ABSTRACT
Objective: This study aimed to explore and verify the antigout activity of pachyman on alleviating the hyperuricaemia of rats and the underlying mechanisms were examined.
Methods: 60 males Wistar rats were randomly divided into blank group, model group, PM high-, medium- and low-dose groups and benzobromarone (BBR) groups. Adenine combined with allantoxanic acid potassium salt was used to replicate hyperuricemia model in rats, and intragastric administration began from the third week, once a day for 30 consecutive days. Serum uric acid, IL-1β, TNF-α inflammatory factors, SOD, GSH-Px oxidation factors, and the expression of TKR4, NF-κB p65 protein were determined.
Results: Compared with the blank group, adenine combined with allantoxanic acid potassium salt could successfully replicate the hyperuricemia model (P<0.01). PM can remarkably reverse hyperuricemia and show a dose-dependent response. The effect of PM medium- and high-dose groups was similar to or better than BBR. PM significantly reduced TLR4/ NF-κB expression(P<0.01), and the serum levels of IL-1β, TNF-α, SOD and GSH-Px were also reduced(P<0.01), which was demonstrated the antigout effects of PM.
Conclusion: PM can effectively treat gout by regulating the TLR4/ NF-κB pathway to inhibit inflammation and play an antioxidant role.
Keywords: Gout; Hyperuricemia; Pachyman; Serum Uric Acid; NF-κB p65
Abbreviations: BBR: Benzbromarone; ELISA: Enzyme-Linked Immunosorbent Assay; GAPDH: Glyceraldehyde-3-phosphate Dehydrogenase; GSH-PX: Glutathione peroxidase; IL-1β: Interleukin 1 Beta; MSU: Monosodium Urate; NF-κB p65: Nuclear Factor-Kappa B p65; PM: Pachyman; PMSF: Phenyl Methane Sulfonyl Fluoride; PVDF: Polyvinylidene Fluoride; SOD: Superoxide Dismutase; SUA: Serum Uric Acid; TLR4: Toll-like Receptor 4; TNF-α: Tumor Necrosis Factor-α
Introduction
Gout, as a metabolic disorder that results from sustained elevation of serum urate levels (hyperuricaemia), the incidence of gout has been increasing for decades [1]. The continuous elevation of serum uric acid forms monosodium urate (MSU) crystals, which deposit in tissues and joints subsequently induces an inflammatory response [2]. This is the most common form of inflammatory arthritis globally. Inadequate urate excretion has been demonstrated to induce hyperuricemia [3,4]. Therefore, reducing serum uric acid (SUA) level and treating inflammation have become the goal of treating gout in clinic. The nuclear factor NF-κB pathway has long been considered a prototypical pro-inflammatory signaling pathway, largely based on the role of NF-κB in the expression of pro-inflammatory genes including cytokines, chemokines, and adhesion molecules [5]. There is wildly known that the TLRs/ NF-κB signal transduction pathway is one of the main pathways involved in gout, which can directly regulate the expression of pro-inflammatory factor IL-1β [6]. And IL-1β can activate other pro-inflammatory cytokines and including tumor necrosis factor (TNF)-α, which are critical for the initiation and propagation of the inflammatory response. Also, there is a large amount of evidence that reactive oxygen species overproduction and oxidative stress are crucial in the pathogenesis of gout [7,8].These factors and pathways all play extremely notable roles in the regulation of goutassociated immune and inflammatory responses. The suppression of these pro-inflammatory mediators has been found to reduce the severity of the inflammatory reaction [9].
Clinically, non-steroidal anti-inflammatory drugs, colchicine and uric acid-lowering drugs are mostly used to reduce and control gout attacks. However, these drugs may lead to adverse reactions such as gastrointestinal reactions, liver and kidney damage, bone marrow suppression and so on, which greatly limits the use of these drugs. Although alternative drug options exist, such as modified uricase and interleukin-1 inhibitors [10], patients with gout still have not been effectively treated. The new strategies for alleviating gout attacks require good efficacy and safety, but the current treatment of gout attacks is still short of ideal drugs. Herbal drugs have been applied to treat gout in Traditional Chinese Medicine for more than 2000 years [11]. In recent years, the satisfactory effect has been got in that classical traditional Chinese medicine formulas isolated from some herbs are used to treat gout [12]. Pachyman (PM) is one of the main components of poria coco, and it has a variety of pharmacological effects, including immunomodulatory [13], antitumor [14], anti-oxidation [15,16], anti-inflammatory [17] and anti-depression [18]. Poria cocos is one of the top 10 commonly used drugs in Chinese herbal medicine for clinical treatment of gout. But so far, no relevant studies have reported the efficacy and mechanism of PM in the treatment of gout. Hyperuricemia is a major cause of the increase and development of gout. Therefore, in this study, hyperuricemia was used as a model to study the efficacy and mechanism of PM in the treatment of gout.
