ABSTRACT
Bacterial infections have a large impact on public health. The use of medicinal plants in the management and treatment of bacterial infections has shown some promises. Zapoteca portoricensis of Fabeceae family is a perennial shrub found in Tropical Africa where it is used locally in treating many bacterial infections. The aim of this work was to investigate the antibacterial properties of the root extract of Zapoteca portoricensis against Streptococcus pyrogenes and other various bacterial infections. Serial solvent extraction of the root was done using chloroform, ethyl acetate, acetone and ethanol, but only ethanol fraction of the crude extract was active against both gram-negative and gram-positive bacteria used including; Streptococcus pyrogenes, E. coli, Salmonella typhi, Klebsiella pneumonia and Bacillus subtitis. The result showed significant zone of inhibition of Streptococcus pyrogenes and other bacteria treated with the crude ethanolic extract of Zapoteca portoricensis. The minimum inhibitory concentration (MIC) of the crude extracts are; 25, 100, 6.25, 50, 25, 6.25 mg/ml for Staphylococcus aureus, Streptococcus pneumonia, Streptococcus pyrogenes, E.coli, Klebsiella pneumonia and Salmonella typi respectively. Therefore, Zapoteca portotricensis may be a potent broad spectrum phytotherapy for the treatment of Streptococcus pyrogenes and other related infections.
Keywords: Streptococcus Pyrogenes; Zapoteca Portotricensis; Infections; Inhibition; Antibacterial
Introduction
Bacterial infections have a large impact on public health Nguyen, et al. [1]. Diseases can range from mild to severe and sometimes can be deadly if left untreated Tamar, et al. [2]. According to report, more than 2.8 million antibiotic resistant bacterial infections occur in the USA each year, and more than 35,000 people die as a result Li [3]. Additionally, according to center for disease control study, the cost to treat multidrugresistant bacterial has been estimated to be $4.6 billion annually Van Duin [4]. Despite the efforts to combat multidrug resistant bacterial with synergistic drugs, the report of multiple drug resistance in medically important strains of bacteria (such as Streptococcus pyrogene, Streptococcus pneumonia, Klebsiella pneumonia, Staphylococcus aureus, Salmonilla typhi and Bacillus subtilis) is still too high Wernli, et al. [5]. The persistent increase in antibiotic resistant strain of bacteria has led to the development of more potent synthetic antibiotics Gajdács [6]. However, these new antibiotics are scarce, costly and so not affordable particularly in developing countries Liu [7]. This therefore, makes compliance to these antibiotics difficult Gajdács [6]. Therefore, there is need for continuous search for new, effective and affordable antimicrobial agents. Local medicinal plants provide a source of new possible antibiotics that may be potent for some of these bacteria Arwa [8]. One of these very important plants is Zapoteca portoricensis Ikeyi [9]. Zapoteca portoricensis is a perennial shrub with a climbing stem which is woody at its base Ikeyi [9]. This plant belongs to the family of Fabeceae and genus Zapoteca, it is commonly called dead awakener (English) Agbafor [10]. However the Yorubas call it Ule, while the Igbos in Nsukka area, where the plant is majorly used by traditional doctors, calls it Azonta Agbo [11]. Zapoteca portoricensis is widely distributed in tropical rain forest, it is found in tropical Africa: Nigeria, Ghana, Togo, and the Caribbean Islands especially British Virgin Island, Dominican Republic, Grenada, Haiti, Jamaica and Puerto Rico Nwodo [12]. The plant has been used traditionally to treat diseases like tonsillitis (sore throat), fever, convulsion, breast engorgement, stomach disorder, purgative, and amenorrhoea (Joshua [13]). Aqueous and alcoholic extracts of the leaf have been reported to be used in the treatment of gastro intestinal disorders, spasmodic and in the treatment of tonsillitis Ukwe [14].
Reported phytochemical analysis of the roots indicated the presence of a number of secondary metabolites such as saponins, resins, glycosides, flavonoids, alkaloids, terpenoids and steroids Ikeyi [9]. Recently, there has been suggestion on the antimicrobial and anti-inflammatory potentials of Zapoteca portoricensis but no scientific investigation has been carried out to verify these claims Esimone [15]. Therefore, this study investigated the antibacterial potentials of Zapoteca portoricensis root extract against some medically important strains of bacteria.
Materials and Methods
Materials
Plant Materials
Field Collection of Plant Material: Plant roots were collected from areas surrounding Nru village, Nsukka. The plant was identified by Mr Alfred Ozioko of the Department of Botany, University of Nigeria, Nsukka. The materials were cleaned of adhering soil and dust in the field by shaking and were dried at room temperature for one week.
