ABSTRACT
Topical ophthalmic solutions are widely used as a way to deliver lubrication or medication for the treatment of extensive variety of eye diseases or discomforts. In order to maintain the sterility and stability of these products, as well as prevent microbial growth during the in-use period, ocular solutions have historically been packaged with a preservative. However, as some preservatives have been shown to have potentially adverse outcomes in patients, in recent years there has been a large market interest in preservative-free products, particularly for patients who must use daily eye drops long term. Products which do not possess preservatives to keep products sterile must then rely on sophisticated packaging to maintain product integrity. Here, we review the multi-dose preservative-free packaging currently available to consumers and discuss the differences in their design.
Introduction
Topical ocular solutions are widely used and commonly recommended for a variety of ocular diseases. Some preparations are used chronically by patients, for months or, very often, for years. In 1953 Alcon Research, Ltd released its trademarked DROPTAINER® eye drop dispensing bottle, which quickly became the standard packaging design for eye care products [1]. Following this, the majority of products now come packaged in similar multiuse bottles, with enough volume meant to last for a month or more. In order to protect the product after being opened by the patient, solutions were traditionally packaged with a preservative to act as an antimicrobial agent which either kills or suppresses microbial growth, or both. Historically, the most commonly used preservative in multi-dose eye drops has been benzalkonium chloride (BAK). This preservative is a powerful quaternary ammonium compound which is highly effective against most potential ocular pathogens. It is typically used in concentrations varying from 0.004% to 0.02% in solution. However, there are indications, particularly in patients who must use eye drops for chronic conditions, that preservatives may lead to tolerability issues. These issues, which are the result of continuous exposure, can include in ocular surface changes, ocular discomfort, tear film instability, conjunctival inflammation, subconjunctival fibrosis, epithelial apoptosis, corneal surface impairment, subclinical inflammation, and potential risk of failure in surgical interventions [2]. Current studies indicate that long-term adverse effects of preserved products are predominately linked to the use of benzalkonium chloride, and clinical data suggests that the use of preservative-free products or products with less harmful preservatives are indicated for patients with chronic ocular conditions [2,3].
Some innovations have been tested to combat BAK in eye drops, such as the development of modified tip of an eye drop dispenser which removes BAK out of solution as it is being dispensed [4]. However, due to a low number of preservative-free clinical trials, there is not yet evidence to suggest that preservative-free eye drops necessarily have higher safety profile compared to preserved forms in clinical practice, and reservations regarding preserved eye drops remain unique to chronic use [5]. Furthermore, there are more eye drop preservatives now on the market than only BAK. Notably, polyquaternium-1 (PQ-1) has been found via multiple tests to be extremely safe while also highly effective as a sterility maintenance agent, [6] with no differences reported between preservativefree or PQ-1-preserved eye drops after LASIK surgery [6]. Other eye drop preservatives on the market include polyhexamethylene biguanide, sodium perborate, stabilized oxychloro complex, and Sofzia®, which are largely not associated with any negative side effects [6]. In terms of medication or product effectiveness, there is no evidence to suggest that preservative-free artificial tears are more effective than preserved artificial tears [7]. However, many patients and clinicians are interested in using preservative-free products because of the perceived safety benefits. A preservativefree product must inherently rely on the packaging of the product to maintain sterility of the solution between uses and developing an easy-to-use and affordable packaging system which maintains complete product sterility has been a unique challenge for the ocular health field.
Finally, there are major bottle-use aspects which must be taken into consideration with any eye drop container. These include angle of drop instillation as well as the force required to dispense one drop. Studies have found that the vast majority of glaucoma patients, despite being long-term eye drop users, struggle to properly place the drop on the eye. This is due to the drop landing outside of the globe of the eye following difficulty with the angle of the bottle and the angle of the head required for dispensing, dispensing too many drops at once, holding the dropper at an angle which alters the drop volume, or touching the tip of the bottle to the eye or surrounding tissue [8-10]. Although, while it’s important that containers be user friendly, it has been found that educational resources instructing patients to apply their eye drops correctly mitigates many issues with unintentional noncompliance [11]. Patients also find difficulty with either understanding or complying with single-use package directions and are likely to re-use single-use medications if they are able to [12]. A recent investigation of 21 different eye drop bottles found that long-term eye drop users struggle with the squeeze force required to exert one drop at a time, and that bottles on the market vary widely in terms of force required [13]. Squeeze also can change during the in-use period as the product volume decreases. Therefore, in the mission to provide patients with effective ocular topical medication, it is important to note that packages must not only be able to keep contents safe from microbial contact but must also be user-friendly enough to allow the patient to medicate themselves at regular intervals without difficulty.
Testing Required for Preserved Products
Preserved products undergo rigorous antimicrobial testing to ensure that products which come in contact with microbial contamination are able to neutralize the pathogenic threats. The Committee for Proprietary Medicinal Products (CPMP) has published Notes for Guidance on In-Use Stability Testing of Human Medicinal Products (CPMP/QWP/2934/99) [14] which approaches the protocols for defining selection of batches, test design, test storage conditions, test parameters, and test procedures for solutions in multidose containers. This Guidance is applicable to both preserved and preservative-free products in multidose containers, but still does not identify set requirements for sterility, stability, particulate matter, pH, or endotoxins. Also, while the Food and Drug Administration has outlined recommendations for labeling preservative-free multi-dose injectable medications, [15] no specific guidance exists for MDPF ocular administrations. Preserved products must also be tested in accordance with the United States Pharmacopeia (USP) preservative efficacy test, [16] the USP antimicrobial effectiveness test, [17,18] and the International Standards Organization protocol 14730 for preservative effectiveness test standards [19,20].
