ABSTRACT
Introduction: Chronic bowel disease (IBD) has a chronic recurrent course with frequent complications and requires multiple monitoring and control. IBDs not only affect the gut, but also have extra-intestinal involvement in the oral cavity. Saliva is increasingly being investigated because it is an easily accessible body fluid that reflects various physiological and pathological conditions in the human body.
Purpose: Comparative study of salivary cell composition in patients with IBD and evaluate the effect of intestinal inflammatory activity on it.
Material and Methods: Subjects: 54 patients (40 UC and 14 CD) hospitalized with IBD and 80 healthy individuals (43 women and 37 men) as controls. Material: Unstimulated native saliva is collected in the morning by a passive droplet and sediment diluting with a suitable diluent. Routine laboratory indicators for the activity of the inflammatory process have also been investigated. An automatic enumeration and identification of salivary cells of a FUS-100 urinary sediment analyzer (DIRUI) is performed, followed by visual validation. Statistics: A non-parametric Man-Whitney test is used to estimate differences between groups.
Results and Discussion: Salivary erythrocytes, leukocytes, and epithelial cells are significantly higher in IBD patients than those in the control group, possibly due to chronic inflammatory disease. In contrast, the bacterial count in the patient’s saliva is significantly smaller. A possible explanation for this discrepancy is that the treatment of patients with various bacteriostatic and bactericidal agents leads to oral dysbiosis. Patients’ stratification by age, sex, disease duration, and type of medication did not reveal significant differences between the subgroups.
Conclusion: Saliva is a biological fluid that could be useful for novel approaches to laboratory or clinical diagnosis, and monitoring and management of patients with IBD.
Abbreviations: IBD: Inflammatory Bowel Disease; CD: Crohn’s Disease; UC: Ulcerative Colitis; RBC: Red Blood Cells Erythrocytes; WBC: White Blood Cells; EC: Epithelial Cells; oPMN: Oral Polymorphonuclear Cells; bPMN: Blood Polymorphonuclear Cells; CP: Calprotectin, FC: Faecal Calprotectin; GIT: Gastro- Intestinal Tract; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein
Introduction
The oral cavity reflects the overall condition of the body. Signs of systemic disease are commonly manifested in the oral cavity before the systemic disease itself suspected. The various oral tissues (lips, tongue, gingiva, mucous surfaces, teeth) and fluids (saliva and cervical fluid) are involved in the presentation of disease state [1]. Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) with multifactorial origin including the immune system (autoimmune response and autoantibodies against organ-specific cellular antigens shared by the gastrointestinal tract and other systems), genetic sensitivity and environmental factors (diet, use of antibiotics or NSAIDs and the presence of enteral infections) [2]. Both the intestinal mucosa and the oral mucosa contain a large number of immune cells that make up the so-called mucosal immune system, functioned to preserve the body’s constancy against antigens that have penetrated the digestive system [3]. The commensal microbial flora promotes immune processes at the level of secretion of antimicrobial peptides, regulatory and effector immune cells [4]. This symbiosis with the microbiome helps maintain homeostasis, while dysbiosis induces altered immune responses with an inflammatory response [5]. CD and UC can engage not only the mucosa of the gut, but also have extra-intestinal manifestations in the oral cavity and other organs (joints, skin, eyes, bile ducts). Oral lesions as a clinical manifestation were first described in 1969 by Dudeney [6]. The incidence ranges from 0.5% to 30% most commonly seen in Crohn’s disease (cheilitis, ulcerations, fissures and glossitis) [7]. Oral manifestations in 5-10% of cases may be the first sign of bowel disease [8] or precede the appearance of intestinal lesions by a year or more [9]. Some of the oral changes are considered disease-specific and others are non-specific (aphthous stomatitis, pyostomatitis), but they can help diagnose and monitor the activity of the process [10,11].