Materials and Methods
Reagents and Chemicals
Pachyman was purchased from Solarbio(Shanghai, China). 97% allantoxanic acid potassium salt and adenine were purchased from Sigma-Aldrich (Shanghai, China). Benzbromarone (BBR) was purchased from Heumann Pharma (Germany). Uric acid assay kits, Total superoxide dismutase (SOD) assay kit, Glutathione peroxidase (GSH-PX) assay kit, Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α and IL-1β were purchased from Jiancheng Biotech (Nanjing, China). Antibodies, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), toll-like receptor 4 (TLR4) and nuclear factor kappa B (NF-κβ), were purchased from Abcam, UK.
Animals
A total of 60 males Wistar rats (200±20 g) were purchased from the Laboratory Animal Center, Chengdu University of Traditional Chinese Medicine. All animals were housed five per cage under controlled conditions (12 h light/dark cycles with a temperature of 22±2˚C and a humidity of 60-70%), fed with a standard laboratory chow and had free access to water for the duration of the experiment. All animal care and experimental procedures were approved by the Animal Care Ethics and performed in accordance with the guidelines of this committee.
Grouping and Drug Administration
Using adenine combined with allantoxanic acid potassium salt to create a model of hyperuricemia after 1 week of adaptation, and the rats were randomized into 6 groups, each containing 10 animals: blank group, model group, BBR group, PM high-, mediumand low-dose groups. Except for the blank group was administered an equal volume of normal saline by gavage once per day, adenine (0.1 g/kg) and 97% allantoxanic acid potassium salt (0.1g/kg) were administered by gavage to each group for seven consecutive days to induce the hyperuricaemic model. The benzbromarone group received 20 mg/kg benzbromarone, and an equal volume of normal saline was administered to the model and blank groups. PM high-, medium- and low-dose groups were 80mg/kg, 40mg/kg, 20mg/kg, respectively. The dose was 10ml/kg. Administration was performed once per day by gavage, and the treatment period was one month.
Uric Acid Assays
The levels of uric acid in the serum were assayed by kits according to the manufacturer’s instructions.
Proinflammatory Cytokines Assay
The levels of IL-1β and TNF-α in the serum were determined using commercial Rat IL-1β and TNF-α ELISA kits, respectively, according to the manufacturer’s protocols. In short, 100 μl of the diluent standard or serum samples were added into the coated wells and incubated at 37˚C for 2h. Following removal of the supernatant, 100 μl biotinylated antibody solution was added and incubated for 1h at 37˚C. Following washing three times, 100 μl avidin-peroxidase complex solution was added and incubated for 1h at 37˚C. Following washing, 90 μl tetramethylbenzidine color solution was added and incubated in the dark for 30 min at 37˚C. Finally, 50 μl stop solution was added to terminate the reaction, and the optical density at 450 nm was measured using a plate reader.
Estimation of Oxidative Stress
The activities of SOD and GSH-Px in the serum were determined using commercial assay kits obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions.
Western Blot
Part of the kidney tissue samples was immersed in precooled RIPA buffer containing phenyl methane sulfonyl fluoride (PMSF) inhibitor and homogenized at 4°C. Then, the homogenate was centrifuged at a speed of 13,000 r/min for 10 minutes and the supernatant was collected. Following, a BCA protein determination kit was used to detect the protein concentration of the sample. These protein samples were mixed with the sample buffer (4 :1) and boiled for 10 minutes, the proteins were electrophoresed on 10% SDS-PAGE and transferred to PVDF membranes for 1 hour. The membranes were blocked in 5% skim milk powder dissolved in TBST for 2 hours. Then, the membrane was incubated overnight with TLR4 (1:1,000), NF-κB p65 (1:1,000), and GAPDH (1:1,000) antibodies at 4°C. Following washing three times, the membranes were incubated with a suitable secondary antibody conjugated with HRP for 2 hours at room temperature and then washed the membrane three times again. The protein bands were detected by an ECL kit and analysed by ImageJ software.