This ISO protocol oversees challenges involving the most common microorganisms found in eye drop contamination: Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Candidate albicans, and Aspergillus niger. Following these rigorous standards, studies have indicated that preserved multi-use eye drops which make it to the market are consistently well packaged, possess optimal clarity, lack particulate matter, are sterile, and mostly have high preservative efficacy [21]. However, these same studies have indicated that preserved eye drops on the market may possess endotoxin levels above 0.25 EU/mL [21]. Also, despite these various testing standards and protocols which indicate a largely safe and stable market availability of preserved eye drops, there have been some recalls of topical eye products due to reports of mold [22] and lack of sterility [23]. Indeed, studies of both opened and unopened preserved products have found evidence of microbial contamination, indicating that the included preservatives may not always be sufficient [24,25]. These findings point to the necessity of eye drop containers which can help maintain the sterility of the product until use, regardless of preservation status.
Antimicrobial Maintenance of Preservative-Free Products Depends on the Packaging
While there is lacking evidence for a large difference between the medical effectiveness of preserved versus non-preserved products themselves, the type of dispenser has been repeatedly shown to be critical in terms of patient use, compliance, medication delivery, and sterility. Importantly, it has been shown that MDPF eye drops are at risk of contamination via microbial ingress by pathogenic organisms [26,27]. This is particularly true for patients who cannot or will not attempt to use aseptic technique when applying their eye drops [27]. Contamination rates are also affected by the angle of the dropper and nozzle geometry: adjusting instillation angles to 90° and using a sharp nozzle geometry vs. a round nozzle geometry has been shown to reduce contamination of the drop [28]. Thus, it is clear that package design affects both patient compliance and stability of the product at the same time. The following preservativefree packages (Figure 1) described in this review all universally attempt to deliver a sterile, easily accessible, single-drop dose reliably, in a way that is simple for the user to dispense and easy to keep clean. The multi-dose containers aim to do this even after the package has been opened and are intended for multiple uses over a period of weeks to months. However, it is important to note that all of these packages still require the patient to raise the arm, tilt the head back, and use gravity as the means of drop delivery, which some find difficult [29]. Additionally, the reader will note that there are two predominate technological designs to the available MDPF packages: a highly complex valve release system which does not allow for contaminated liquid or air to come in contact with the product, or a fine filtration system which prevents contaminants from air or water interacting with the product.
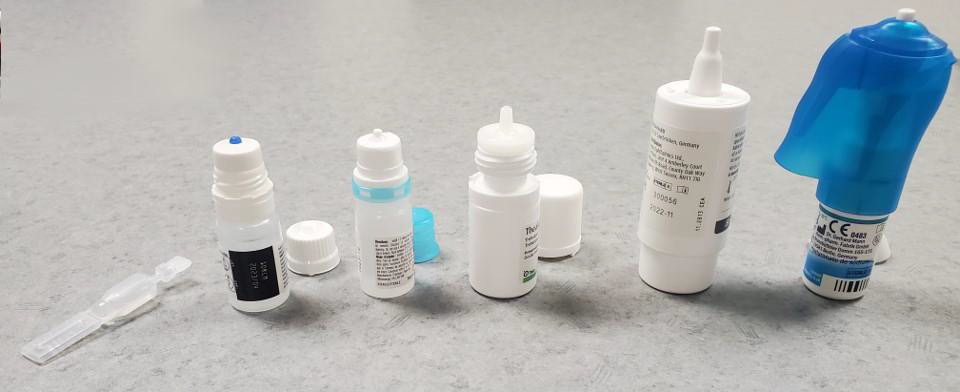
Figure 1: Samples of preservative-free containers on the market. From left to right: single-dose container, Novelia® Multidose bottle, Aptar Pharma’s Ophthalmic Squeeze Dispenser, ABAK® bottle, COMOD® system, and the 3K® system with Side Actuation Device.
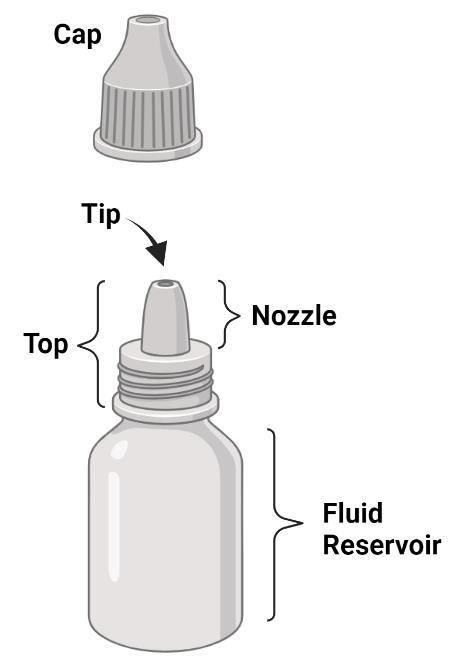
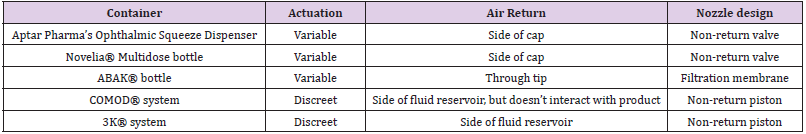
For the purpose of discussion this review, the parts of a bottle will be assigned universal terms. Some manufacturers refer to parts of a bottle differently, despite it being the same piece. Here we will be discussing the tip (the part of the bottle which is last touched by the drop as it exits the bottle), the nozzle (the apparatus which product flows through directly, and which measures and establishes drop volume), the top (the entire section which houses the filtration device and/or nozzle), the cap (which is removeable and can be placed over the entire top), and the fluid reservoir (which houses the product when it is not in use). Please refer to (Figure 2) for these depictions. There are also important aspects of each preservative-free package design which will be discussed, such as variable versus discreet actuation of each drop (variable actuation results in the ability of the patient to produce more than one drop per squeeze, while discreet actuation indicates that only one drop of defined size is possible per squeeze), how air is compensated in each bottle, and the basic design of the nozzle design which contributes to the microbial stability of the packaging.
