing a series of concentrations of peptides and further incubated for 24, 48 or 72 h respectively. Finally, 10 μL of the CCK-8 reagent was added into each well, and OD at 450 nm was measured using a multimode microplate reader after incubation for 4 h at 37°C.
Results and Discussion
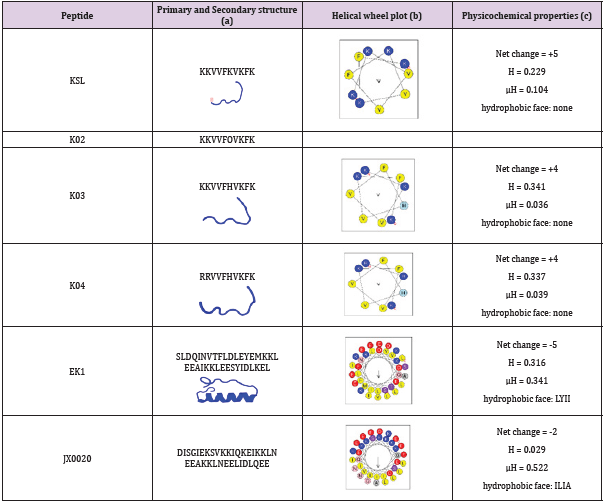
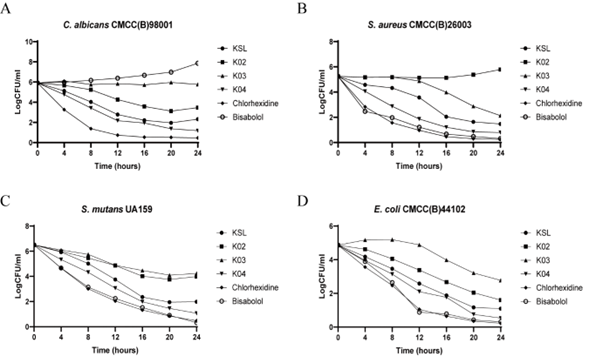
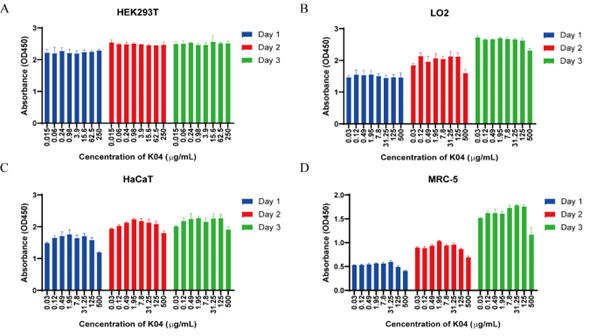
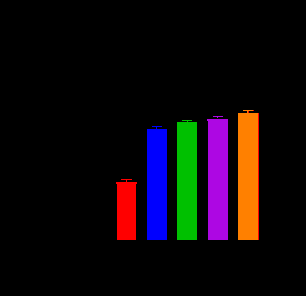
K04 demonstrated antifungal and antibacterial activity. Previous study had investigated the influence of different amino acid residues of decapeptide on the antifungal activity through screening the combinatorial peptide libraries composed of seven amino acids (Lys, Leu, Val, Pro, Ser, Phe, and Gln). The positively charged amino acid at the N terminus and the Lys at sixth position confer to the highest level of antifungal activity. In this study, we constructed three analogs of KSL (Table 1) by replacing the amino acid residue at the first, second and sixth position. KSL and its analogs K02, K03, K04 (Table 1) were generated by solid-phase synthesis in high purity (> 95% by HPLC). Some physiochemical properties, such as their secondary structures, hydrophobicities (H), hydrophobic moments (μH) and net charge (at neutral pH) were listed in Table 1. Similar to KSL, these analogs did not form perfect amphipathic α-helical structure in a wheel diagram, but the mutation of Lys to His at the sixth position increases the hydrophobicities (H) and decreases hydrophobic moments (μH). To test the in vitro antifungal and anti- bacterial activity, KSL and its analogs were co-cultured with microbial cells for different times. Then, the live microbial cells were enumerated by plate count method (CFU/mL). As shown in Figure 1, among the three KSL analogs, K04 was the best anti-microbial agent and showed higher anti-microbial activity when compared to its native peptide KSL. To evaluate possible toxic effect of K04 peptide with healthy eukaryotic cells, different concentrations of K04 peptide were prepared and added into the cell culture media of human embryonic kidney cell 293T, hepatocyte cell line LO2, epidermal keratinocyte line HaCaT and fetal lung fibroblast cell MRC-5 for three days, respectively. As shown in Figure 2, the CCK-8 assays demonstrated that K04 caused almost no influence on the viability of cell lines tested at the concentration below 125 μg/mL. Above results indicated that the membrane-active peptide K04 improved the anti-microbial activity by introducing three amino acid mutation.