Statistical Analysis
In this experiment, SPSS 19.0 software was used for data processing. The graphs were drawn with GraphPad Prism 7.0. All data were subjected to one-way analysis of variance (ANOVA) and expressed by mean ± standard deviation (SD). LSD t-tests were applied when the homogeneity of variance assumptions was satisfied; otherwise, Dunnett’s t-test was used. P < 0.05 was statistically significant.
Results
PM Affects the levels of UA in Hyperuricemia Rats
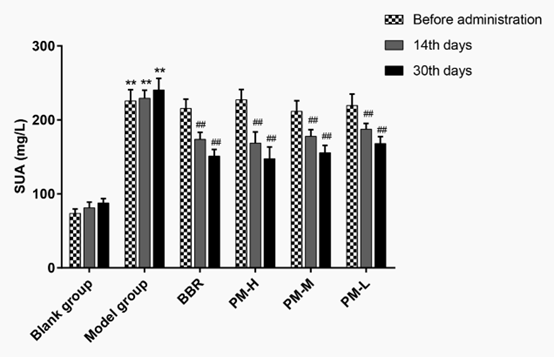
As shown in Figure 1, before administration, except for the blank group, the serum uric acid (SUA) level was significantly higher than that of the blank group after 7 consecutive days of administration of modelling agent in the other groups (P <0.01), which indicated that the model of hyperuricemia was successfully established. On the 14th and 30th day after administration, SUA in the model group was maintained at a high level (P <0.01), which indicated that adenine combined with allantoxanic acid potassium salt could successfully replicate the hyperuricemia model. Compared with model group, SUA level was dramatically decreased in both BBR group and PM high-, medium- and low-dose groups after 14 days of continuous administration. Furthermore, after 30 days of continuous administration, the effect was even more significant. We also know that PM high- and medium-dose groups were better than or equal to BBR group. There was a dose-response relationship between high, medium and low doses of PM. It follows that PM alleviated the hyperuricemia.
Figure 1: Changes of PM to SUA in hyperuricaemia rats. SUA: Serum uric acid; BBR: Benzbromarone; P < 0.01 compared with the blank group; ## P < 0.01 compared with the model group.
Effects of PM on the levels of IL-1β and TNF-α
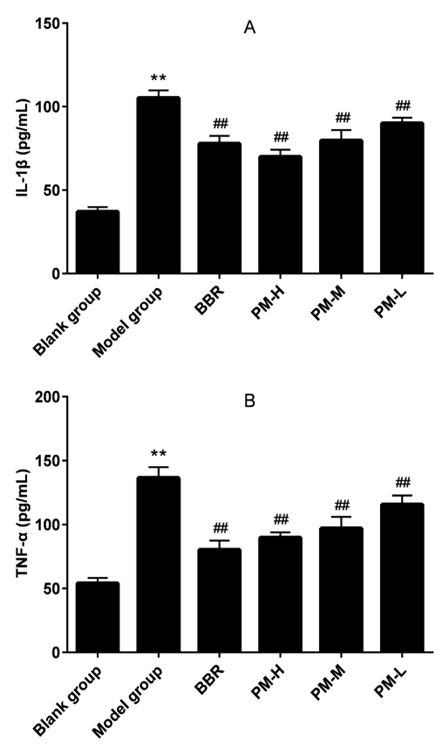
The level of IL-1β and TNF-α in the serum were shown in Figure 2. The model of hyperuricemia was successfully established that result in a significant increase of IL-1β and TNF-α levels in the serum, which indicated that the hyperuricemia model can produce a significant inflammatory response. Compared with the model group, treatment with BBR and all doses of PM reduced IL- 1β and TNF-α towards the normal level (P <0.01). Here, the PM group demonstrated an anti-inflammatory effect, and the antiinflammatory effect became more pronounced with increasing dose.
Figure 2: The effects of PM on IL-1β (A) and TNF-α (B) in hyperuricaemia rats. IL-1β: Interleukin 1 Beta; TNF-α: Tumor Necrosis Factor-α; BBR: Benzbromarone; PM-H: high dose of Pachyman; PM-M: medium dose of Pachyman; PM-L: low dose of Pachyman. **P < 0.01 compared with the blank group; ##P < 0.01 compared with the model group.