Figure 2: Description of the pieces of an MDPF container, which include the cap, tip, nozzle, top, and fluid reservoir.
Single-Use Containers
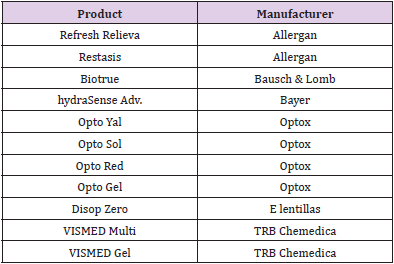
Single-use eye drop dispensers are the gold standard in pathogen-free preservative-free dispensing, as they can be aseptically assembled through blow-fill-seal and are kept completely closed until the patient is ready to use them (Figure 3). These packages have been on the market for many years, in both plastic and glass options. Also, while they are grouped in this review with preservative-free packaging, it is important to note that they are not multi-dose containers. They are not meant to be re-used after the first use and are therefore reliably sterile when opened. Studies show that patients are usually able to use these single-use containers as well as or better than multi-use dispensers [30]. However, both product manufacturers and patients report concerns regarding single-unit doses. These include cost (as more packaging and eye drop solution per dose is required), [7,31,32] waste, [33] and convenience, [33] as it is easier to store a single multi-use bottle in a preferred location than to ensure the patient has the correct number of unit-dose pipettes with them every day. Additionally, these unit-doses are often packaged with more volume than is needed for one dose, so patients may attempt to save the remaining liquid for a future use even though the packaging is not intended to keep the contents sterile after opening, thereby providing the opportunity for self-contamination [3]. Finally, the majority of the MDPF bottles are designed to deliver an exact volume of a drop every time. In general, these single-use containers are not able to deliver an exact drop volume [34]. The following companies provide products in unit-dose formulations (Table 1).
Figure 3: Single-dose containers, which are meant to be single use and discarded after they are opened [34]. Image taken from Alcon.com [34].
