ABSTRACT
Helicobacter pylori is a spiral-shaped, Gram-negative, microaerophilic flagellate. It may cause duodenal/gastric ulcer disease, gastritis, gastric adenocarcinoma, mucosa-associated tissue lymphoma, and primary B-cell gastric lymphoma. The delay in creating an H. pylori vaccination has led to novel treatment options. Eradication treatment is difficult since there are so many medication combinations with different effectiveness and toxicity. Herbal medicines have recently been proposed as a storehouse for innovative bioactive chemicals mostly in existing drug manufacturing landscape. Commercial viability of highly effective medication has not yet been shown after a number of research have been conducted to find highly active and well-tolerated medicines. These include extended DDS that increases the anti- h.pylori activity of antimicrobials in the stomach and can be used peptic ulcer disease therapy. Since most antibiotics are unstable in acidic environments, a gastro protective GRDDS is needed. Bacteriophages may replace antibiotic treatment due to their host specificity and restricted range of action. Gene editing tools may cleave species-specific sections of the bacterial DNA and can act as antimicrobial. Zinc fingers, PNAs, and CRISPR-Cas systems are used to modify genes. Studies have also shown the antibacterial activity of metallic nanoparticles, indicating their usage in medical devices.
Keywords: Helicobacter Pylori; Crispr- Cas Systems; Antibiotics; Bacteriocin; Bacteriophages; Nanotechnology
Abbreviations: NPS: Nanoparticles; H. Pylori: Helicobacter Pylori; MALT: Mucosa- Associated Tissue Lymphoma; DDS: Drug Delivery Systems; CRISPR-Cas 9: Clustered Regularly Interspaced Short Palindromic Repeats And CRISPR-Associated Protein 9; PPI: Proton Pump Inhibitor; Sno2: Tin Oxide; AgNPs: Silver Nanoparticle; Au: Gold, Zno: Zin Oxide
Introduction
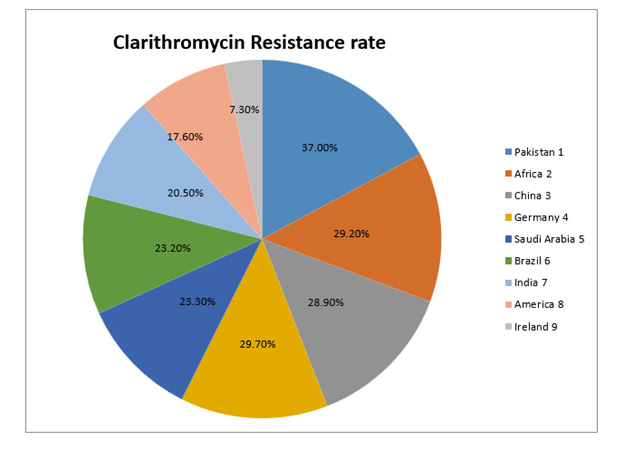
Helicobacter pylori is a spiral-shaped, microaerophilic, Gramnegative flagellate bacterium. It is suspected of playing a role in the development of diseases such as duodenal/gastric ulcer disease, gastritis, gastric adenocarcinoma, mucosa-associated tissue lymphoma (MALT), and primary B-cell gastric lymphoma [1]. It is important to keep in mind, in light of the pharmacological treatment that is being administered at the present time, that the primary drugs being administered may not have an impact, mostly as a result of drug resistance. Since the efficacy of empirical treatment has decreased and the rate of H. pylori eradication is directly dependent on the strain’s susceptibility to the antibiotics that are currently in use, it is known that H. pylori is a high priority group that is in urgent need of new antimicrobials. This is because [2,3]. Alterations in the enzymatic systems may lead to metronidazole resistance mechanisms [4]. These changes interfere with the normal growth and development of microorganisms. This antimicrobial displays the most prevalent antibiotic resistance seen in H. pylori (20-95 percent): 99.5 percent in Asia, 79.4 percent in America, 83.0 percent in Europe, and 57.0 percent in Oceania. Because it has such a high incidence of resistance, clarithromycin is the antimicrobial treatment of choice for eradicating H. pylori; nonetheless, this poses a significant problem at the time (0-50 percent). Changes in genes that encode a domain of one of the subunits of the prokaryotic ribosome, in addition to other enzymes connected to the process of protein synthesis, are responsible for its resistance mechanism, which may be found in reference number [5]. The development of resistance to amoxicillin is linked to structural alterations, such as variations in penicillin-binding proteins [6]. In H. pylori, resistance to levofloxacin, rifampicin, and furazolidone is believed to be low and unimportant; nonetheless, the levels of all of these antimicrobial resistances are growing with time [7,8].
Therefore, gaining a knowledge of the mechanism of resistance as well as the prevalence of the antibiotics employed in the eradication of H. pylori is essential for the search for new medications and better therapy. Novel medicines may be of assistance in the management of H. pylori patients, even in the face of the development of drug resistance. In recent years, a number of different drug delivery systems (DDS) have been created with the purpose of delivering medications to the stomach in a more specific manner. Extended residence periods of DDS in the stomach may lead to local action in the upper GI tract, such as in the treatment of peptic ulcer disease. This can also contribute to enhanced bioavailability for medications that are largely absorbed easily upon release in the GI tract. Because of problems with the stability of most antibiotics in an acidic environment, a GRDDS that is gastro-protective to the encapsulated medication is necessary in order to circumvent this problem. Antimicrobial peptides, which are substances generated by cells as a consequence of innate immunity in order to create protection against several infections, are another possibility that has been investigated and looked into as a possible treatment option. They are able to influence cellular membranes as well as intracellular activities [9] (Table 1).