Oral manifestations in IBD patients may be due to other causes, such as drug reactions, infections, malnutrition, adherence to specific diets, complications (inflammatory activity, dyselectrolytemia anemia, and hypovitaminosis) [1]. The relationship between changes in the oral cavity and IBD has not been sufficiently clarified. The inflammatory response, autoimmune genesis, dysbacteriosis, and infection are thought to be factors leading to specific changes in the oral cavity, most pronounced in the activity of inflammatory bowel mucosa [12]. The oral cavity and its oral fluid are an integral part of the gastro-intestinal tract (GIT), have a common phylogenetic and morpho-functional origin and structure, obey general neurohumoral regulation and, in addition to digestive function, have a protective role for the digestive system [13]. Saliva is an aqueous secretion with a slightly acidic to neutral pH of 6.0-7.0 and includes dissolved inorganic ions and various organic substances, including proteins (mucins, immunoglobulins, enzymes), epithelial cells, bacteria, leukocytes and food residues [14]. Oral fluid is a mirror of the metabolic, functional, hormonal and emotional state of the body [15]. Investigation of saliva as a non-invasive biological material may answer the question whether the pathological process and inflammatory activity in IBD can influence and alter its cellular content and functions. These cellular and functional alterations could serve us to monitor patients’ status. The aim of the present study is to investigate the salivary cell content and composition in patients with IBD and evaluate the effect of intestinal inflammatory activity on it.
Material and Methods
Subjects
The study included 54 patients (30 women and 24 men) with IBD (40 with UC and 14 with CDs), mean age 43.9±14.7 (range: 19 to 74 years) admitted to the gastroenterology wards at the University Hospital “St. Marina” and Military Hospital - Varna during September 2017 – May 2019 for the diagnosis of IBD or exacerbation of the disease, as well as for routine control colonoscopies. The diagnoses of UC and CD of the examined patients were made based on the criteria of ECCO Consensus 2019, year (European Crohn’s and Colitis Organization), including a complex of anamnestic, clinical, laboratory and instrumental studies. All participants enrolled in the study have signed informed consent. The local Ethical Committee approved the study (Protocol No 64/13.07.2017). As a control group, 80 healthy subjects (43 women and 37 men) aged between 20-65 years (mean: 43.1±10.8 years, corresponding to that of the patient group; p>0.05) undergoing routine prophylactic examinations, including dental status at the Military hospital-Varna were examined. The exclusion criteria were inflammatory changes in the mouth and dental procedures within 48-72 hours before the examination. The preliminary requirements for the collection and primary treatment of biological material have been clarified and respected. Unstimulated native saliva is collected in the morning by a passive droplet.
Collection Protocol
In all patients, saliva was collected under the same conditions: on an empty stomach (in the morning from 8-10 h) without the use of tonic drinks (coffee) and smoking. Unstimulated saliva is used. The oral fluid is collected in special sterile containers (conical bottom and graduated) by passively repeatedly removing the amount collected in the mouth within 5-10 minutes to a total amount of 2-3 ml. For reliable results, patients were instructed to comply with the following conditions: More than 30 minutes have elapsed since the last meal, drink, chewing gum or the last toothbrush and toothbrush cleaning. Mouth 5 min before the test, rinse twice with grunt for 10 sec. with saline or mineral water.
Material Processing
The saliva is hypotonic, lytic cellular processes are accelerated, so the material is quickly processed within 15-20 minutes. Until then, the samples are refrigerated at 4°C. On the graduated scale of the containers, we count the amount of saliva collected. We spin the containers at 2500 rpm for 5 minutes. The supernatant was separated. Resolve the sediment with dilute (dilution buffer for biological material at FUS 100 cell count) of the same amount to the original volume. We homogenize well. Cell counting is performed by the FUS 100 automated urinary sedimentation system (DIRUI). The method is based on flow cytometric microscopic high-speed images to enumerate and identify the formed elements in the sample. The pictures of the sample cells are compared to a database of images of various morphologies and crystals embedded in the instrument software and classified according to shape, structure and size. The number of images is calculated as count/μL. The morphological characteristics of the different cellular elements obtained by the automatic analysis are also verified visually. Routine laboratory methods were used for the assay of the inflammatory markers: WBC count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). All IBD patients were tested for FC, a specific biomarker for intestinal inflammation, by the quantitative immunochromatographic method Point-of-Care Test (Quantum Blue®fCAL, BÜHLMANN).