Aptar Pharma’s Ophthalmic Squeeze Dispenser
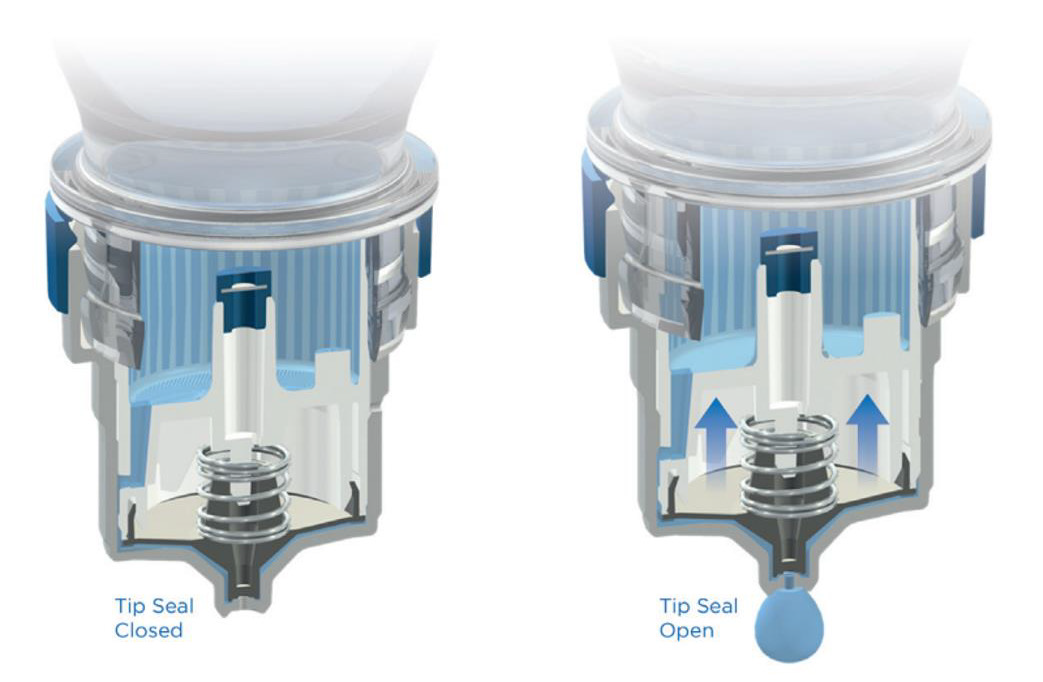
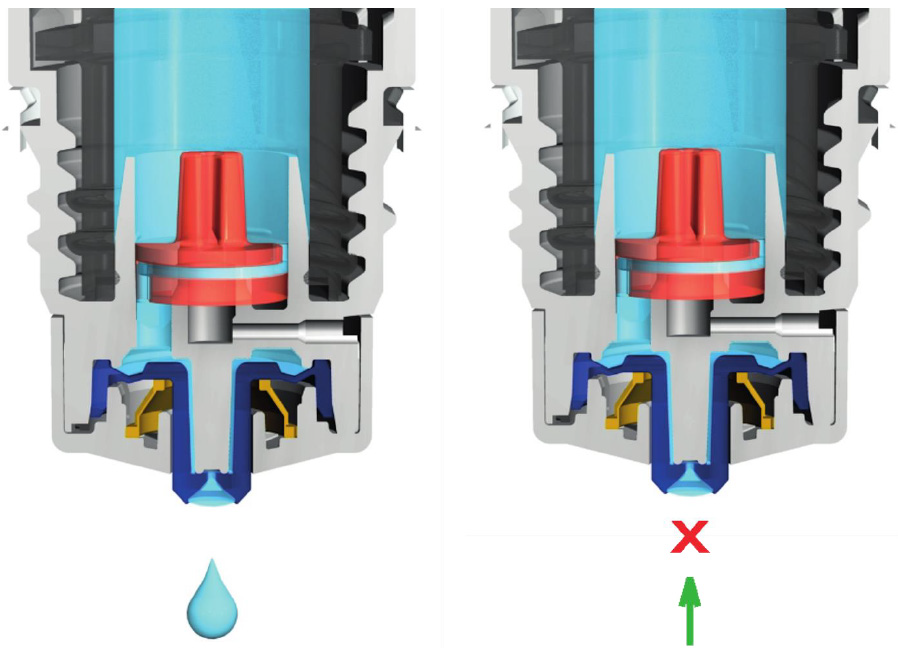
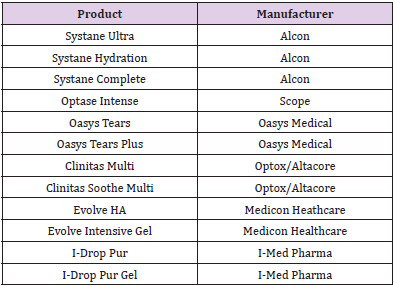
The Aptar Pharma’s Ophthalmic Squeeze Dispenser was the first MDPF ocular formulation delivery system to deliver an FDAreviewed formulation, with Allergan’s Restasis Multidose [35,36]. This bottle delivers a drop of a defined size (based on bottle size and product viscosity) following a variable actuation and comes in 7ml and 10ml sizes. The Aptar bottle uses a dual channel system to release a drop from the tip (Figure 4). Upon squeezing the bottle a sealing membrane opens, allowing the liquid in the dual channels to pass to the tip and form the drop. This bottle relies on air intake occurring through a small hole in the side of the top. Incoming air passes through a 0.2μm sterilizing membrane filter to allow decontaminated air to equalize the pressure in the bottle. Any liquid remaining on the bottle tip is meant to be shaken off or will evaporate with or without the cap being closed. The snapfit closure of the top of the bottle to the reservoir is designed to provide a tight connection and prevent deliberate disassembly. Aptar Pharma developed their own testing method, the Tip Seal Integrity Test, based on the protocols designed for preserved antimicrobial efficacy testing such as USP <51> and Ph. Eu. 5.1.3 [35]. This testing regimen is highly similar to the tests performed for the Novelia® packaging: [37] the tip of the Aptar bottle is directly challenged with liquid contaminants containing 106 CFU/ mL of Pseudomonas aeruginosa, Staphylococcus aureus, or Candida albicans over four days. The contents of the packaging are then observed for sterility up to 96 hours after the last contamination challenge [35]. The Aptar Ophthalmic Squeeze Dispenser claims to be the ‘leading device for preservative-free prescription and overthe- counter products’. Several companies use this packaging for their MDPF formulations, a list of which can be found in (Table 2).