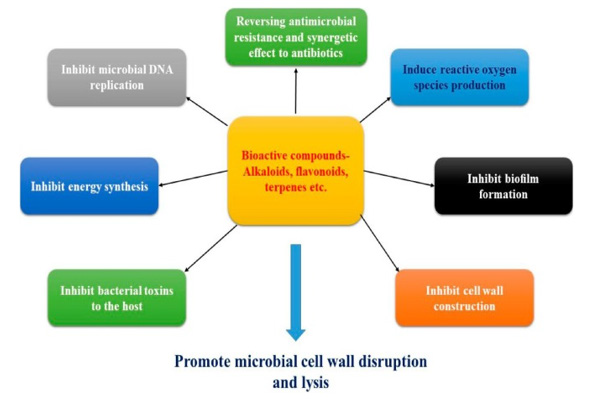
Also, many studies have been performed on a great number of plant varieties. Natural products exhibit their own anti-H. Pylori actions via different mechanisms. While therapeutic agents have either ant secretory or healing effects, prophylactic compounds produce their effect via their antioxidant and anti-inflammatory mechanisms. Bacteriophages can provide a valid therapeutic alternative and can substitute antibiotic therapy phages possess very unique qualities such as Host specificity and narrow spectrum of its activity disturbs the microbiota negligibly and have very good safety parameters [10-19]. Phages are tolerated very safely by the human body as they replicate inside specific bacterium and has no interaction with human body cells or its molecular mechanisms [20], Bacteria also develops resistance towards phages like antibiotics but engineering new phages are much easier than inventing new antibiotics [21]. Gene editing techniques are also very interesting because of their capacity to target and cleave particular regions within the bacterial genome in a way that is species-specific. This may lead to antimicrobials that have the narrowest range imaginable. Zinc fingers [22-25], transcription activation-like effector nucleases [26], peptide nucleic acids [27], RNA interference (RNAi) [28], and CRISPR-Cas systems [29-35] are the techniques that are used for editing genes. Particulate systems are needed to penetrate the mucus barrier. This is necessary in order to get around the limits of mucoadhesive systems. Many recent studies also reported the antimicrobial efficacy of metallic nanomaterials, suggesting their potential use in medical devices Figures 1-5.
Antibiotics a Gold Standard for H. Pylori Eradication
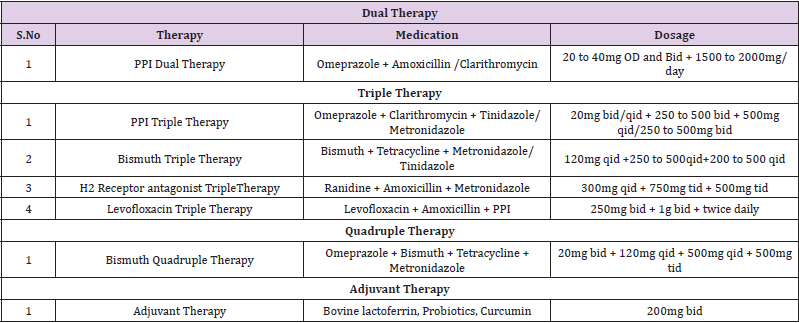
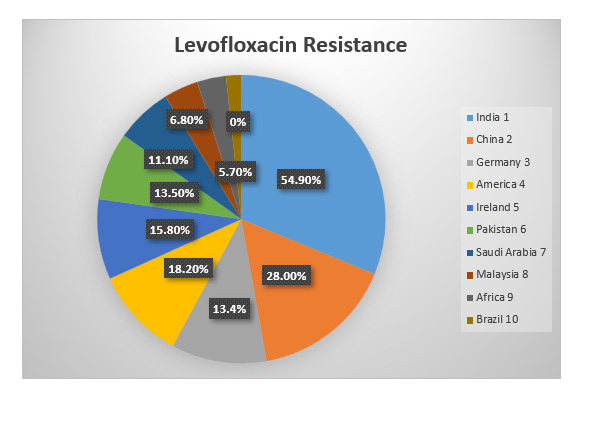
The current gold standard for the eradication of H. pylori infections in adults is a treatment regimen consisting of either triple or quadruple combination therapy. The use of a proton pump inhibitor (PPI) as a pH-control pharmaceutic in conjunction with the concurrent or sequential administration of two antibiotics (clarithromycin, metronidazole, or amoxicillin) over a period of one to two weeks constitutes triple therapy. Unfortunately, the increasing prevalence of antibiotic resistance is posing a threat to the efficacy of this treatment [36]. Antibiotic resistance can be caused by escape mutations, drug inactivation, and drug efflux pumps, and altered membrane permeability. However, recalcitrant and recurrent infections can also be caused by antibiotic tolerance caused by the presence of biofilm-embedded or dormant, no replicating bacteria [37-40]. Metronidazole was shown to be the most resistant of the H. pylori antibiotics studied, followed by clarithromycin and amoxicillin, with levofloxacin and amoxicillin having the second-highest rates of resistance at 18.94% and 14.67%, respectively. A 2-year assessment of H. pylori eradication effectiveness and best treatment regimens is conducted by the Maastricht group in Europe [41]. Recent years have seen an increase in the recommendation of 2-week treatment and quadruple therapy, which includes bismuth or another antibiotic (such as tetracycline, levofloxacin, or furazolidone), as a first-line therapy [41-45] Figures 6-10. Because of the high dose and lengthy duration of these medications, patient noncompliance is a major contributor to treatment failure. Rescue therapy is an option if treatment fails, however it is only indicated for individuals who have had three or more unsuccessful treatments [46].
A combination of rifabutin and a high-dose PPI is often administered [47]. The antibiotic moenomycin, a transglycosylase inhibitor that prevents peptidoglycan production in Gram-positive bacteria and exhibits strong efficacy against H. pylori and stomach ulcers, has also been found to be beneficial [48-50]. Antibacterial efficacy against multidrug-resistant organisms suggests that moenomycin may be a suitable rescue antibiotic [51]. Prolonged therapy with broad-spectrum antibiotics may have negative health consequences on the commensal microbiome, in addition to diminishing eradication efficiency. As well as causing shortterm problems such as Clostridium difficile blooms, antibioticinduced dysbiosis can also have long-term effects on health, including the development of inflammatory bowel syndromes and the acceleration of metabolic diseases such as weight gain, fat accumulation, and Type 2 diabetes [52,54]. In paediatric H. pylori therapy, this is a major issue because of the harm that broad-spectrum medicines may do to the gut flora. Adults with H. pylori-positive peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, or who are at elevated risk of developing gastric cancer or who are in remission get eradication treatment. For the first time, researchers have shown that the prevention of stomach cancer is possible in healthy, uninfected persons who have been infected with H.pylori, at least in areas with high rates of gastric cancer [55]. It seems doubtful that large-scale H. pylori eradication initiatives will be implemented until new therapeutic alternatives, or a vaccine are produced, since this would likely worsen the emergence of medication resistance. It is very clear that the existing treatment choices are coming under increasing amounts of stress, and it is imperative that new antibiotics, other medicines, or a vaccination be developed Figures 11-13.