Equipment and Instrument: Incubator (Beckman Coulter Co., Indianapolis, Indiana, USA), Oven (WMT-CNC Industrial Co. Ltd., Chizhou, Anhui, China), pH meter (Hanna Instruments, Woonsocket, Rhode Island, USA), Electric balance (Adams Equipment Inc., Oxford, England, UK), Refrigerator, Autoclave, Rotary evaporator (All Thermo-cool Public Ltd Co., Ilupeju, Lagos, Nigeria), Spectrophotometer, (Spectrum Laboratories, Stamford, Connecticut, USA), Infrared spectrometer, Nucear magnetic resonance spectrometer (All Gallenkamp Co., London, England), Whatman No 1 filter paper (Zibo Xinsu Chemical Industry Ltd., Zibo, Shandong, China).
Methods
Extraction Procedure: The roots were washed and air dried at room temperature for two weeks. The dried roots were pulverized using a mechanical grinder and the weight of the root powder was 550g. The powder was macerated in 3.5 litres of absolute ethanol (Figure 1). The mixture was kept for three days under room temperature. Filtration was done using Wattman No. 1 filter paper. The resulting extract was concentrated under fan at room temperature to avoid denaturation of the active ingredients to obtain a semi-solid mass. The extract was stored in a refrigerator at -4°C one week.
Fractionation of the Crude Extract: The crude ethanol extract was fractionated using serial solvent fractionation method Jamil, et al. [16]. The dried crude extract weighed 6.5 g. This dried extract was mixed with silica gel (60 GF for column), in the ratio of 1:2 (w:w). The solvents: chloroform, ethyl acetate, acetone and ethanol respectively, were used to wash the mixture. For a particular solvent the washing was done until a colourless filtrate was obtained, then the solvent was changed to another one in the order above, after air drying the mixture. The fractions were concentrated by allowing the solvents to evaporate under room temperature.
Preliminary Phytochemical Analysis: The crude ethanol extract of the roots were subjected to phytochemical analysis according to the method outlined by Harborne [17]. The phytochemical analysis was done to detect the presence of secondary metabolites, such as alkaloids, tannins, saponins, resins, flavonoids, steroids, glycosides and terpenoids.
Test for Alkaloids: A quantity of 0.2 g of the extract was added to 5 ml of 2% hydrochloric acid and heated on a boiling water bath for 3 min, it was allowed to cool and then filtered. A portion of the filtrate (1ml) was treated with 2 drops of Dragendorff’s reagent, the appearance of a red precipitate indicated the presence of alkaloids. Test for Glycosides: Two gram of the extract was mixed with 30 ml of water and heated on a water bath for 5 min and filtered. About 5 ml of a mixture of equal parts of Fehling’s solutions A and B was added to 5 ml of the filtrate until it turned alkaline (tested with litmus paper) and then boiled on a water bath for 5 minutes. A brick-red precipitate indicated the presence of glycosides.
Test for Flavonoids: Ethyl acetate, 10 ml, was added to 0.2 g of the sample and heated on a water bath for 3 minutes. The mixture was allowed to cool and filtered. A volume of 4 ml of the filtrate was shaken with 1 ml of dilute ammonia solution. The layers were allowed to separate. Absence of a yellow colour in the ammoniacal layer indicated the absence of flavonoids.
Test for Resins: A quantity of 0.2 g of the sample was extracted with 15 ml of 96% ethanol. The alcoholic extract was then poured into 20 ml of distilled water in a beaker. A precipitate occurring indicated the presence of resins.
Test for Tannins: A quantity, 2 g of the sample was boiled with 5 ml of 45% ethanol for 5 minutes. The mixture was cooled and then filtered; the filtrate was then treated with lead sub-acetate solution. To 1 ml of the filtrate 3 drops of lead sub acetate solution was added. A none-red gelatinous precipitate indicated the absence of tannins.
Test for Saponins: A quantity of 0.1 g of the extract was boiled with 5 ml of distilled water on a water bath for 5 min. The mixture was filtered hot and allowed to cool. To 1 ml of the filtrate, 2 drops of olive oil was added and the mixture shaken vigorously. The formation of emulsion indicated the presence of saponins.