Novelia® Multidose Bottle with Pureflow Technology
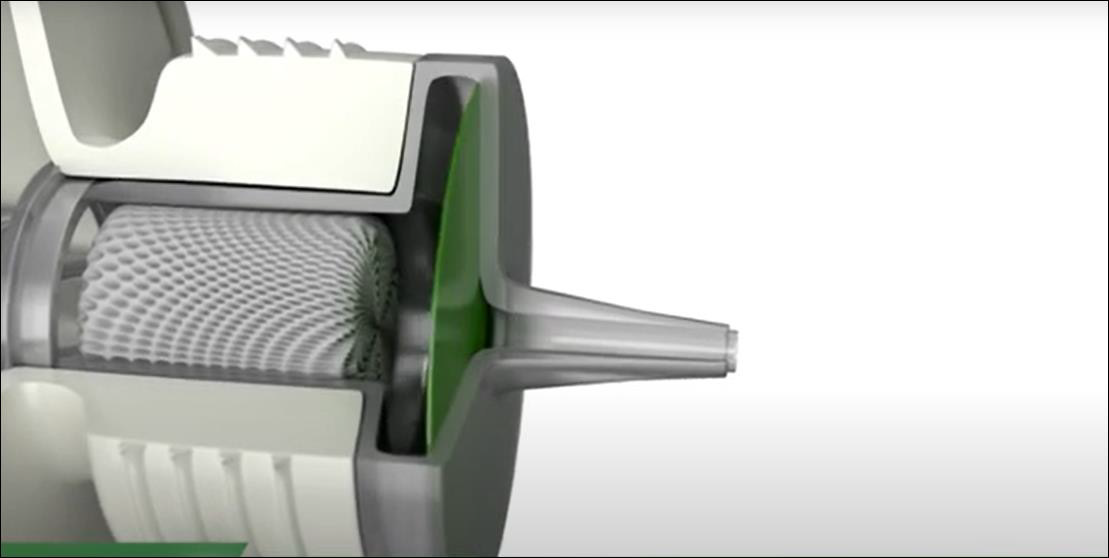
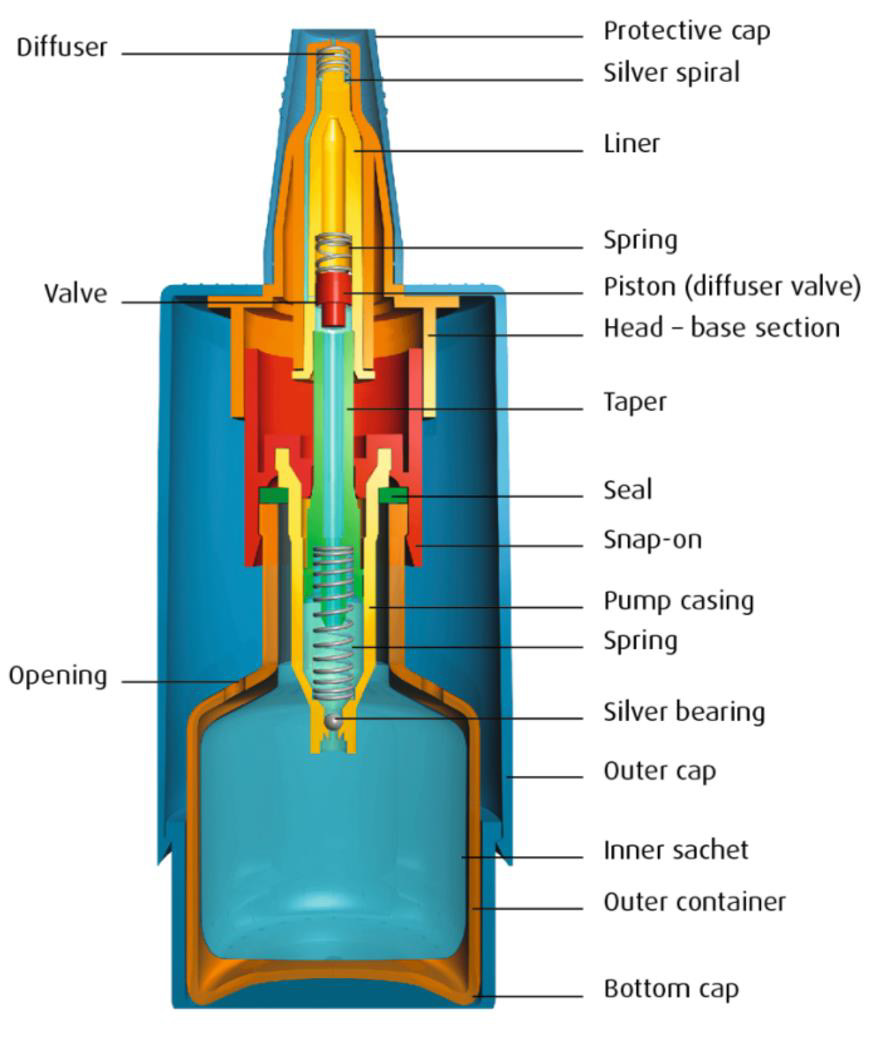
The Novelia® bottle, [38] a registered trademark of Nemera La Verpilliére, comes in several different sizes (5mL, 7.5mL, 11 mL, and 15mL) to allow for varied in-use periods, and different nozzles which can be best selected for solution viscosity and desired drop size (which can range from 28μl to 46μl). This package design utilizes a highly technical mechanical pathway for the drop to flow through (Figure 5) and is also a variable actuation package. This pathway prevents microbial ingress via both the blocked fluid return and the filtered air intake port on the side of the bottle. Upon the exit of the drop from the bottle, the exit valve closes immediately to prevent contaminated liquid or air returning via the drop delivery mechanism. A small amount of liquid (about 20% of a normal drop) may remain on the tip of bottle. Nemera highlights the placement of silver ions which are embedded in the cap prevent microbial growth. Any remaining liquid is also further prevented from contamination via the cap closing. Once the cap is closed the remaining liquid also dries, further minimizing the likelihood of bacterial growth. This bottle has an actuation force which is highly similar to preserved droptainers on the market [13,39]. However, it should be noted that containers which have an air intake, such as this one, do increase their actuation force over use as their volume is replaced with air [40]. The Novelia® system underwent a dispensing study wherein a drop from the bottle was added once a day for 60 days to 5% sheep blood-agar medium to identify any potential pathogens growing in the contained solution [41]. Every day twice a day the caps of the eye drop bottle were opened, left off for 20 seconds, and then closed again, to simulate potential air contamination of the tip. This study demonstrated that the contents of the Novelia® bottle showed no signs of bacterial contamination during the 60 day study period [41]. The Novelia® Multidose bottle is used by several manufacturers to contain their preservative-free eye drop formulations. This information is listed in (Table 3).