Figure 1: Time course of KSL and its analogs-induced killing of C. albicans (A), S. aureus (B), S. mutans (C) and E. coli (D).
Figure 2: Cell viability of HEK293T, LO2, HaCaT, MRC-7 induced by K04 after Day1, Day 2 and Day3 in CCK-8 assay.
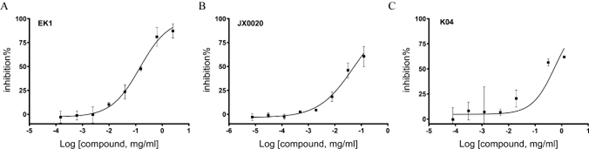
Each appearance of such as Alpha, Beta, Delta, Omicron mutant sets off a new epidemic of infections. And new variants have been found that make the vaccine less effective. In order to prevent reinfection of the virus, in addition to vaccines, new drugs that can block the fusion of the virus with cells need to be developed. Since SARSCoV- 2 spike protein-mediated membrane fusion played important role on the virus infection, spike protein became an important target for the development of preventative and therapeutic drugs. As an optimized form of OC43-HR2P, EK1 peptide was reported to be a promising pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike [11]. In this study, we constructed JX0020 peptide derived from HR2 region on the basis of the 6-helix bundle structure of SARS-CoV-2 Spike protein and introduced ten mutations (N6E, A7K, V10K, N11K, D17K, R18K, V22E, N25K, S29E, L36E), at the i to i + 3 or i to i + 4 positions to form intramolecular E-K or K-E salt bridges. Then, we used the pseudo virus assay, a good model to study the process of virus entry into the target cell, to assess the inhibitory activity of JX0020 against SARS-CoV-2 pseudo virus infection with EK1 as positive control and K04 as negative control. As showed in Figure 3, EK1 demonstrated antiviral activity against pesudotyped SARS-CoV-2 infection with IC50 value of 0.143 mg/mL, while JX0020 exhibited improved fusion inhibitory activity with IC50 value of 0.049 mg/mL.
Interestingly, K04 also showed mild antiviral activity with IC50 value of 0.43 mg/mL (Fig. 3C). Then, the synergic effect between K04 and JX0020 on the inhibition of SARS-CoV-2 infection was investigated. As showed in Figure 4, JX0020 improved the inhibitory activity with the increase of concentration from 15 μg/mL to 150 μg/mL in the absence of K04. In addition, when combined with a relatively low amount of K04 peptide (10 μg/mL), JX0020 significantly decreased the effective inhibitory concentration from 150 μg/mL to 15 μg/mL by exhibiting the comparative inhibition activity. These results indicated that K04 had a strong synergism with JX0020 against SARS-CoV-2 pseudovirus infection. A number of studies have shown that KSL and its analogs have significant antibacterial activities against a variety of bacteria and fungi. However, there have been no reports on KSL or its analogs antiviral or co-antiviral activities so far, and the specific mechanism needs further study.