Test for Terpenoids and Steroids: A volume (9 ml) of absolute ethanol was added to l g of sample and refluxed for 5 min and filtered. The filtrate was concentrated to 2.5 ml on a boiling water bath and then 5 ml of hot water was added. The mixture was allowed to stand for 1 hr and the waxy matter was filtered off. The filtrate was extracted with 2.5 ml of chloroform using a separating funnel (Figure 2). To 0.5 ml of the chloroform extract in a test tube, 1 ml of concentrated sulphuric acid was carefully added to form a lower layer. A reddish brown interface showed the presence of steroids. Another 0.5 ml of the chloroform extract was evaporated to dryness on a water bath and heated with 3 ml of concentrated sulphuric acid for 10 min on a water bath. A grey colour indicated the presence terpenoids.
Test for Acidic Compounds: A quantity, 0.l g was placed in a clean dry test tube and sufficient water added. This was warmed in a hot water bath and then cooled. A piece of moist litmus paper was dipped into the filtrate and the colour change on the litmus paper was observed. A red coloration of the litmus paper indicated the presence of acidic compounds.
Test for Proteins: A volume, 5 ml of distilled water was added to 0.1 g of the extract. This was left to stand for 3 hrs and then filtered. To 2 ml portion of the filtrate was added 0.1 ml Million’s reagent. It was shaken and kept for observation. A yellow precipitate indicated the presence of proteins.
Test for Carbohydrate: A quantity, 0.1 g of the extract was shaken vigorously with water and then filtered. To the aqueous filtrate was added few drops of Molisch reagent, followed by vigorous shaking again. Then, 1 ml of concentrated sulphuric acid was carefully added through the side of the test tube to form a layer below the aqueous solution. A brown ring at the interface indicates the presence of carbohydrate.
Test for Reducing Sugar: A quantity, 0.1 g of extract was shaken vigorously with 5ml of distilled water and filtered. Equal volumes of Fehling’s solutions A and B were added to 1 ml portion of the filtrate. The mixture was shaken vigorously. A brick red precipitate indicated the presence of reducing sugars.
Test for Fats and Oil: A quantity of 0.1 g of sample was pressed between filter paper and the paper observed. A control was also prepared by placing 2 drops of olive oil on filter paper. Translucency of the filter paper indicates the presence of fats and oil.
Test microorganisms: The clinical isolates were obtained from the Faculty of Veterinary Medicine, University of Nigeria, Nsukka, Enugu State, Nigeria. Test isolates were Streptococcus pyogenes, Streptococcus pneumonia, Bacillus subtilis, Salmonella typhi, Staphylococcus aureus, E. coli, Klebsiella pneumonia.
Preparation of culture media: The materials for the experiment were sterilized in an autoclave. They were allowed to cool before being used. Nutrient agar, pH 7.4 (Oxoid laboratories, England, United Kingdom) was used in the study. A quantity, 5.6 g of dried nutrient agar was weighed. This was dissolved in 200 ml of water. The suspension was well mixed by stirring in the water. The solution was heated to dissolve and to purify media. The media were sterilized by autoclaving at 50°C. The plates were thereafter inoculated with test microorganisms and incubated at 37°C for 24 hours.
Determination of antimicrobial activity: The antimicrobial activities of the crude extract were determined using NCCLS method (WHO [18]). For determination of antibacterial activity, bacterial cultures were adjusted to 0.5 McFarland turbidity standards and inoculated to 15 cm diameter nutrient agar plates. The crude ethanol extract of the plant’s roots was dissolved in dimethyl sulfoxide (DMSO); (500 mg of the extract was constituted with 5 ml of 100% DMSO to prepare stock solution). The concentration of stock was 100 mg/ml. Different concentrations (50 mg/ml, 25 mg/ml, 12.5 mg/ml, 6.25 mg/ml), of the plant root extract were prepared using serial dilution in DMSO. The control had DMSO alone without any extract. The method used was agar well diffusion method (Arwa, et al. [8]). Briefly, microorganisms from growth on nutrient agar incubated at 37°C for 18 h were suspended in saline solution, 0.85% NaCl and adjusted to a turbidity of 0.5 Mac Farland standards (108 cfu/ml). The suspension was used to inoculate 15cm diameter Petri plates with a sterile non-toxic cotton swab on a wooden applicator. Six millimeter diameter wells were punched in the agar and filled with fractions. The dissolution of the extract was aided by 1% (v/v) DMSO which did not affect microorganisms’ growth, according to our control experiments. Commercial antibiotic, streptomycin was used as positive reference standard to determine the sensitivity of the strains. The extract was introduced into the wells. Plates were incubated in at 37°C for 24 hr. Antibacterial activities were evaluated by measuring inhibition zone diameters. The experiments were conducted twice. All tests were performed in duplicate and the antibacterial activity was expressed as the mean diameter of inhibition zones (mm) produced by the plant root extracts.