ABAK® Bottle
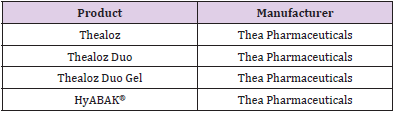
The ABAK® system is one of the most mechanistically simple MDPF packaging systems [42] The ABAK® system uses a neutral microporous pad to regulate the flow of air and fluid in and out of the bottle. It also uses a 0.2μm hydrophilic polymer membrane which is also rendered hydrophobic by a patented surface treatment (Figure 6). This microporous pad and membrane allow for variable actuation. Compared to the other MDPF packages, there is a relatively large amount of liquid held in the top, in the space between the hydrophilic polymer membrane and the microporous pad, compared to other top and nozzle designs. The hydrophilic polymer membrane allows fluid to flow out of the bottle, but not back in, and removes contaminants from any air which returns to the bottle. This bottle holds 10mL of sterile MDPF solution and is intended for up to three months of use. Similar to other MDPF bottles, the manufacturer touts that this dropper is designed to deliver a consistent dose of 30μL of liquid each time the dropper is used, and that this bottle is designed to produce 300 drops (dependent on product viscosity). This bottle is noted as being affected by high altitudes, and may produce an irregular efflux of drops, leak, or prevent uniform dosing if used in flight [43]. Additionally, one study found that when the outside of the nozzle is contaminated with Pseudomonas aeruginosa, the resulting drop is often also contaminated [28] This nozzle contamination resulting in drop contamination was true also for preserved products, except for the Systane bottle [28] Thea Pharmaceuticals is the primary user of the ABAK® multidose bottle. The eye drop formulations which use this bottle are in (Table 4).
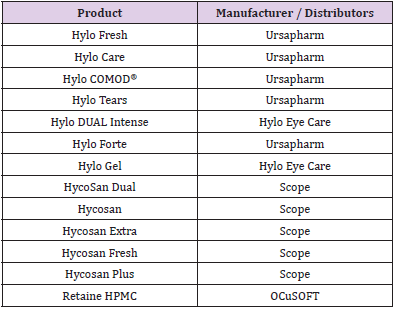
COMOD® System
The COMOD® system, a registered trademark of Ursa Pharm, [44] has been shown to be suitable for preservative-free products [45]. The COMOD® name stands for “Continuous Monodose”, as this is another bottle which has been designed to produce exactly one drop of a specified volume per squeeze. Unlike the previous packages discussed, this bottle is designed to produce a discreet drop for every actuation applied, meaning only one drop can come out at a time. This bottle (Figure 7), similar to the Novelia® and Aptar packages, relies on a multi-part mechanical nozzle to deliver the sterile drop. This begins with a ball valve in the base portion of the dropper, followed by a spring and a fluid pathway. The nozzle of the bottle is sealed by a spring and piston. Once the piston is depressed, fluid can flow through a side capillary, and out of the bottle. This piston also prevents backflow of fluid to prevent contamination of the sterile bottle fluid contents. This system also uniquely relies on a flexible inner package containing the liquid, and air space between the flexible inner packer and the hard outer shell. This air space is where air will be returned after fluid is dispensed, and this air does not come in contact with the fluid. Also, the next dose of the eye drop formulation rests in the nozzle. The negative pressure of the ball valve draws this next dose into the nozzle after a current dose has been released [46]. The manufacturer describes that this bottle is also designed to release 300 drops (dependent on product viscosity) in a 10ml bottle, and has been shown to keep contents sterile for up to 6 months [44]. Similar to the ABAK® system, a study in Brazil found that when the outside of the nozzle of this bottle is contaminated with Pseudomonas aeruginosa, the resulting drop is also contaminated.28 However, a study using surgical patients examined used COMOD® bottles and found that no bottles exhibited “serious contamination”, indicating that the bottle prevented microbial ingress into unpreserved eye drops [45]. The products which use the COMOD® system are listed in (Table 5).
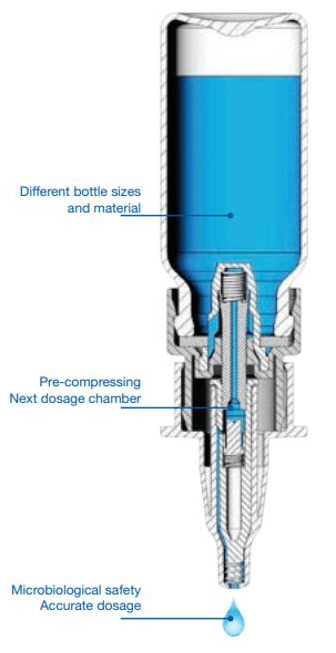
3K® Ophthalmic Multidose System
The 3K® system, [47] a registered trademark of Aero Pump, [48] is highly similar to the COMOD® System: it relies on a long, dualspring loaded dosing chamber (Figure 8). Liquid flows through a leak-proof valve, which prevents the backflow of liquid. When the chamber is released, the next dose of product is drawn into the chamber. This creates a discreet actuation device. This pump also features a silver coil at the pump opening to further provide antimicrobiological properties. Similar to the other MDPF containers, this product features a specified dose volume when the next dose is drawn into the chamber. Unlike the COMOD® system, the dispensed product is compensated with an inflow of air which passes through a special filter matrix via an air intake in the side of the bottle. These dispensers come in three varieties of squeeze mechanisms: the finger sleeve, the comfort grip finger sleeve, and the side actuation device (Figure 8). They also come in 5ml and 10ml package sizes. The products which use the 3K® system are listed in (Tables 6 & 7) [49].