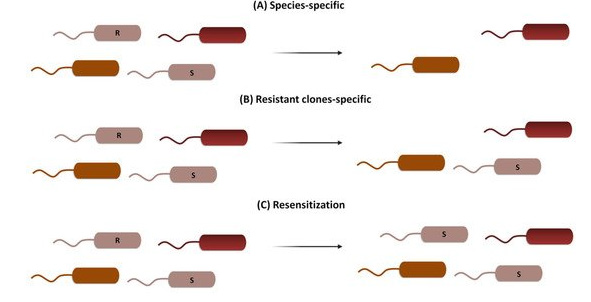
Figure 6: There are three distinct ways to fight antimicrobial resistance (AMR). The remaining members of the microbial community are unaffected by the use of species-specific targeting, which eliminates both vulnerable and resistant clones of the same species. Through the deliberate targeting of resistance genes, the resensitization process can convert resistant clones into vulnerable ones [200].
New Strategies in Antibiotic Delivery For Treating H. Pylori Infection
It has been more common in recent years to design drug delivery systems (DDS) that specifically target the stomach. Peptic ulcer disease therapy and enhanced bioavailability for medications that are rapidly absorbed when released in the GI system may be achieved by extending the time DDS remains in the stomach. The inability of most antibiotics to remain stable in an acidic environment may be circumvented by using a GRDDS that is gastroprotective against the encapsulated medication. This will allow for the problem to be solved. The floating and mucoadhesive tactics are the gastroretentive approaches that are claimed to be used the most often. The use of low-density polymers or gas-generating agents is required for floating DDS to function properly. Because these formulations are less dense than the contents of the stomach, they are able to float in the stomach for a longer period of time without having an effect on the pace at which the stomach empties, and they slowly release the drug. Treatment with conventional formulations may on occasion result in the elimination of H. pylori; however, treatment with these formulations commonly fails to eliminate the bacteria from the gastric fundus and the body of the stomach, which results in re-colonization. Antibiotics should be more successful as a therapy if they are in touch with a wider surface area of the stomach, which may be accomplished by directing antibiotics to the fundus of the stomach using floating gastroretentive formulations. Mucoadhesive DDS are manufactured using polymers that are capable of sticking to the mucus lining of the gastrointestinal system via the establishment of noncovalent bonds [53-58]. This adhesion to the stomach lining improves the gastric residence of the formulation, which in turn improves the local delivery of the medicine as well as the bioavailability of the drug that has been administered. Because each of the gastroretentive techniques has its own set of drawbacks (for example, floating systems need a significant amount of fluid to be present in the stomach, and mucoadhesive systems are vulnerable to gastric mucus turnover rates), researchers typically investigate the use of multiple gastroretentive techniques in tandem with one another in order to achieve gastroretention. In the event that one of the methods does not work, it is possible that the other will. This will guarantee that the formulation is kept in the stomach. There have been several studies written and published that concentrate on the mechanism of drug delivery systems that are targeted to the stomach [58-70].
Simultaneous Delivery of Antibiotics Using Liposomes
In the wake of recent advancements in nanotechnology, the idea of using liposomes in gastrointestinal tract–targeted medication delivery systems has come into clearer focus (DDS). Liposomes having the potential of entrapping both hydrophilic and hydrophobic medicines, with the hydrophilic drugs remaining entrapped in the bilayer region while the hydrophobic drugs are entrapped in the aqueous environment [70-73]. Liposomes are useful in the treatment of infections due to the fact that they are equivalent to cell membranes [73-77]. Research has been conducted on the prospect of utilising liposomes to cure infections caused by H. pylori, and it has been shown that this might be accomplished. Liposomes that were loaded with antimicrobial medications such as ampicillin and MET were oriented towards H. pylori, and the interactions between the liposomes and H. pylori were investigated [78]. H. pylori is a bacterium that causes stomach ulcers. It is likely that the integration of certain ligands at the surface of the liposome would enable for precise targeting of H. pylori, which would enhance the GRT of the drug. VacA is a protein that is released by many different H. pylori strains, and it is the protein that is responsible for destabilising the phospholipid membrane that is present in epithelial cells [79-81]. Therefore, it is hypothesised that the release of the encapsulated medicine might be aided by the vacuolating impact of the protein if the liposomes are in close vicinity to the bacteria. This is based on the fact that the closeness of the two entities is necessary. Compared to liposomes generated using DPPC, Epikuron 170® (Cargill, Minnesota, United States) derived liposomes exhibited a greater degree of repulsion toward positively charged molecules (1,2-dipalmitoyl-sn-glycero3- phosphocholine).
The EE of MET was much lower for Epikuron 170 (1.4 percent) compared to the DPPC ones (11.2 percent); however, the encapsulation of ampicillin was not significantly impacted by the alteration in phospholipid composition (10 percent [DPPC] versus 13.9 percent [Epikuron]). It has been found that H. pylori has a very strong affinity for cholesterol [82,83], which helps to explain why liposomes have such a strong attraction for the bacterium. This was confirmed as the different liposome formulations demonstrated varying degrees of affinity for the H. pylori suggesting that liposomes are ‘stuck’ around the bacteria. This affinity was determined using fluorescence intensity due to both the bacteria and liposome. The interactions of the liposomes with other bacteria such as Staphylococcus or Escherichia coli strains showed no evidence of interactions, suggesting the presence of cholesterol in liposomes was likely the main reason for the H. pylori–liposome interactions. This liposome–bacteria interaction resulted in the killing of the bacteria and the ampicillin-loaded liposomes demonstrated antibacterial effects [78]. The incorporation of fucosyled glycolipids in the vesicle membrane led to an interaction between the liposome and the spiral and coccoid forms of the bacteria. The formulated liposomes demonstrated promising results against H. pylori infection [78]. A further study was carried out on liposomes in which cholesteryl tetraethylene glycol oside were incorporated as model ligands for H. pylori adhesins to study the effect of gastric conditions on stability of the liposomes [84]. These liposomes were stable with the pH of the internal aqueous environment being close to pH 4 even when the external environment was exposed to gastric pH condition between pH 1.2 and 2. This presents a means of enhancing the stability of the antibiotics to be delivered which are known to be generally unstable in the acidic environment of the stomach [85]. A GRDDS incorporating with AMX and MET was designed for the eradication of H. pylori. This system was prepared by alternating coatings of polyanion (poly[acrylic acid]; PAA) and polycation (poly[allylamine hydrochloride]; PAH) using liposomes as the core. It was observed that the multilayered system exhibited prolonged drug release in simulated gastric fluid compared with conventional liposomes suitable for drug delivery for eradication of H. pylori infection. These composite nanocapsules containing the combination therapy of AMX and MET had anenhanced potential in H. pylori eradication when compared with conventional DDS in a mouse model [84].