Effects of PM on the Anti-Oxidant Status of the Serum of Hyperuricemia Rats
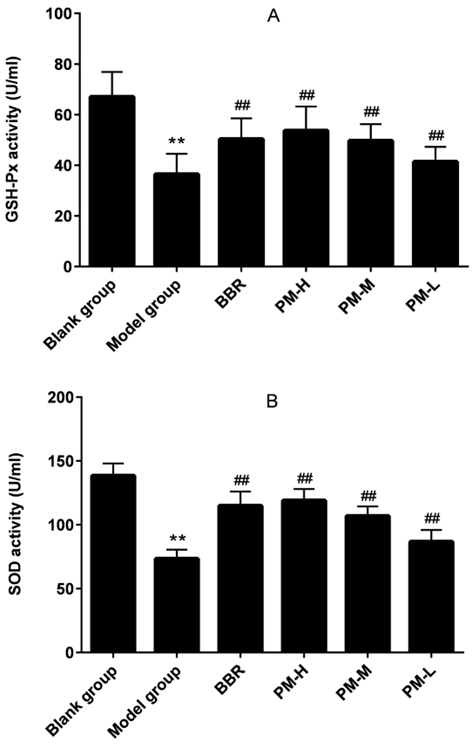
The production of inflammation breaks the body’s dynamic equilibrium state and accelerates the production of oxygen free radicals, which further affect the body’s function and metabolism, resulting in a decrease in the body’s resistance [19,20]. Therefore, we measured the antioxidant effect of PM in hyperuricemia by measuring SOD and GSH-Px. Compared with the blank group, the establishment of hyperuricemia model led to significant decreases in the activities of SOD and GSH‑Px. Treatment with PM was able to significantly improve SOD and GSH-Px levels at any tested dose. Meanwhile, the PM treatment dose dependently improved the antioxidation, which indicated that PM reduced hyperuricemia induced oxidation (Figure 3).
Figure 3: The effects of PM on GSH-Px (A) and SOD (B) in hyperuricaemia rats. GSH-Px: Glutathione peroxidase; SOD: Superoxide dismutase; BBR: Benzbromarone; PM-H: high dose of Pachyman; PM-M: medium dose of Pachyman; PM-L: low dose of Pachyman. **P < 0.01 compared with the blank group; ##P < 0.01 compared with the model group.
Effects of PM on TLR4 and NF-κB p65 Protein Levels in Hyperuricemia Rats
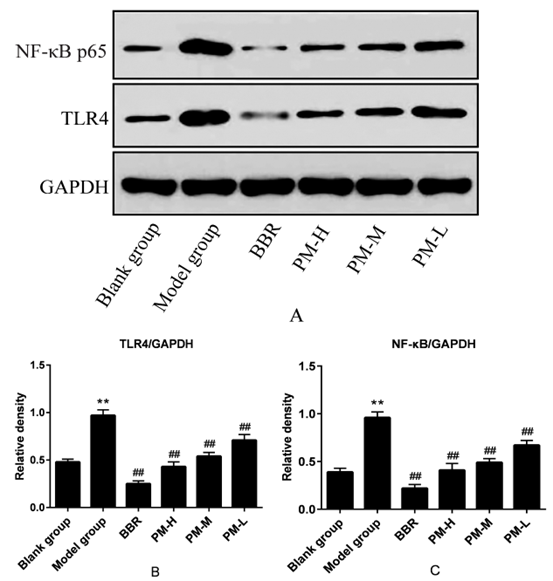
The results are shown in Figure 4. Compared with the blank group, the expression levels of TLRs and NF-κB p65 protein in the model group were significantly up-regulated, and the differences were statistically significant (P <0.01). Compared with the model group, BBR and PM high-, medium- and low-dose could significantly down-regulate the expression of TLR4 and NF-κB p65 protein in a dose dependent manner, and the differences are statistically significant (P <0.01). The results inferred that PM may play an antiinflammatory and anti-oxidant role by regulating TLRS /NF-κB signaling pathway, and then play an anti-hyperuricemia role.
Figure 4: The effects of PM on TLR4 and NF-κB p65 protein levels in hyperuricaemia rats. TLR4: Toll-like Receptor 4; NF-κβ: Nuclear factor-kappa β; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; BBR: Benzbromarone; PM-H: high dose of Pachyman; PM-M: medium dose of Pachyman; PM-L: low dose of Pachyman. ** P < 0.01 compared with the blank group; ##P < 0.01 compared with the model group.