Use of Infra-Red and NMR Spectroscopy to Determine the Functional Groups in the Ethanol Fraction
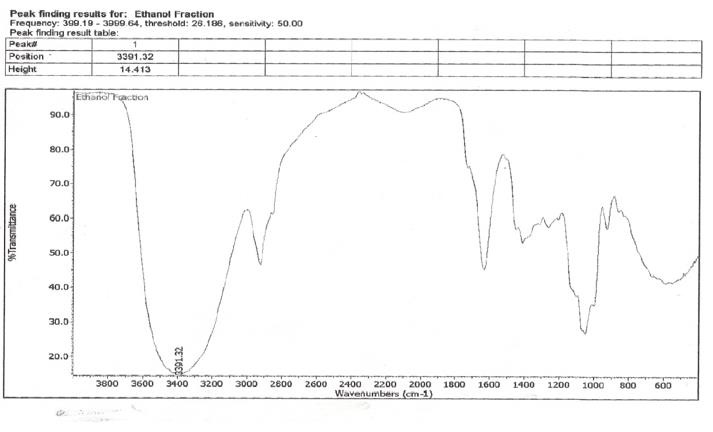
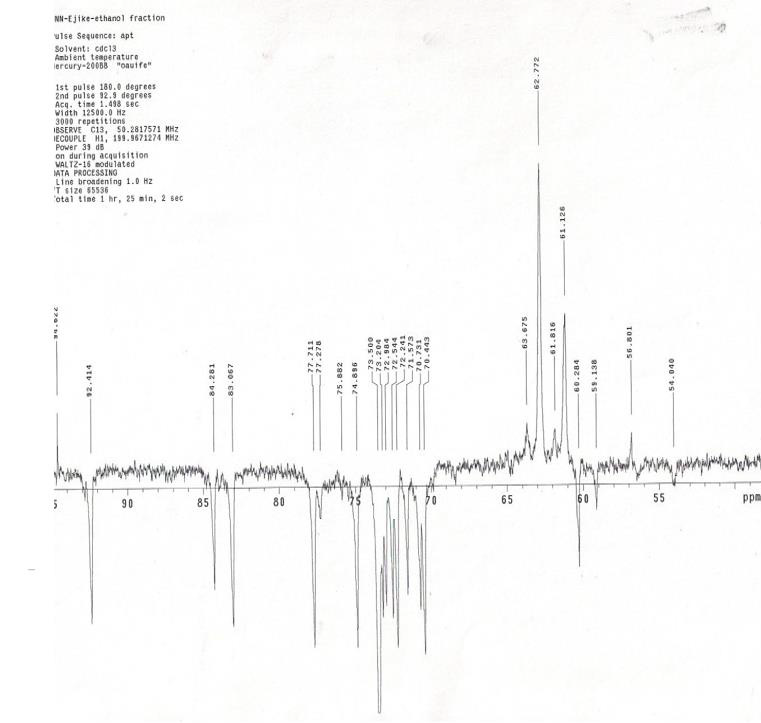
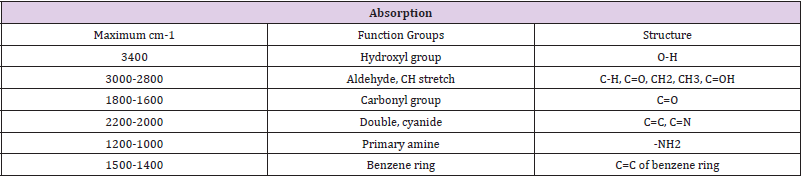
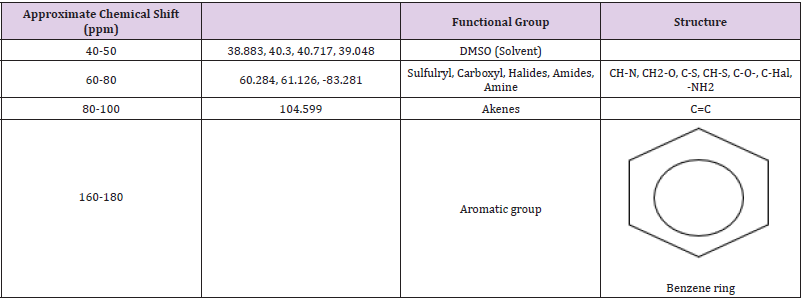
Infrared spectroscopy was carried out on the ethanol fraction to determine the functional group present in the fraction. The extract was dissolved in ethanol and ethanol was used as a control. Infrared spectra were obtained between the frequencies: 399.19 to 3999.64, characteristic peak of the functional group were detected and shown in (Table 5). The NMR spectroscopy was carried out on the samples, using DMSO as the solvent and teramethyl silane as an internal standard, at frequency of 199.9671071MHz at ambient temperature. The characteristic peaks of the functional groups were detected and shown in (Table 6).