Phytochemicals Role in Combating H. Pylori Infections
Exploration in the realm of medicinal plants has been pushed by the search for novel anti-H. Pylori medicines. Numerous investigations on a very large number of different plant kinds have been carried out. Natural products each have their own unique anti-H. Pylori effects that they accomplish via a variety of ways. Prophylactic substances, on the other hand, create their impact via antioxidant and anti-inflammatory processes, as opposed to the ant secretory or healing actions that are associated with therapeutic medicines [85-89].
Mechanisms of Medicinal Plants as Anti-H. Pylori: Many natural compounds have anti-H. Pylori potentials. These potentials may be realised via a variety of methods, including urease inhibition, DNA damage, reduction of protein synthesis, and anti-inflammatory actions. In addition to the anti-H. Pylori actions that certain enzymes, such as dihydrofolate reductase and myeloperoxidase N-acetyltransferase, are responsible for [90-92].
Bioactive Compounds in Plants and their Anti-Hyplori Applications: Effective urease inhibitors include a number of natural and synthetic compounds, including sulforaphane, an isothiocyanate that is derived from crucifers4, allyl isothiocyanates5, flavonoids and their corresponding reductive derivatives6, quercetin and its analogues7, and some synthetic thiosemicarbazones [93]. It was discovered that caffeic acid phenethyl ester, one of the primary components of propolis, acts as a competitive inhibitor of H. pylori peptide deformylase (HpPDF). HpPDF is an enzyme that catalyses the removal of the formyl group from the N-terminus of nascent polypeptide chains, which is essential for H. pylori survival [94]. Caffeic acid phenethyl The following is a list of other targets for the treatment of H. pylori infection:
a) the type II dehydroquinase (DHQ2), which is the third enzyme of the shikimic acid pathway9.
b) glutamate racemase, which provides d-glutamate for the construction of N-acetylglucosamine-N-acetylmuramic acid peptidoglycan subunits10.
c) H. pylori -hydroxyacyl-ACP (FabZ), an important enzyme involved in the bacterial type [95].
Natural Flavonoids Against Helicobacter Pylori Infection: Natural flavonoids have shown significant antibacterial activity against H. pylori (MIC 8 g/mL). Flavonoids inhibited the essential function of HsrA, an OmpR-like orphan response regulator that synchronises metabolic functions and virulence with the availability of nutrients and cell division. Chrysin, apigenin, kaempferol, and hesperetin bind to HsrA with micromolar dissociation constants and 1:1 stoichiometry. These flavonoids were also able to influence other identified molecular targets in H. pylori. Flavonoids are effective antimicrobials against Helicobacter pylori infections. Chrysin reduced CLR’s MIC value by eight times (FIC = 0.125) and MTZ activity by sixteen times. Myricetin suppressed gene expression in the morphological transition from spiral to coccoid forms [96,97].
Alkaloids Against H. Pylori Infections: Rhizoma Coptidis, also known as Huanglian in Chinese, is one of the most often used traditional Chinese remedies for the treatment of H. pylori-related gastrointestinal illnesses. It is the rhizoma of Coptis chinensis Franch., which bears the name Coptis chinensis Franch. It would appear that the distinctive structure of alkaloids is very important to the actions that they are responsible fo [98].
Isothiocyanates (Itcs) Against Helicobacter Pylori: Isothiocyanates are a class of volatile organosulfur chemicals that also go by the name ITCs. They are formed as a byproduct as a result of an interaction that takes place between plant glucosinolates and the myrosinase enzyme. This interaction takes place in plants. These chemical substances are now under investigation for their potential as antibacterial therapy options [89]. The urease that is linked with Helicobacter infections is inhibited by the Isothiocyanates, which also reduces the inflammatory response that occurs as a result of these infections. [99]. Extremely electrophilic and fast to interact with amines, thiols, and hydroxyls, the carbon atom that makes up the ITC group (N=C=S) may be found in the formula. In spite of the fact that the antibacterial processes of ITCs have not been fully understood, it has been hypothesised that the antimicrobial activity of ITCs may be associated with their interaction with proteins [90]. Cysteine is an amino acid that is necessary for the structure of proteins, the function of protein regulators, and the stability of proteins achieved by a number of different approaches. It is well knowledge that ITCs aim for the cysteine residue in P-ATPase in bacteria in order to obstruct the ATP binding sites on that enzyme (E. coli) [91].