Statistical Analysis
Descriptive statistical analysis, non-parametric T-test for comparison of the mean values and Spearman correlation test were used for data processing. Values of p <0.05 were considered significant.
Results
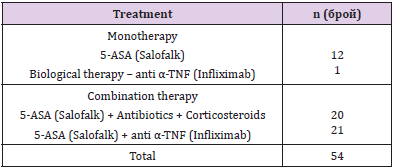
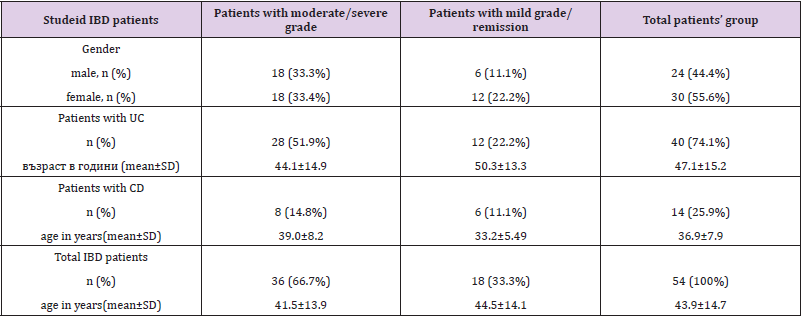
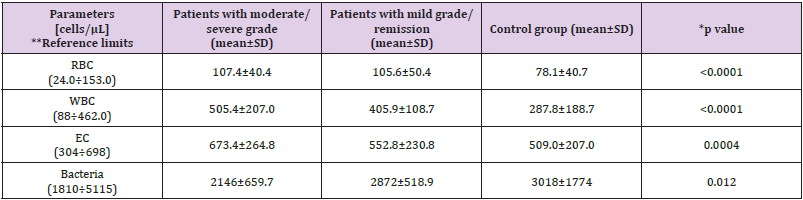
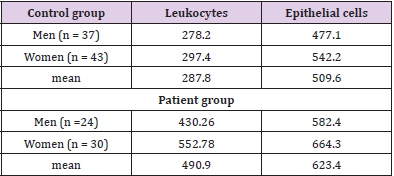
The mean disease duration was 7.44±9.51 (1-31) years. All patients are on therapy (mono- or combination therapy) given in (Table 1). The pharmacological management of IBD aims to reduce inflammation and maintain remission. Therapy includes sulfasalazine, 5-aminosalicylates, glucocorticoids and immunosuppressants, and a series of biological agents, monoclonal antibodies (adalimumab, golimumab, infliximab). The classification of patients with IBD according to disease activity/severity is given in (Table 2). It is based on the indices used to evaluate activity or disease severity: for CD - CDAI (Crohn’s Disease Activity Index), and for UC - Mayo index. According to these indices, we divided the patients included in the study into two groups. The first group was patients with active disease (CDAI> 220 and Mayo index ≥2), and the second group was patients with mild or remission. The number of cellular elements (epithelial cells, erythrocytes, leukocytes and bacteria) are presented in (Table 3). (Figure 1) Leukocytes and epithelial cells, as significant components of the mucosal epithelial barrier, are most affected by changes in physiological and pathological conditions. Physiological factors such as gender and age have been found to influence the values of these parameters in both the control and patient groups. The comparative analysis showed that the number of cells in the norm had a gender variation. Women have slightly higher values than men.
Table 3: Cellular elements in unstimulated saliva in the three study groups.