Result
Extraction and Fractionation
The percent extract yield of the plant roots was 1.56%. After fractionation the following fractions were obtained: chloroform fraction, acetone fraction, ethyl acetate fraction and ethanol fraction.
Result of Phytochemical Analysis and Macronutrients in the Crude Extract of the Plant Roots
Phytochemical screening of the plant roots showed that the plant contains alkaloids, glycosides, reducing sugar, carbohydrates, steroids, terpenoids, saponins, proteins, and fats and oil. The roots lacked flavonoids, tannins and acidic compounds. The chemical classes of compounds in the plant roots as indicated by the phytochemical analysis are listed in (Table 1).
Table 1: Phytochemical constituents of the plant.
Note: Absent -, Present, Low concentration ++ Moderate concentration +++ High concentration ++++.
The Antimicrobial Activities of the Crude Ethanol Extract
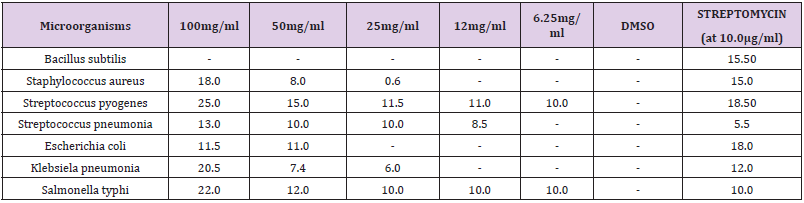
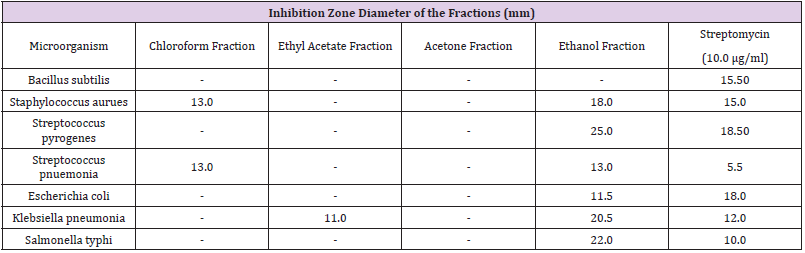
(Table 2) shows the inhibition zone diameter (IZD) of the crude ethanol extract on the microorganisms used. The crude ethanol extract of the plant roots was found to possess antimicrobial activities on both Gram-positive and Gram-negative bacteria. The crude extract was active on S. Pyogenes, Streptococcus pneumonia, Klebsiella pneumonia, Staphylococcus aureus, Salmonella typhi and E. coli. The crude extract was not active on Bacillus subtilis. There were significant differences (p<0.05) between the standard antibiotics and crude extract.
The Minimum Inhibitory Concentrations (Mic) of the Crude Ethanol
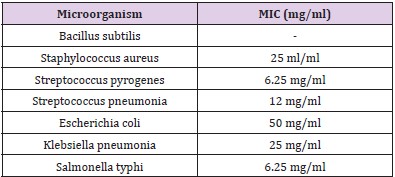
MICs of the crude extract are 25 mg/ml, 100 mg/ml, 6.25 mg/ml, 50 mg/ml, 25 mg/ml 6.25 mg/ml for Staphylococcus aureus, Streptococcus pneumonia, Streptococcus pyogenes, E. coli, Klebsiella pneumonia and Salmonella typhi respectively (Table 3).
Antimicrobial Activities of the Fractions
The chloroform fraction was active on Staphylococcus aureus and on Streptococcus pneumonia, but it was not active on other microorganisms. The ethyl acetate fraction was active only on Streptococcus pneumonia while acetone fraction did not show any significant activity on the microorganisms used. The ethanol fraction showed significant activity on all the bacteria except Bacillus subtilis. Only ethanol fraction of the crude extract was active on both Gram-negative and Gram-positive bacteria (Table 4).
Infra-Red and Nuclear Magnetic Resonance Spectra
When scanned in infra-red, the H1 at 199.9671071 MHz was observed and the highest peaks were between 2-6 ppm, the following groups present are shown in (Table 5). The 13CNMR showed peaks between 180-56 ppm. It was noticed that the following groups are present in the fraction; aromatic group, sulfuryl group, halogen group, carboxyl group and some double and triple bonded carbons.