Sulforaphane:
A wide number of plants, like the Diplotaxis harra, may be a source of the chemical sulforaphane, which can be found within the ITCs. Significant anticarcinogenic and antibacterial capabilities, most notably against H. pylori, have been shown by it. Since this chemical has also been found to be effective against S. aureus and Listeria monocytogenes, it may be a good candidate for functioning as a novel naturally occurring antibacterial agent. This was shown by the fact that both of these bacteria were killed by the chemical [92].Role Of Curcumin in Treatment of H. Pylori Mediated GIT Diseases:
The rhizomes of turmeric, also called curcuma longa, are the source of the pigment curcumin, which is a well-known polyphenolic pigment with a bright yellow colour. Curcumin is generated from turmeric [100]. In particular, India makes extensive use of it both as a spice and as a food colouring additive [101]. These compounds have the potential to have a number of beneficial effects on human health, including anti-inflammatory, anti-cancer, and anti-microbial properties [102,103]. Both the in vitro studies and the preclinical trials gathered data regarding the impact that the treatment had on H. pylori as well as the diseases that were brought on by the bacteria. Curcumin is a keto-enol tautomer when seen from the perspective of its chemical structure. Despite this, the enol form of the molecule is more stable than the keto form, and this is the case for both the solid and the liquid forms of the substance. Curcumin has a colour of yellow when the pH is acidic, while it has a hue of red when the pH is basic. Two aromatic rings and two carbonyl groups that are not saturated with carbonyl are present in the polyphenolic chemical substances. This molecule’s stability may be attributed to its central hydroxyl group, which is denoted by -OH [104,105].Bacteriophages and Its Antibacterial Activity
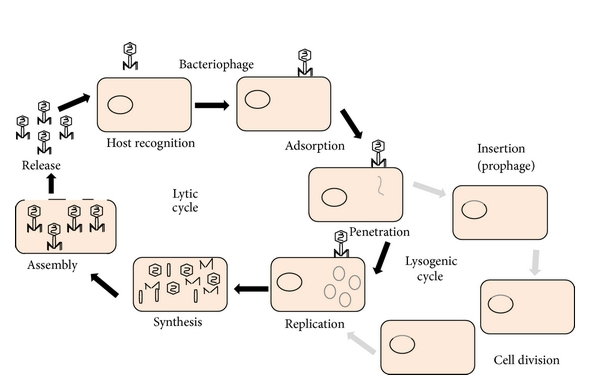
Bacteriophages were discovered by Frederick Twort in 1915 and Felix d Herelle in 1917 independently [106]. Bacteriophage therapy is using of Bacteria specific viruses or phages to eradicate unwanted and unrestrained infectious diseases [106]. Bacteria that have been attacked by obligatory lytic phages are unable to sustain its viability,on the other hand certain antibiotics just stops bacterial groth e.g Macrolides which permits bacteria to evolve itself [107- 109]. When phages infect bacterial cells and during its killing process they replicate and increase in number by autodosing itself [110], at molecular level phages consist mostly of nucleic acids and proteins which are inherently nontoxic towards humans [111-113], phages being macromolecules can interact and activate human immune systems and resulting very harmfull immune responses, about this very little evidence exist which is not really considered during phage therapy as hardle [114-116], to overcome activation of human immune system it is adivisble to use highly specific bacteriophages infecting only bacteria of infection [14]. Phages being highly specific against specific bacterial strains can have very little impact on Normal flora which is present in our GIT [117,118].
Phages as an Alternative Therapy For H. Pylori
The Helicobacter pylori being very plastic in its genetic makeup and the irrational use of antibiotics facilitate spread of resistance against antibiotics [119]. Antibiotic resistance is on peak phages still provide a valid therapeutic alternative and can substitute antibiotic therapy phages possess very unique qualities such as Host specificity and narrow spectrum of its activity disturbs the microbiota negligibly and have very good safety parameters [120,121]. Phages are tolerated very safely by the human body as they replicate inside specific bacterium and has no interaction with human body cells or its molecular mechanisms [122], Bacteria also develops resistance towards phages like antibiotics but engineering new phages are much more easier than inventing new antibiotics [123], the increased acidity of stomach and different gastric enzymes has the ability to change the structural components of phages thus reducing its production rate and its infecting power of the bacterium of interest [124,125]. The increased antibiotic resistance has told us the failure of traditional treatment *proton pump inhibitors along with clarithromycin and amoxicillin or metronidazole* against H. pylori [126,127].
Phages Exploited Against Helicobacter Pylori
The phages which are specifically isolated against H.pylori are named as* phi HPE1 and *phi HPE2 and their host ranges were investigated against four different strains of H.pylori in which all were susceptible to either *phi HPE1 or *phi HPE2 [26], another H.pylori specific phage was isolated which was named KHP30 [27], despite its very fruitful characteristics still there is no available collection of phages specifically against H.pylori and very little research work and scientific literature is available about H.pylori phages the given phages which are isolated uptill now are fully characterised and the next target is to study in detail and examine the virulence factors responsible in eradicating H.pylori [128].
Phages and Antibiotic Synergism
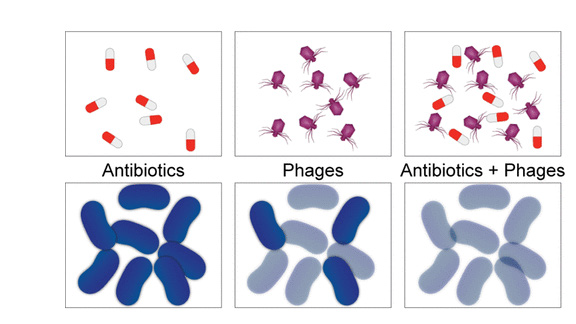
Infections which are caused by antibiotic resistant bacteria lead to reconsideration of phages an alternative therapy beyond antibiotics to combat such infections, still using phages alone is not that much effective as it is used in combination with antibiotics as a combined regimen because a huge number of studies support this concept because the combined regimen reduces the chances of resistance to either of them *antibiotics, phages. interference between antibiotic and phages may be positive, negative or even neutral, while designing the dual therapy of antibacterial choosing the phage ,and antibiotics type, their mixing ratios must also be carefully evaluated [129], it has been shown that using antibiotics below the inhibitory concentrations can enhance the antibacterial effect of phages and also enhances the proliferation power of phages as a result the phage mediated killing of bacteria is increased which is actually called phage antibiotic synergy or PAS [130], using phages and antibiotics combined the benefit of such combined strategy might be stronger bacterial killing and less chances of resistance towards phages or antibiotics used [131]. The use of biotechnology to discover remedies for illnesses caused by multidrug-resistant bacteria, such as those caused by antibiotic resistance, is referred to as phage therapy. As an alternative to the use of antibiotics in the treatment of certain disorders, phage therapy has recently been a topic of study in Western medicine [132]. The current surge in interest was sparked in large part by Polish research that was published for the first time in 1985 and included the use of phages in the treatment of 114 instances of suppurative bacterial infections in children, which were then subjected to scientific investigation.