Note: *Differences in the number of cell elements in the three study groups were evaluated using One way ANOVA Bonferroni correction
**The reference limits were determined for the purposes of our study in a representative group of clinically healthy persons from the Bulgarian population. The selection of persons from the reference group (n =186) was carried out randomly - from persons passing to MMA-Varna, subject to a routine annual preventive examination or expert assessment, without subjective and objective deviations (healthy persons) and normal dental status.
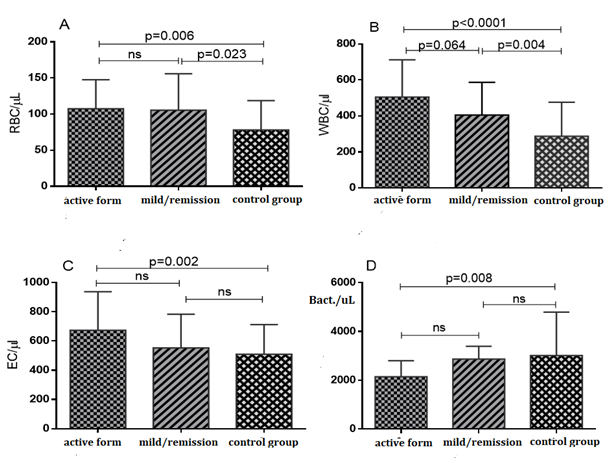
Figure 1: Comparison of salivary cell elements in the three studied groups
A) Erythrocytes,
B) Leukocytes,
C) Epithelial cells and
D) Bacteria (One way ANOVA with Bonferroni correction is attached).
The data are presented in (Table 4). Similar observations are described by Rijkschroeff, et al. [16]. Many studies show that the oral mucosa is sensitive to the effect of sex hormones (estrogens and progesterone). Different periods in a woman’s life, such as puberty, pregnancy, and menopause, are associated with changes in their levels affect oral mucosa. Donald, et al. [17,18] disclose wellexpressed rhythmic changes in the oral cavity cells, coinciding with changes detected in vaginal smears, thus reflecting the hormonal state of the menstrual cycle [17,18]. We also find a moderate positive correlation (r=0.358; p=0.009) between age and increased oral leukocytes, but not with epithelial cells. The number of leukocytes in the mouth decreases in proportion to the reduction of teeth due to their inability to migrate through the gingival crevices. The mean disease duration was 7.44±9.51 (1-31) years. We found no relationship between disease duration and cellular element values. They are influenced by the activity of the inflammatory process.
Table 4: Values of leukocytes and epithelial cells in control and patient groups and their sex dependence.
Discussion
Cells found in the oral fluid have different origins. Leaving the ductus of the parotid gland, the saliva contains no cellular elements [19]. They are added by buccal mucosa, migration of leukocytes and erythrocytes from different areas of the oral mucosa. The largest is the abundance of epithelial cells that are usually excreted in chewing processes. The oral cavity is covered by squamous epithelial cells, which are a barrier against mechanical, chemical damage and pathogenic microbiological invasion [20]. The normal mucosal epithelium is a multilayer flat with high regenerative capacity. Due to its involvement in the digestive process, however, it remains un keratinized or partially corrosive in certain places (such as the intricately arranged mucous membrane of the tongue with the papillae available) and secretes mucus to facilitate the passage of food. It is already known that epithelial cells are not passive bystanders, but are metabolically active and able to respond to external stimuli by synthesizing several cytokines, adhesion molecules, growth factors, chemokines and matrix metalloproteases [21]. Morphologically, squamous cells have a small nucleus and a large polygonal cytoplasm with numerous granulations. Cell sizes are 85-125 μm, while those of the gingiva and periodontium are smaller in size. According to Watanabe, the number of epithelial cells in the saliva is 4x105/ml. [22].