Figure 3: Inhibitory activity of EK1 (A), JX0020 (B), and K04 (C) peptide in pseudovirus infection assay against SARS-CoV-2.
Conclusion
In summary, our results indicated that K04, as a novel membrane- active peptide, demonstrated antifungal, antibacterial activity and synergic effect with membrane-fusion inhibitor without cytotoxicity to eukaryotic cells. Antimicrobial peptides were paid more attention as the potential substitutions for common antibiotics. Our results suggested that K04 might be a promising drug candidate in the development of multi-functional antimicrobial agent.
Acknowledgement
This work was supported by grant from the State Key Laboratory of Pathogen and Biosecurity (SKLPBS2119).
References
- Karim SSA, Karim QA (2021) Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 398(10317): 2126-2128.
- Ze-Jun Wang, Hua-Jun Zhang, Jia Lu, Kang-Wei Xu, Cheng Peng, et al. (2020) Low toxicity and high immunogenicity of an inactivated vaccine candidate against COVID-19 in different animal models. Emerg Microbes Infect 9(1): 2606-2618.
- Prabhu S Arunachalam, Alexandra C Walls, Nadia Golden, Caroline Atyeo, Stephanie Fischinger, et al. (2021) Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 594(7862): 253-258.
- Lulu Bravo, Igor Smolenov, Htay Htay Han, Ping Li, Romana Hosain, et al. (2022) Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet 399(10323): 461-472.
- Suzannah J Rihn, Andres Merits, Siddharth Bakshi, Matthew L Turnbull, Arthur Wickenhagen, et al. (2021) A plasmid DNA-launched SARS-CoV-2 reverse genetics system and coronavirus toolkit for COVID-19 research. PLoS Biol 19(2): e3001091.
- Altmann DM, RJ Boyton (2022) COVID-19 vaccination: The road ahead. Science 375(6585): 1127-1132.
- Nick Andrews, Julia Stowe, Freja Kirsebom, Samuel Toffa, Tim Rickeard, et al. (2022) Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med 386(16):1532-1546.
- Ke Wang, Wei Chen, Zheng Zhang, Yongqiang Deng, Jian-Qi Lian, et al. (2020) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 5(1): 283.
- Claudio Fenizia, Silvia Galbiati, Claudia Vanetti, Riccardo Vago, Mario Clerici, et al. (2021) SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells 10(6).
- Berend Jan Bosch, Byron EE Martina, Ruurd Van Der Zee, Jean Lepault, Bert Jan Haijema, et al. (2004) Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc Natl Acad Sci U S A 101(22): 8455-8460.
- Shuai Xia, Meiqin Liu, Chao Wang, Wei Xu, Qiaoshuai Lan, et al. (2020) Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30(4): 343-355.
- Shuai Xia, Qiaoshuai Lan, Yun Zhu, Chao Wang, Wei Xu, et al. (2021) Structural and functional basis for pan-CoV fusion inhibitors against SARS-CoV-2 and its variants with preclinical evaluation. Signal Transduct Target Ther 6(1): 288.
- Yanxing Cai, Wei Xu, Jiayi Tang, Najing Cao, Qiaoshuai Lan, et al. (2021) A bivalent protein targeting glycans and HR1 domain in spike protein potently inhibited infection of SARS-CoV-2 and other human coronaviruses. Cell Biosci 11(1): 128.
- SP Concannon, TD Crowe, JJ Abercrombie, CM Molina, P Hou, et al. (2003) Susceptibility of oral bacteria to an antimicrobial decapeptide. J Med Microbiol 52(Pt 12): 1083-1093.
- SY Hong, JE Oh, M Kwon, MJ Choi, JH Lee, et al. (1998) Identification and characterization of novel antimicrobial decapeptides generated by combinatorial chemistry. Antimicrob Agents Chemother 42(10): 2534-2541.

 Research Article
Research Article