Table 7: Summary of major aspects of each MDPF container. Variable actuation results in the ability of the patient to produce more than one drop per squeeze. Discreet actuation indicates that only one drop of defined size is possible per squeeze.
Conclusion
Overall, there are several highly sophisticated and affordable MDPF packages available on the market, which are used by a wide variety of topical ocular formulation producers [Table 7]. These MDPF containers still require a patient to tilt the head back while dispensing, which patients find difficult, although these designs do make concerted attempts to reliably dispense one consistent drop at a time without requiring an abundance of squeeze force. While each manufacturer is responsible for the sterility and stability of these products, there is lacking evidence that any of these packages are susceptible to microbial ingress or contamination when used within the suggested time period. However, the largest amount of published information regarding the safety and sterility of these packages are in reference to the Novelia® Multidose Bottle, followed by the Aptar Ophthalmic Squeeze Dispenser. It is clear that the Novelia® packaging is able to withstand both the likely microbial challenges in real-world scenarios, as well as more significant and severe challenges which could occur.
Financial Disclosure
All authors are employees of Alcon Research, LLC.
References
- (2022) DROPTAINER Trademark Details. JUSTIA Trademarks.
- Baudouin C, Labbé A, Liang H, Pauly A, Brignole Baudouin F, et al. (2010) Preservatives in eyedrops: the good, the bad and the ugly. Progress in retinal and eye research 29(4): 312-334.
- Bagnis A, Papadia M, Scotto R, Traverso CE (2011) Antiglaucoma drugs: The role of preservative-free formulations. Saudi Journal of Ophthalmology 25(4): 389-394.
- Hsu KH, Gupta K, Nayaka H, Donthi A, Kaul S, et al. (2017) Multidose Preservative Free Eyedrops by Selective Removal of Benzalkonium Chloride from Ocular Formulations. Pharm Res 34(12): 2862-2872.
- Figus M, Agnifili L, Lanzini M, Manuela Lanzini, Lorenza Brescia, et al. (2021) Topical preservative-free ophthalmic treatments: an unmet clinical need. Expert Opin Drug Deliv 18(6): 655-672.
- Walsh K, Jones L (2019) The use of preservatives in dry eye drops. Clin Ophthalmol 13: 1409-1425.
- Ribeiro M, Barbosa FT, Ribeiro LEF, Sousa Rodrigues CF, Ribeiro EAN, et al. (2019) Effectiveness of using preservative-free artificial tears versus preserved lubricants for the treatment of dry eyes: a systematic review. Arq Bras Oftalmol 82(5): 436-445.
- Gupta R, Patil B, Shah BM, Bali SJ, Mishra SK, et al. (2012) Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma 21(3): 189-192.
- Sklubalova Z, Zatloukal Z (2005) Systematic study of factors affecting eye drop size and dosing variability. Pharmazie 60(12): 917-921.
- Sam Oyerinde OA, Onyekwelu OM, Musa KO, Olufisayo TA, Ibukunoluwa TA, et al. (2021) Assessment of eye drop instillation techniques among patients with primary open angle glaucoma in a Nigerian tertiary hospital. International Ophthalmology 42(4): 1031-1040.
- Davis SA, Carpenter DM, Blalock SJ, Donald L Budenz, Charles Lee, et al. (2019) A randomized controlled trial of an online educational video intervention to improve glaucoma eye drop technique. Patient Educ Couns 102(5): 937-943.
- Seal DV (2005) Multiple use of single use solutions: a dangerous practice. The British journal of ophthalmology 89(6): 783.
- Moore DB, Hammer JD, Akhtari R, Beck J, Sanders S, et al. (2016) Squeeze Me if You Can: Variability in Force Requirements to Extract a Drop From Common Glaucoma Bottles. Journal of glaucoma 25(9): 780-784.
- (2001) Note for guidance on in-use stability testing of human medicinal products. The European Aggency for the Evaluation of Medicinal Products.
- (2018) United States Food and Drug Administration: Selection of the Appropriate Package Type Terms and Recommendations for Labeling Injectable Medical Products Packaged in Multiple-Dose, Single-Dose, and Single-Patient-Use Containers for Human Use Guidance for Industry.
- (2006) United States Pharmacopeia (USP) Antimicrobial effectiveness testing. J. U S Pharm 29: 2499-2500.
- Sutton SV, Porter D (2002) Development of the antimicrobial effectiveness test as USP chapter <51>. PDA J Pharm Sci Technol 56: 300-311.
- Sutton S (2022) The Antimicrobial Effectiveness Test.