Positive therapeutic results were obtained in 109 (95.6 percent) of the cases; patients had a wide range of bacterial infections caused by the pathogenic Staphylococci, Klebsiella, Escherichia, Proteus, and Pseudomonas bacteria. Positive therapeutic results were obtained in 109 (95.6 percent) of the cases [133]. Phage formulations were administered to patients who were suffering from a broad array of ailments that were antibiotic resistant. Patients as little as one week old and as elderly as 86 years old have been cared after at this facility. These examinations revealed that more than ninety-two percent of patients had been successfully treated [134]. In a different trial that the same group carried out, phage was used as a therapy for suppurative chronic skin infections. After receiving therapy with phage, the researchers discovered that 77 percent of the patients demonstrated symptoms of improvement in their condition. On the other hand, there were instances in which the phage therapy was unsuccessful for 1.7 percent of patients [135]. After some time, the same group of researchers revealed the findings of a more extensive study that included a greater number of patients [136]. 94 people with antibiotic-resistant septicemia received phage treatment. Wound infections, gastroenteritis, sepsis, osteomyelitis, dermatitis, empyemas, and pneumonia were among them. 14.9% of phage patients were unsuccessful [137]. Complete recovery was attained in 85.1 percent of the cases, which is an excellent rate. The Polish researchers reported a success rate of between 80 and 95 percent [138,139]. Patients suffering from infections that are unresponsive to antibiotic therapy have roughly a forty percent chance of benefiting from treatment with phage. The vast majority of patients at the facility were not subject to any kind of active observation by the medical staff. 2005 saw the establishment of the institution’s very own phage therapy centre, which is now in charge of providing direct patient care [140]. The results of the study have been validated by a research project carried out in Britain more recently, but which was otherwise quite similar to the study. Additionally, the remarkable efficacy of phages against Escherichia coli, Acinetobacter spp., Pseudomonas spp., and Staphylococcus aureus was shown and validated by this research [141-143].
CRISPR-Cas: A New Concept of Antimicrobials
Pharmaceutical corporations have little interest in participating in the antimicrobial medication market. The attention of pharmaceutical corporations is often directed toward more profitable areas like chronic disorders. Antimicrobial resistance (AMR) is a significant issue for researchers since there is a risk that antimicrobial treatments would become less effective as a consequence of its development [144-149]. Because of the conditions in this environment, new methods of battling pathogenic microorganisms have emerged, each of which employs a distinct mechanism of action. Antimicrobial peptides, bacteriophages, metal nanoparticles, and tools for altering genes are all included in these efforts [150]. Among them are zinc fingers, which are able to target and cleave certain areas within the genome of bacteria in a manner that is species-specific [151,152], transcription activationlike effector nucleases (TALENs) [153], peptide nucleic acids [19], RNA interference (RNAi) [154], and CRISPR-Cas systems [144,155]. The first three rely on protein–DNA interactions to confer specificity, which means that protein engineering is necessary for the creation of the fourth method. Because of this, reshaping the effector proteins in order to adapt them to new targets is a tough task that is both costly and time-consuming. However, the specificity of CRISPR-Cas is accomplished by interactions between RNA and DNA. Since RNA engineering is far less expensive, it is an ideal option for a new concept of antibiotics based on gene editing [156], due to the fact that it is such a good candidate. CRISPR-Cas can be utilised in the following ways:
(i) It can be directed to cleave species-specific genes to treat acute infections, resulting in the deployment of the bacteria of interest while maintaining the host’s microbiome unaltered [157].
(ii) It can be directed to cleave drug-resistance genes, eliminating bacteria harbouring them while maintaining the viability of the wild-type susceptible clones and thus decolonizing patients [158].
Probiotic Therapy in Helicobacter Pylori Infection
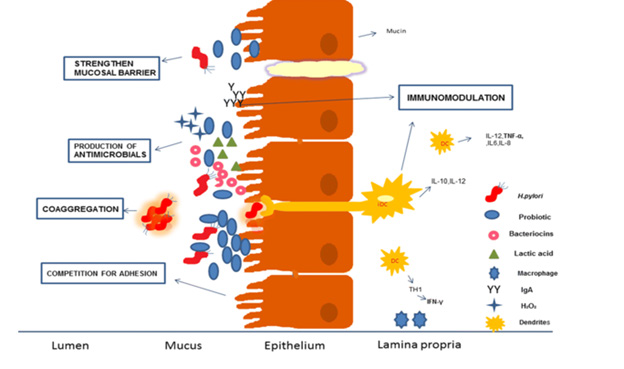
Infections caused by H. pylori have been the subject of substantial research into the use of probiotics as a complementary therapy to antibiotics [159]. Probiotics have been shown in a number of studies to have significant therapeutic promise for the treatment of a variety of gastrointestinal disorders [160]. According to one definition, probiotics are “living micro-organisms that offer a favorable influence on the health of the host when provided in enough quantity” [161]. There are a wide variety of microbial species that have the potential to operate as probiotics. Some of them include Lactobacillus, Bifidobacterial, Saccharomyces, and Streptococcus, among others; however, Lactobacillus and Bifidobacterial are the ones that get the most research. Stabilizing the intestinal microflora by inhibiting pathogens is one of the primary functions of probiotics. This function is primarily attributed to probiotics’ ability to outcompete pathogens for food and binding sites [162,163], as well as their production of antimicrobial substances and immunomodulatory effects [164]. In addition to possessing qualities that inhibit the growth of pathogens, probiotics must also be able to withstand conditions of high pH and bile salts and colonise the surfaces of the gastrointestinal tract in order to be considered among the most promising and possible probiotic candidates. Because of these qualities, researchers have been interested in studying new strains in order to obtain a better understanding of the qualities that distinguish them from others [165]. Numerous studies demonstrate probiotics may reduce antibiotic adverse effects, improve H. pylori eradication, and reduce cell harm [166]. Not every probiotic strain improves H. pylori eradication therapy, although some do. In a trial of H. pylori infection in children following conventional treatment, 30% were re-infected after 2 years probiotics as an eradication adjuvant or vaccine delivery mechanism would be highly effective. In prior research, probiotics’ potential against H. pylori in in vitro, in vivo, and clinical trials was documented, but not as an effective vaccine delivery vehicle [167].