Discussion
This research was carried out to determine the antimicrobial potentials of Zapoteca portoricensis root extract on streptococcus pyrogen and other bacteria. The result show that the root extracts of Zapoteca portoricensis had antimicrobial effect on streptococcus pyrogen, E. coli, Staphylococcus aereus, Klebsiella pneumonia and Salmonilla typhi except Bacillus subtilis. The reason for the antimicrobial effect of the root extract of Zapoteca portoricensis might be because it possesses phytochemical compounds that might have antimicrobial properties (Ikeyi [19]). This is quite true because the phytochemical of the root extract of the plant showed the presence of phytochemicals such as alkaloids, glycosides, reducing sugar, terpenoids, saponin and resins which have been reported to have antimicrobial activities and could serve as scaffolds for important antibacterial drugs (Ikeyi [19]). Specifically, among the phytochemical compound found in the root extract of Zapoteca portoricensis is alkaloids (Agbafor [10]). Alkaloids have been suggested to have good antimicrobial activity against both gram-negative and gram-positive bacterial (Cushnie, et al. [20]). The presence of alkaloids in high concentration could have likely contributed to the antimicrobial activity of the ethanolic root extract of Zapoteca portoricensis. The phytochemical components seen in these study corroborates with the results of (Agbafor [10]) who also confirmed the presence of alkaloids, glycosides, terpenoids and saponin in the root extract of Zapoteca portoricensis. In order to determine the level of antimicrobial activity of the root extract of Zapoteca portoricensis on the bacterial tested, the crude extract of the plant was used to determine the inhibitory zone diameter of the plant at different concentrations. The result showed that the zone of inhibition of the different bacterial by the plant extract were concentration dependent and was highest at the concentration of 100 mg/ml. Among all the bacterial tested, the inhibitory zone diameter of the plant extract on streptococcus pyrogen at the concentration of 100 mg/ml was found to be the highest. This suggests that streptococcus pyrogen has higher sensitivity to the root extract of Zapoteca portoricensis than other bacterial tested. The variations in the sensitivity of the bacterial to the root extract of Zapoteca portoricensis could be due to differences in the cell wall composition of the bacterial (Nguyen, [1]).
Streptococcus pyrogen might possess a cell wall that allows for easy permeability of the chemical constituents of Zapoteca portoricensis root extract and thus allowing for the inhibition of the cell wall synthesis of the bacterial (Gajdács [6]). It has also been suggested that the cell wall of Streptococcus pyrogen lack cell membrane, an attribute that could aid in easy uptake of antimicrobial phytochemicals in Zapoteca portoricensis (since there will be no selective permeability) (Tamar, et al. [2]). This result corroborates with (Agbafor [21]) who reported high inhibitory zone diameter of Streptococcus pyrogenes, Staphylococcus aureus, E. coli and Klebsiella pnuemonia by root extract of Zapoteca portoricensis. Generally, the leaf extract of Zapoteca portoricensis had inhibitory effect on all the bacterial tested except Bacillus subtilis. The mechanism of inhibition of these bacterial could vary, such as inhibition of the protein synthesis, disruption of membrane structure, inhibition of folate coenzymes, inhibition of nucleic acids, inhibition of peptidoglycans, deterioration of cytoplasmic pH, increase permeability of plasma membrane, prevention of extracellular and intracellular microbial enzyme production, interruption of bacterial metabolic pathways and disruption of plaque and biofilm formation (Mahon, et al. [22]) (Lin, et al. [23]). Depending on the morphological and physiological structure of the bacterial, the demonstration of antimicrobial activity against both gram-positive and gram negative bacteria by Zapoteca portoricensis may be indicative of the presence of broad spectrum antibiotic compounds in the extract (Reygaert [24]). Different fractions of the leaf extract of Zapoteca portoricensis were assayed in order to determine which fraction has higher antimicrobial activity. The result showed that only the ethanol fraction had significant activity on both the gram-negative and gram-positive bacteria. This might be due to better solubility of the active component of Zapoteca portoricensis in ethanol and suggests that ethanol might be the best solvent of extraction of antimicrobial compounds in Zapoteca portoricensis (Nwodo, et al. [25]). This corroborates with the result of (Nwodo, et al. [25]) who reported better solubility of Zapoteca portoricensis in ethanol than other solvents. Next, investigation was carried out to determine the minimum inhibitory concentration (MIC) of the root extract of Zapoteca portoricensis on the bacterial.