Several studies have tracked the number and change in morphology of epithelial cells in screening and diagnosis of precancerous conditions and oral cancer [23]. Inflammatory lesions and periodontal disease also lead to an increase in the number of squamous cells secreted. Our study found that the control group had higher values of epithelial cells, given by some researchers as the norm. This can be explained by the presence of dental bridges and dental crown, whose finding in the oral cavity increases with age. Smoking is also an essential factor acting irritably on the oral mucosa and a significant risk factor for inflammatory and neoplastic processes. In a previous study, we found a statistically significant difference in the number of epithelial cells and leukocytes in smokers compared to non-smokers. Of the subjects in our control group, smokers were 36, representing 45%, and 23 (43%) from the patient group. We observed an increased number of epithelial cells in patients with IBD, which was statistically significant relative to the control group (p=0.0192). However, no such dependence was observed between the two patient groups. The finding is believed to be due to the chronic inflammatory process, dehydration and poor nutrition, especially during relapse. Little is known about the factors that affect the relative number of epithelial cells and leukocytes in the oral cavity. The buccal mucosa is relatively permeable and has a rich blood supply, with continuous flow and migration of leukocytes from the gingival fluid through the gingival gap in the saliva [24].
Healthy buccal mucosa contains several cells involved in immune function, including lymphocytes (T cells), polymorphonuclear cells (including neutrophils and eosinophils), and mast cells [25,26]. They protect the oral cavity and are an element of the innate immune response. Leukocyte counts in norm and pathology have been the subject of several studies. It is estimated that about 1 – 4x105 cells are normally found in 1 ml of saliva. Their number has inter-individual but also circadian intra-individual variation [19]. Leukocytes are predominantly represented by polymorphonuclear cells (PMNs) - 90% and 10% monomorphonuclear cells (MMNs). The major representatives of MMNs are B lymphocytes (60%), T cells (about 20-30%) and macrophages (~10%), which are integral parts of mucosa-associated lymphoid tissue (MALT). Its role is to induce an immune response to specific antigens from the environment and to develop local immunity. In a healthy state, approximately 30,000 oral PMNs (oPMN) per minute arrive through gingival cervical fluid (GCF), which inflows into the oral cavity from the periodontal sulcus [27]. Eighty percent of oPMNs are viable and functional in the gingival gap and play a phagocytic and antibacterial role. In the hypotonic saliva, oPMNs undergo rapid lytic changes. Functional PMNs are paramount to innate immunological processes, including maintenance of oral health.
In acute and chronic inflammatory processes in the oral cavity, the amount of oPMN increases. Rijkschroeff, et al. [16] report significantly higher expression of CD11b in oPMNs compared to circulating blood PMNs in healthy individuals, suggesting their facilitated migration through oral mucosal tissues. There are insufficient studies on the effects of systemic diseases on salivary cell composition and oral hemostasis. We found a statistically significant difference (p<0.0001) in leukocyte counts between the control group and patients with IBD, but there was no similar relationship between the two patient groups (with activity and mild/remission). The active involvement of oral mucosa in the immunological response and inflammation most likely causes leukocyte growth in the saliva. We did not find a correlation between the total number of circulating blood leukocytes and the number of salivary leukocytes. Although bPMNs reflect the general inflammatory state of an individual, the number of oPMNs varies greatly depending on local influences in the oral cavity [16,28]. A similar weak correlation was observed between oPMN and C-reactive protein as a marker of inflammation. Corticosteroid therapy probably affects the content of cellular elements in the oral fluid, causing increased permeability and migration of erythrocytes and leukocytes through the gingival crevices. Calprotectin is a cytoplasmic protein secreted by the degranulation of neutrophil leukocytes and monocytes locally at the site of inflammation.