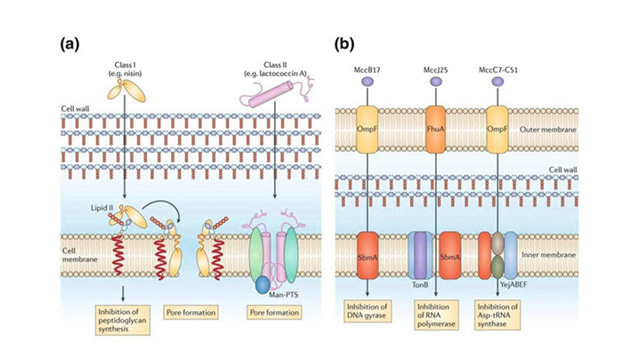
Bacteriocins Role in H. Pylori Treatments
Bacteriocins are proteinaceous molecules that are synthesised by ribosomes. At certain doses, they exhibit potent antibacterial action. Bacteriocins have many functions. They do not have a colour, odour, or taste, which is another factor that contributes to the versatility of their possible applications. As a result of the proteinous structure of these antimicrobial peptides, they are also susceptible to being broken down by proteolytic enzymes [168- 172]. Because they are effective against certain specific kinds of bacterial illnesses, bacteriocins are often referred to as “designed drugs.” Escherichia coli is one of the Gram-negative bacteria and lactic acid bacteria that may be found, however there aren’t very many cases of either of them [173].
Treatment Of Peptic Ulcer Caused by H. Pylori Through Bacteriocin
It has been proposed that bacteriocins produced by Pediococcus acidilactici BA28 might be used in the development of topical therapies for personal care products. Ulcers of the stomach and duodenum are often accompanied by high levels of the anaerobic strain of Helicobacter pylori [174].
Muco-Penetrating Systems for H. Pylori Eradication
When the mucosal gel is thick and viscoelastic, it does not let antimicrobial medications to enter through it in a consistent manner. However, mucoadhesion causes an increase in the amount of time those particles spend in the stomach. The swelling of the polymer may make it more difficult to dock it in stomach mucus, reducing its mobility and, as a result, its capacity to penetrate mucus [175]. In addition, stomach motility and proteolytic activities increase mucus turnover, shortening gastric residence time. Thus, adhesion to mucus might prevent the system from penetrating the mucus layer and infiltrating the underlying epithelia [176]. H. pylori infection can only be treated if the medicine is delivered directly to the infected area using a particle system that penetrates the mucus barrier. Several studies have discovered the existence of particle systems that may pass through the mucosa. PEG-coated polystyrene-based non-adhesive nanoparticles may penetrate the sputum of individuals with cystic fibrosis, according to the researchers [177], Biodegradable PEG-PSA (poly sebacic acid)- based biodegradable nanoparticles quickly penetrate the human mucus barrier, [178]. insulin-loaded polyethylene glycol grafted chitosan (PEG-g-chitosan) nanoparticle for nasal absorption [179]. PLGA nanoparticles coated with DNA for gene delivery in gastric mucus (poly lactide co-glycolic acid) [180]. By sheltering the cationic charge, mucin fibres may play a key role in particle penetration, since they reduce the ability of particles to adhere to mucous membranes. Particles smaller than the mucin fibre mesh have been shown to have high mucin penetration [181]. Biopolymer nanomaterials were also reported for the purpose of stomach-specific administration of medicines in the context of H. pylori elimination [182]. Chitosan was employed because of its bioadhesive and antibacterial qualities, and heparin was employed because of its anticoagulation characteristic, in order to hasten the healing of ulcers and the regeneration of mucosal tissue. For the purpose of stomach mucosal adhesion, the penetration of nanoparticles deep into the mucus layer, and the efficient administration of medication near epithelial cells, the particulate system has to have a smaller size of less than 200 nanometers and a low zeta potential value [183,184]. According to the results of a research, nanoparticles may maintain their stability in acidic environments and are able to shield the antibiotic ingredient. It’s possible that the protonation of amino groups [NH3] in chitosan caused a greater particle size for nanoparticles at pH 1.2–2.5 than it did at pH 4.5–6.5. This was seen when comparing particle sizes at both pH ranges [185]. Investigators have demonstrated that gastric mucus may function as a transport medium for nanomaterials when chitosan is employed. This might culminate in the distribution of antimicrobial therapies, which would be helpful in the elimination of Helicobacter pylori as well as other infections, such as MRSA and MRK [186].
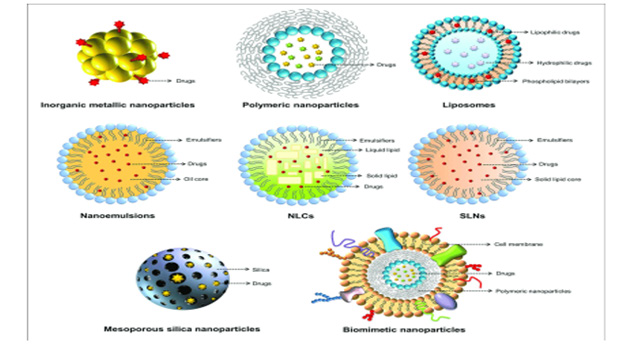
Nanotechnology-Based Treatment Approaches
The advent of nanotechnology has ushered in a new era of global progress by opening up previously inaccessible avenues of exploration within the realm of biomedical research. It is possible to provide NPs to every biological system through the respiratory, gastrointestinal, parenteral, and intraocular routes, respectively. They engage with the cell wall or membrane and then move the medicine through the cellular structure [187-191].