The result showed that Streptococcus pyrogenes and Salmonella typhi had the lowest MIC scores indicating that less concentration of ethanolic root extract of Zapoteca portoricensis is required for inhibition of these bacterial (Nkechukwu, et al. [26]). This suggests that, ethanolic root extract of Zapoteca portoricensis might be a more effective antimicrobial agent on Streptococcus pyrogenes and Salmonella typhi than other bacterial tested. The efficacy of phytochemicals in the plant extracts as antimicrobial agents with little or no side effect depends on the structure of the compounds interacting with the pathogen. The infrared spectrum of the ethanolic root fraction of Zapoteca portoricensis showed a broad band at 3400cm-1, 2900cm-1, 2100cm-1, 1600cm-1, 1000cm- 1, 1400cm-1 and 900cm-1 indicating the presence of hydroxyl, aldehyde, cyanide, carbonyl, primary amine, alkene and aromatic group respectively. In addition, the 13CNMR spectra showed peaks between 60-80 ppm and 100-110 ppm indicating the presence of sulfulryl, carboxyl, halide, amide, alkene and aromatic groups. The NMR spectra corroborates with the infrared spectrum which also indicated the presence of amine, alkene and aromatic groups. Earlier studies have shown that functional groups such as alkene, aldehylde, carbonyl, carboxyl and aromatic groups exerts good antibacterial activity and play an important role in antibiotic action (Siyanbola, et al. [27]) (Jabamalairaj, et al. [28]). Since the infrared spectra and NMR showed the presence of these functional groups in ethanolic root fraction of Zapoteca portoricensis [28]. It suggests that these functional groups possess great inhibitory effect on the bacteria tested and could be responsible for the inhibition shown by Zapoteca portoricensis root extract.
Conclusion
In this work, the antimicrobial potentials of Zapoteca portoricensis root extract was evaluated, it was found that ethanolic root extract of Zapoteca portoricensis had inhibitory effect on Streptococcus pyrogene, Staphylococcus aereus, E. coli, Klebsiella pneumonia and Salmonella typhi except Bacillus subtilis. Infrared and NMR spectra of the root extract also showed the presence of functional groups such as alkene, aldehylde, carbonyl, carboxyl and aromatic groups which might have played a significant role in the inhibitory effects of Zapoteca portoricensis on streptococcus pyrogene and other bacterial tested.
Acknowledgement
Thanks to the Department of Biochemistry, University of Nigeria, Nsukka for providing the chemical reagents and equipment for this study.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.
Funding Statement
No funding was received for this work.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval Statement
The study was conducted in accordance with the regulations and ethical approval of the Ethics and Biosafety Committee of the Faculty of Biological Sciences, University of Nigeria, Nsukka.
References
- Nguyen J, Fernandez V, Stocker R, Uwe Sauer, Martin Ackermann, et al. (2021) A distinct growth physiology enhances bacteria growth under rapid nutrient fluctuations. Nature Communications 12(1): 3662.
- Tamar E, Koler M, Vaknin A (2016) The role of motility and chemotaxis in the bacterial colonization of protected surfaces. Scientific Report 6: 19616.
- Li B, Webster T J (2018) Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopaedic infections. Journal of Orthopaedic Research 36(1): 22-32.
- Van Duin D, Paterson D (2016) Multidrug resistant bacteria in the community: trends and lessons learned. Infectious Disease Clinics of North America 30(2): 377-390.
- Wernli D, Jørgensen PS, Harbarth S, Scott P Carroll, Ramanan Laxminarayan, et al. (2017) Antimicrobial resistance: the complex challenge of measurement to inform policy and the public. PLoS Med 14(8): e1002378.
- Gajdács M (2019) The concept of an ideal antibiotic: implications for drug design. Molecules 24(5): 892.
- Liu J (2020) Tackling the global non-prescription use of antibiotics. Lancet Infectious Disease 20(2): 169-170.
- Arwa PS, Onyango JC, Nyunja RO (2008) Phytochemical compounds and antimicrobial activity of extracts of Rhoicissus plant (Rhoicissus reoilli) (Plench). Plant Sciences Research, 1(3): 68-73.
- Ikeyi AP, Okagu IU, Ezeanyika LUS, Alumanah EO (2020) Zapoteca portoricensis root crude methanol extract and its fractions normalizes aberrations associated with benign prostatic hyperplasia in rats. All Life 13(1): 360-372.
- Agbafor KN, Ogbanshi ME, Akubugwo EI (2014) Phytochemical screening, hepatoprotective and antioxidant effect of leaf extract of Zapoteca portoricensis. Advanced Biological Sciences 4(1): 35-39.