Faecal calprotectin (FC) has in recent years become a marker reflecting the activity of the inflammatory mucosal response in IBD. Majster, et al. [28] examined calprotectin in saliva from patients with IBD, finding elevated values in both stimulated and unstimulated saliva. In our patients, we routinely examine FC for disease monitoring. By correlation analysis, we checked whether there was a relationship between oral leukocyte count and FC as a marker of inflamed intestinal mucosa. A weak correlation r=0.2043 was found. For comparability, the correlation between the number of salivary leukocytes and salivary calprotectin should be used. The normal amount of erythrocytes in the oral fluid is negligible, although there is no accurate literature data on their normal count per milliliter. Most studies look at the increase in red blood cells and the presence of salivary blood in fissures, lesions, periodontal or cancerous diseases in the oral cavity [29,30]. Our study found equally higher erythrocyte values in both patient groups, regardless of the inflammatory process activity. This is most likely due to side effects of medicines leading to increased permeability of the vessels in the oral cavity, ecchymoses and bleeding. It is more commonly observed in patients with mono or combination therapy with Infliximab (a TNFα inhibitor) [31]. The oral cavity and gastrointestinal tract are highly colonized sites [16]. Bacteria are the most numerous cells, represented mainly by normal microflora, but pathological species can also be found.
In healthy individuals, the microflora have a symbiotic relationship with the host organism and possess important and unique functions, including metabolic function. The human oral microbiome is regarded as a community of more than 300 species of microorganisms, with a total salivary total of 106-108 CFU/ml (colony-forming unit) [32]. Gram positive bacteria predominate, and anaerobic bacteria are about 10 to 100 times the amount of aerobes [33]. In the study group, especially those with inflammatory process activity, the bacterial count was statistically significantly lower. During the relapse, antibiotic therapy is included as part of the treatment and treatment of the inflammatory process. Most likely, antibiotics lead to microflora suppression by reducing their population or leading to dysbiosis. The thesis about the reducing role of AB against the oral microflora is confirmed by the fact that in the second patient group and without antibiotic therapy, the number of salivary bacteria is almost similar to that of the control group.
Conclusion
Oral health and homeostasis, maintaining oral hygiene is crucial for patients with IBD during their treatment and remission. The concept of enumeration of cellular elements in saliva with the FUS 100 automated system may be proposed as a possible screening method for the indication of inflammatory changes in the mouth. A rapid, non-invasive qualitative and quantitative assessment of cellular composition would be a new approach in laboratory or clinical diagnosis and monitoring and management of patients with IBD.
Author Contributions
The primary and corresponding author was responsible for conducting the research and investigation process. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geraldine N (2014) Systemic Disease Manifestations in the Oral Cavity. Osteopathic Family Physician 6(3): 16-21.
- Lee SH, Kwon JE, Cho ML (2018) Immunological pathogenesis of inflammatory bowel disease. Intest Res 16(1): 26-42.
- Chang John T (2020) Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med 383: 2652-2664.
- Chung H, Kasper DL (2010) Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Current opinion in immunology 22(4): 455-460.
- Hisamatsu T, Kanai T, Mikami Y, Yoneno K, Matsuoka K, et al. (2013) Immune aspects of the pathogenesis of inflammatory bowel disease. Pharmacology & therapeutics 137(3): 283-297.
- Dudeney TP (1969) Crohn's disease of the mouth. Proc R Soc Med 62(12): 1237.
- Annese V (2019) A Review of Extraintestinal Manifestations and Complications of Inflammatory Bowel Disease. Saudi J Med Med Sci 7(2): 66-73.
- Plauth M, Jenss H, Meyle J (1991) Oral manifestations of Crohn's disease. An analysis of 79 cases. J Clin Gastroenterol 13(1): 29-37.
- Stavropoulos F, Katz J, Guelmann M, Bimstein E (2004) Oral ulcerations as a sign of Crohn's disease in a pediatric patient: A case report. Pediatr Dent 26(4): 355-358.
- Zois C (2010) Neurologic manifestations in inflammatory bowel diseases: Current knowledge and novel insights. J Crohn’s Colitis 4(2): 115-124.
- Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, et al. (2005) Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol 11(46): 7227-7236.