Discussion
Gout is a treatable disease resulting from a disturbance in purine metabolism. Uric acid is often synthesized by purine metabolism, hyperuricemia is easily formed when uric acid is produced too much or excreted too little, which further lead to gout disease. Mats Dehlint et al reports of the prevalence and incidence of gout were ranged from a prevalence of <1% to 6.8% and an incidence of 0.58-2.89 per 1,000 person-years. BBR, colchicine, Febuxostat, Topiroxostat and Lesinurad are the main clinical drugs, but they are associated with kidney damage, liver toxicity, cardiotoxicity, drowsiness and other side effects. Thus, the development of safer and more active agents, especially Chinese herbal medicine extracts, is urgently needed. According to Zhu’s, et al. [21] research we know that uric acid can be further degraded into allantoin by uricase in rodents, but not in humans. Therefore, to reduce the interspecies difference between humans and rats, we used the method described in previous studies to establish hyperuricemia model by intragastry of 97% potashallantoinate and adenine. Our results demonstrate that this approach is very successful in establishing a model of hyperuricemia. In this study, poria cocos is one of the most used traditional Chinese medicines for the treatment of gout in clinic, and PM is main component of poria cocos. Studies have shown that poria cocos can exert anti-inflammatory [17,22] and anti-oxidative [23] pharmacological effects by regulating the NF-κB pathway [24,25]. Therefore, in this study, we assumed that the PM also had anti-gout effect by lowering uric acid, and then by measuring IL-1β, TNF-α inflammatory factors, SOD, GSH-PX oxidation factor, and the expression of TKR4, NF-κB p65 protein to verify that the PM could regulated TLR4/NF-κB pathway inhibition inflammation, play an anti-oxidant effect, and then have an anti-gout effect.
NF-κB signaling pathway plays a central role in inflammationrelated diseases of gout, which TLR4 pathway could regulate the NF-κB signals [26]. In Figures 2-4, we can obviously see that IL-1β, TNF-α inflammatory factors, SOD, GSH-Px oxidation factor, and the expression of TKR4, NF-κB p65 protein increased dramatically in the model group, which was the same as the result of SUA in Figure 1. This is consistent with previous research [27] that gout is a common inflammatory disease and uric acid can activate NF-κB mediated inflammatory response in hyperuricemia rats. These results indicated that gout can be more intuitively represented by these important pathway proteins, inflammatory cytokines. Compared with the model group, we can see that PM can significantly reduce the expression of inflammatory factors, oxidative factors and TLR4/NF-KB protein. It was demonstrated that PM can treat gout by modulating the signal pathway and inhibiting inflammation.
Conclusion
In conclusion, PM can reduce serum levels of TNF-α, IL-1β, SOD and GSH-PXD in hyperuricemia rats, which may be related to the inhibition of TLR4/ NF-kB mediated immune inflammatory signaling pathway, but the specific mechanism of action needs to be further investigated and confirmed. PM as one of main components of poria cocos has the advantages of safe use and wide range and will have a good application prospect in the field of hyperuricemia.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgement
This research was supported by Natural Science Research Projects of Jiangsu Colleges and Universities (17KJB350002). The assistance of the staff is gratefully acknowledged.
References
- Cui L, Meng L, Wang G, Yuan X, Li Z, et al. (2017) Prevalence and risk factors of hyperuricemia: results of the Kailuan cohort study. Mod Rheumatol 27(6): 1066-1071.
- Chen J, Wu M, Yang J, Wang J, Qiao Y, et al. (2017) The Immunological Basis in the Pathogenesis of Gout. Iran J Immunol 14(2): 90-98.
- Major TJ, Dalbeth N, Stahl EA, Merriman TR (2018) An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol 14(6): 341-353.
- Perez-Ruiz F, Calabozo M, Erauskin GG, Ruibal A, Herrero-Beites AM (2002) Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 47(6): 610-613.
- Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6): a001651.
- Dinarello CA (2010) How interleukin-1beta induces gouty arthritis. Arthritis Rheum 62(11): 3140-3144.