- Agbo MO, Okoye FBC, Nwodo JN (2010) In vivo anti-inflammatory effect of Zapoteca portoricensis. International Journal of Health Research 3(1): 29-35.
- Nwodo NJ, Okoye FBC, Lai D, Debbab A, Brun R, et al. (2014) Two trypanocidal Dipeptides from the roots of Zapoteca portricensis (Fabaceae). Molecules 19(5): 5470-5477.
- Joshua PE, Ezugwu CH, Chilaka F C, Nwodo OFC, Dasofunjo K, et al. (2018) Effect of ethanol extract of Zapoteca portoricensis stem on testosterone-induced benign prostate hyperplasia (BPH) in adult male albino rats. Australian Journal of Basic Applied Science 12(12): 9-18.
- Ukwe CV, Ubaka CM, Adibe MO, Okonkwo CJ, Akah PA, et al. (2010) Antiulcer activity of roots of Zapoteca portoricensis (fam.fabiaceae). JBCP 1(3): 183-186.
- Esimone CO, Onuh PU, Egege MK, Ugoeze KC, Nicholas Chinedu Obitte, et al. (2009) In vitro Evaluation of Lozenges Containing Extracts of Roots of Zapoteca portoricensis (FAM: Fabaceae). Journal of Pharmacology and Toxicology 4(3): 132-137.
- Jamil K, Bakhtiar M, Khan AR, Rubina F, Rehana R, et al. (2009) Synthesis characterization and antimicrobial activitiesof novel organotin compounds. African Journal of Pure and Applied Chemistry 3(4): 66-71.
- Harborne JB (1998) Textbook of Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. 5th Edition, Chapman and Hall Ltd, London 21-72.
- (2000) WHO (World Health Organisation), General Guidelines for Methodologies on Research and Evaluation on Traditional Medicine. WHO/EMD/TRE/: 1-70.
- Ikeyi AP, Ezeanyika LUS, Alumanah EO, Okagu IU (2019) Proximate and phytochemical compositions, and toxicity studies on Zapoteca portoriscensis root methanol extract and its fractions. Pharmacologyonline (1): 50-56.
- Cushnie TP, Cushnie B, Andrew J Lamb (2014) Alkaloids: an overview of their antibacterial, antibiotic-enhancing, and antivirulence activities. International Journal of Antimicrobial Agents 44(5): 1-34.
- Agbafor KN, Akubugwo EI, Ogbashi ME, Ajah PM, Ukwandu CC, et al. (2011) Chemical and antimicrbial properties of leaf extract of Zapoteca portoricesis. Research Journal of Medical Plant p. 1-8.
- Mahon CR, Lehman DC, Manuselis G (2014) Antimicrobial agent mechanisms of action and resistance, In: Textbook of Diagnostic Microbiology, Saunders, St. Louis, USA, pp. 254-273.
- Lin J, Nishino K, Roberts MC, Tolmasky M, Amivov RI, et al. (2015) Mechanisms of antibiotic resistance. Front Microbiology 6: 34.
- Reygaert WC (2018) An overview of the antimicrobial resistance mechanisms of bacteria. Microbiology 4(3): 482-501.
- Nwodo OFC, Joshua PE, Ugwuoke MC, Uroko RI (2015) Anti-Malarial and some Biochemical Indices of the Ethanol Extract of Zapoteca portoricensis Root on Malaria-Infected Mice. Asian Journal of Biochemistry 10 (6): 281-289.
- Nkechukwu IM, Onyeka AL, Calistus DN, Ruth AA, Okechukwu EC, et al. (2014) Antimicrobial Activity of Selected Medicinal Plants of South-Eastern Nigeria on Pseudomonas species Expressing Extended Spectrum Beta Lactamase (ESBL). European Journal of Medicinal Plants 4(11): 1367-1377.
- Siyanbola TO, James OO, Gurunathan T, Sasidhar K, Ajanaku KO, et al. (2015) Synthesis, characterization and antimicrobial evaluation of polyesteramide resin from moringa oleifera seed oil (moso) for surface coating application. Canadian Journal of Pure and Applied Sciences 9(1): 3229-3240.
- Jabamalairaj A, Dorairaj S, Yadav SA, Bathrachalam C (2015) Detection of functional group and antimicrobial activity of leaf extracts of citrus grandis (l.) Against selected clinical pathogens. Indo American Journal of Pharmaceutical Research 5(5): 1642-1648.
- Li B, Webster TJ (2018) Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopaedic infections. Journal of Orthopaedic Research 36(1): 22-32.

 Research Article
Research Article