- Rosenthal RA, Buck SL, Henry CL, Schlech BA (2006) Evaluation of the preserving efficacy of lubricant eye drops with a novel preservative system. J Ocul Pharmacol Ther 22(6): 440-448.
- (2000) European Committee for Standardization. Ophthalmic optics—contact lens care products—antimicrobial preservative efficacy testing and guidance on determining discard date.
- Ezenobi NO, Chinaka CN, Obi EI (2018) Assessment of the Physiochemical and Antimicrobial Quality of Some Antibiotic and Non-antibiotic Eye Drops Marketed in Different Registered Pharmacies in Port Harcourt, Rivers State. Eur J of Biom Pharm Sci 5(3): 4-10.
- (2006) "Alcon recalling Systane Free Liquid Gel drops in U S". Healio Ophthalmology, Ophthalmic Business.
- (2019) "Eyedrops Sold at Walmart, Walgreens, CVS Recalled". WebMD Eyehealth, News.
- Taşli H, Coşar G (2001) Microbial contamination of eye drops. Cent Eur J Public Health 9(3): 162-164.
- Nentwich MM, Kollmann KHM, Meshack J, Ilako DR, Schaller UC, et al. (2007) Microbial contamination of multi-use ophthalmic solutions in Kenya. British Journal of Ophthalmology 91(10): 1265-1268.
- Rahman MQ, Tejwani D, Wilson JA, Butcher I, Ramaesh K, et al. (2006) Microbial contamination of preservative free eye drops in multiple application containers. British Journal of Ophthalmology 90: 139-141.
- Kim MS, Choi CY, Kim JM, Chung HR, Woo HY, et al. (2008) Microbial contamination of multiply used preservative-free artificial tears packed in reclosable containers. British Journal of Ophthalmology 92(11): 1518-1521.
- Da Costa AX, Yu MCZ, de Freitas D, Priscila Cardoso C, Lauren C LaMonica, et al. (2020) Microbial Cross-contamination in Multidose Eyedrops: The Impact of Instillation Angle and Bottle Geometry. Translational Vision Science & Technology 9(7): 7.
- Van Santvliet L, Ludwig A (2004) Determinants of eye drop size. Survey of Ophthalmology 49(2): 197-213.
- Parkkari M, Latvala T, Ropo A (2010) Handling Test of Eye Drop Dispenser—Comparison of Unit-Dose Pipettes with Conventional Eye Drop Bottles. Journal of Ocular Pharmacology and Therapeutics 26(3): 273-276.
- Hedengran A, Steensberg AT, Virgili G, Azuara Blanco A, Kolko M, et al. (2020) Efficacy and safety evaluation of benzalkonium chloride preserved eye-drops compared with alternatively preserved and preservative-free eye-drops in the treatment of glaucoma: a systematic review and meta-analysis. British Journal of Ophthalmology 104(11): 1512-1518.
- Jones L, Downie LE, Korb D, Jose M BDC, Reza Dana, et al. (2017) TFOS DEWS II Management and Therapy Report. Ocul Surf 15(3): 575-628.
- Safarzadeh M, Azizzadeh P, Akbarshahi P (2017) Comparison of the clinical efficacy of preserved and preservative-free hydroxypropyl methylcellulose-dextran-containing eyedrops. J Optom 10(4): 258-264.
- (2022) "Systane Ultra Preservative-Free Eye Drops". Alcon.com.
- (2022) "Ophthalmic Squeeze Dispenser (OSD) Technology Platform". Drug Development & Delivery, by Aptar Pharma.
- (2019) "Delivering on the Growing Need for Topical Preservative-Free Ophthalmic Treatments". On Drug Delivery, Aptar Pharma.
- Patterson B, Crary M, Shannon P (2021) Microbial Ingress Risk Evaluation of NOVELIA Package Containing Systane Ultra. Investigative Ophthalmology & Visual Science 62: 1303-1303.
- Novelia Nemera (2022).
- (2021) Nemera: The Pivotal Role Played by Device Developers to Improve Patient Eye Care. Ophthalmic Drug Delivery.
- (2022) "Novelia, the preservative-free multidose eye dropper". Nemera.net.
- Ozulken K, Cubuk M, Inan N, Acar U, Gocmen J, et al. (2020) The Comparison of Bacterial Contamination and Antibacterial Efficacy of the Anti-Glaucomatous Eyedrops with and without Preservatives. Glokom-Katarakt 15: 118-122.
- (2022) "The ABAK System". Thea Laboratories.
- Da Costa AX, Oliveira EXLD, LaMonica LC, Dos Santos VR, Gomes JAP, et al. (2021) Eye drop performance at high altitude: an "in-flight" problem. Eye (Lond) 35(9): 2631-2632.
- (2022) The COMOD system - the safe solution for your eyes. Ursa Pharm.
- Teping C, Wiedemann B (1994) [The COMOD system. A preservative-free multidose container for eyedrops]. Klin Monbl Augenheilkd 205(4): 210-217.
- (2022) "The HYLO Technology". Candor Vision.
- (2022) "Ophthalmic Multidose Systems: Preservative-Free Solutions". Aero Pump Ophthalmic Multidose Systems.
- (2022) 3K system. Aero Pump.
- (2022) "Ophthalmic Multidose System: Metered Dose for Preservative-free Solutions". Aero Pump Ophthalmic Multidose System.

 Review Article
Review Article