- Kiyani MM, Butt MA, Rehman H, Ali H, Hussain SA, et al. (2019) Antioxidant and anti-gout effects of orally administered zinc oxide nanoparticles in gouty mice. J Trace Elem Med Biol 56: 169-177.
- Krishnan E (2010) Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout Rheumatology (Oxford) 49(7): 1229-1238.
- Stow JL, Low PC, Offenhauser C, Sangermani D (2009) Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology 214(7): 601-612.
- Hershfield MS, Roberts LJ, Ganson NJ, Kelly SJ, Santisteban I, et al. (2010) Treating gout with pegloticase, a PEGylated urate oxidase, provides insight into the importance of uric acid as an antioxidant in vivo. Proc Natl Acad Sci U S A 107(32): 14351-14356.
- Yao R, Geng Z, Mao X, Bao Y, Guo S, et al.(2020) Tu-Teng-Cao Extract Alleviates Monosodium Urate-Induced Acute Gouty Arthritis in Rats by Inhibiting Uric Acid and Inflammation. Evid Based Complement Alternat Med 2020: 3095624.
- Xiao N, Chen H, He SY, Xue CX, Sui H, et al. (2018) Evaluating the Efficacy and Adverse Effects of Clearing Heat and Removing Dampness Method of Traditional Chinese Medicine by Comparison with Western Medicine in Patients with Gout. Evid Based Complement Alternat Med 2018: 8591349.
- Chen X, Zhang L, Cheung PC (2010) Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1-->3)-d-glucan from Poria cocos. Int Immunopharmacol 10(4): 398-405.
- Liu X, Wang X, Xu X, Zhang X (2019) Purification, antitumor and anti-inflammation activities of an alkali-soluble and carboxymethyl polysaccharide CMP33 from Poria cocos. Int J Biol Macromol 127: 39-47.
- Tang J, Nie J, Li D, Zhu W, Zhang S, et al. (2014) Characterization and antioxidant activities of degraded polysaccharides from Poria cocos sclerotium. Carbohydr Polym 105: 121-126.
- Wang N, Zhang Y, Wang X, Huang X, Fei Y, et al. (2016) Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int J Biol Macromol 83: 103-110.
- Huang YJ, Hsu NY, Lu KH, Lin YE, Lin SH, et al. (2020) Poria cocos water extract ameliorates the behavioral deficits induced by unpredictable chronic mild stress in rats by down-regulating inflammation. J Ethnopharmacol 258: 112566.
- Zhang W, Chen L, Li P, Zhao J, Duan J (2018) Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw) Wolf. Int J Biol Macromol 120(Pt B): 1696-1704.
- Murunikkara V, Rasool M (2014) Trikatu, a herbal compound that suppresses monosodium urate crystal-induced inflammation in rats, an experimental model for acute gouty arthritis. Cell Biochem Funct 32(1): 106-114.
- Sabina EP, Rasool M, Mathew L, Ezilrani P, Indu H (2010) 6-Shogaol inhibits monosodium urate crystal-induced inflammation--an in vivo and in vitro Food Chem Toxicol 48(1): 229-235.
- Zhu Y, Peng X, Ling G (2017) An update on the animal models in hyperuricaemia research. Clin Exp Rheumatol 35(5): 860-864.
- Jeong JW, Lee HH, Han MH, Kim GY, Hong SH, et al. (2014) Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 2647 macrophages. BMC Complement Altern Med 14: 101.
- Tian H, Liu Z, Pu Y, Bao Y (2019) Immunomodulatory effects exerted by Poria Cocos polysaccharides via TLR4/TRAF6/NF-kappaB signaling in vitro and in vivo. Biomed Pharmacother 112: 108709.
- Chen YY, Li RY, Shi MJ, Zhao YX, Yan Y, et al. (2017) Demethyleneberberine alleviates inflammatory bowel disease in mice through regulating NF-kappaB signaling and T-helper cell homeostasis. Inflamm Res 66(2): 187-196.
- Liu H, Zhu R, Wang L, Liu C, Ma R, et al. (2018) Radix Salviae miltiorrhizae improves bone microstructure and strength through Wnt/beta-catenin and osteoprotegerin/receptor activator for nuclear factor-kappaB ligand/cathepsin K signaling in ovariectomized rats. Phytother Res 32(12): 2487-2500.
- Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7): 499-511.
- Wang M, Zhao J, Zhang N, Chen J (2016) Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed Pharmacother 83: 975-988.

 Research Article
Research Article