- Ribaldone DG, Brigo S, Mangia M, Saracco GM, Astegiano M, et al. (2020) Oral Manifestations of Inflammatory Bowel Disease and the Role of Non-Invasive Surrogate Markers of Disease Activity. Medicines (Basel) 7(6): 33.
- Groeger S, Meyle J (2019) Oral Mucosal Epithelial Cells. Front Immunol 10: 208.
- Roblegg E, Coughran A, Sirjani D (2019) Saliva: An all-rounder of our body. Eur J Pharm Biopharm 142: 133-141.
- Ahmadi Motamayel F, Davoodi P, Dalband M, Hendi SS (2010) Saliva as a mirror of the body health. DJH 1(2).
- Rijkschroeff P, Loos BG, Nicu EA (2017) Impaired polymorphonuclear neutrophils in the oral cavity of edentulous individuals. Eur J Oral Sci 125(5): 371-378.
- Donald PM, George R, Sriram G, Kavitha B, Sivapathasundharam B (2013) Hormonal changes in exfoliated normal buccal mucosal cells. J Cytol 30(4): 252-256.
- Ziskin De, Moulton R (1948) A comparison of oral and vaginal epithelial smears. J Clin Endocrinol Metab 8(2): 146-165.
- Klein H (1962) Cellular elements in the saliva of infants before and after eruption of teeth. J Dent Res 41: 1017-1020.
- Rachel Bittern, Robin Orchardson (2000) The effect of stimulus form and dimensions on the oral size illusion in humans. Archives of Oral Biology 45(6): 453-459.
- Watanabe J (1951) Effect of Roentgen Irradiation upon Cellular Elements in the Human Saliva. Oral Surg Oral Med Oral Path 4: 89-107.
- Elimairi I, Sami A, Yousef B (2017) Oral Cancer and Potentially Malignant Disorders. In: Srivastava S (Edt.)., Histopathology - An Update. London: IntechOpen.
- Harold Marcotte, Marc C Lavoie (1998) Oral Microbial Ecology and the Role of Salivary Immunoglobulin. A Microbiol Mol Biol Rev 62(1): 71-109.
- Burnett GW, Scherp HW (1962) Oral microbiology and infectious disease; a textbook for students and practitioners of dentistry. (Williams and Wilkins Co), pp. 322-323.
- Squier CA, Kremer MJ (2001) Biology of oral mucosa and esophagus. Journal of the National Cancer Institute Monographs 29: 7-15.
- Moonen CGJ, Hirschfeld J, Cheng L, Chapple ILC, Loos BG, et al. (2019) Oral Neutrophils Characterized: Chemotactic, Phagocytic, and Neutrophil Extracellular Trap (NET) Formation Properties. Front Immunol 10: 635.
- Stephens DJ, Edgar J (1934) Leucocytes in the Saliva in Normal and Abnormal Subjects. Experimental Biology and Medicine 31(7).
- Majster M, Almer S, Boström EA (2019) Salivary calprotectin is elevated in patients with active inflammatory bowel disease. Arch Oral Biol 107: 104528.
- Patil PB, Patil BR (2011) Saliva: A diagnostic biomarker of periodontal diseases. J Indian Soc Periodontol 15(4): 310-317.
- Laskaris G (1994) Color Atlas of Oral Diseases. Portal Saude Direta, Georg Thieme Verlag, Inc. Stuttgart. New York.
- Yuan A, Woo SB (2015) Adverse drug events in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol 119(1): 35-47.
- Schmidt S, Winter J, Gallert C (2012) Long-term effects of antibiotics on the elimination of chemical oxygen demand, nitrification, and viable bacteria in laboratory-scale wastewater treatment plants. Arch Environ Contam Toxicol 63(3): 354-364.
- Contaldo M, Fusco A, Stiuso P, Lama S, Gravina AG, et al. (2021) Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-Intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 9(5): 1064.

 Research Article
Research Article