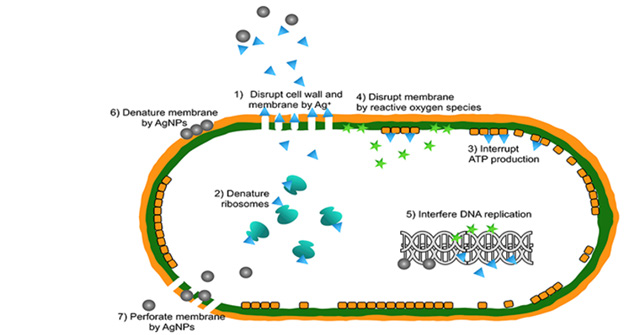
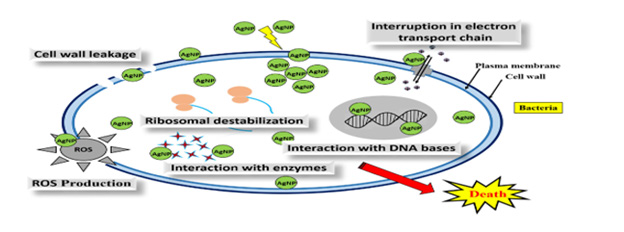
Antimicrobial Efficacy of Metallic Nanomaterials: When bacteria come into contact with nanoparticles of a variety of metal oxides, this results in the creation of reactive oxygen species (ROS). Proteins and DNA, both of which are found on the bacterial cells inside, are susceptible to being harmed by ROS. The appearance of defensive responses may be stimulated when exposed to ROS concentrations below the threshold for lethality [192-195]. Overexpression of extracellular molecules by bacterial cells, such as flagellin, is also included among the bacterial adaptation processes in relation to nanoparticles. These mechanisms allow bacteria to survive in the presence of nanoparticles. Numerous studies have pointed out that nanoparticles have a great antibacterial potential, despite the fact that bacteria already possess mechanisms that allow them to adapt to the effects of being exposed to nanoparticles [195].
AgNPs Antimicrobial Activity: It has been shown that metallic nanoparticles may function well as an antibacterial agent. Against a wide range of Gram-positive and Gram-negative bacteria, silver nanoparticles, often known as AgNPs, have shown strong antibacterial activity. The effectiveness of silver ultra-nanoclusters (UNCs) against H. pylori strains has been shown to increase with decreasing concentrations. Silver nanoparticles create silver ions (Ag+), which adhere to or pass through the cell membrane and wall. Additionally, they have the ability to obstruct DNA replication (DNA) [196-213].
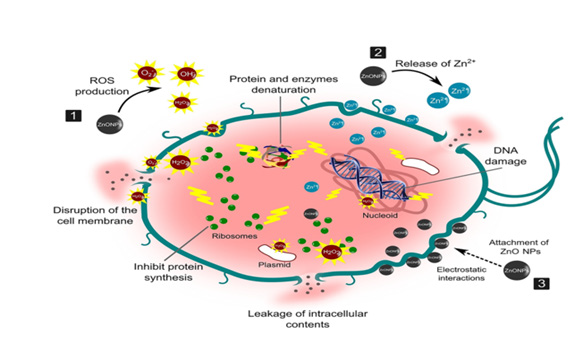
Zno Nanoparticles Antimicrobial Activity: Zinc, which is found as nanoparticles of zinc oxide, has been shown to have a variety of possible applications in the biological realm. They exercise their effects by causing membrane damage in the cells that they penetrate, attaching themselves to proteins and DNA, creating reactive oxygen species (ROS), and interrupting the mechanism that bacteria use to reproduce their DNA.
Gold Nanoparticles: Gold nanoparticles are used in a variety of sectors, including medicine, dentistry, and pharmaceuticals (AuNPs). Photonic crystals, photoluminescent labelling, catalysis, and photodegradation avoidance are all possible applications for AuNPs.
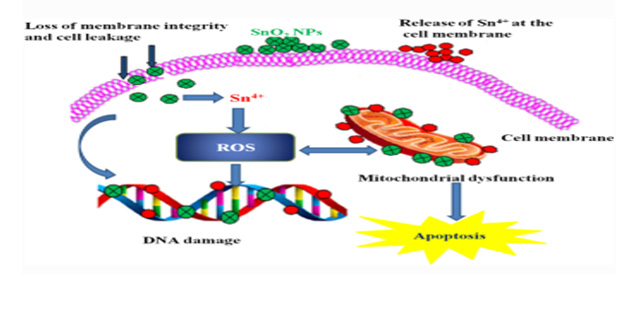
Antimicrobial Activity SnO2 NPs: Antibacterial activity may be exhibited by SnO2 nanoparticles against E. coli and S. aureus. SnO2 NPs have been shown to be effective against a wide variety of microbiological strains; however, the specific mechanism by which this occurs is not fully known. There have been a few different hypothesized mechanisms of action postulated for metal oxide nanoparticles.
Conclusion
There’s no effective approach to prevent and cure H. pylori medication resistance, therefore we recommend complete precautions. Traditional Chinese medicine may cure drug-resistant germs with little adverse effects. The genomic investigations of H. pylori are generating a significant amount of attention, and there is little doubt that they will be followed up on in subsequent research aimed at the development of novel treatments. A treatment that consists of a modest dosage of a single medicine administered over a relatively short period of time and is free of any unwanted effects would be ideal as medical care.
References
- Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N Engl J Med 347(15): 1175-1186.
- Peek RM, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2(1): 28-37.
- Plummer M, Franceschi S, Vignat J, Forman D, de Martel C, et al. (2015) Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 136(2): 487-490.
- Sachs G, Weeks DL, Wen Y, Marcus EA, Scott DR, et al. (2005) Acid acclimation by Helicobacter pylori. Physiology 20(6): 429-438.
- Croxen MA, Sisson G, Melano R, Hoffman PS (2006) The Helicobacter pylori chemotaxis receptor TlpB (HP0103) is required for pH taxis and for colonization of the gastric mucosa. J Bacteriol 188(7): 2656-2665.
- Oleastro M, Menard A (2013) The role of Helicobacter pylori outer membrane proteins in adherence and pathogenesis. Biology 2(3): 1110-1134.
- Salaun L, Linz B, Suerbaum S, Saunders NJ (2004) The diversity within an expanded and redefined repertoire of phase-variable genes in ¨ Helicobacter pylori. Microbiology 150(4): 817-830.
- Goodwin AC, Weinberger DM, Ford CB, Jessica C Nelson, Jonathan D Snider, et al. (2008) Expression of the Helicobacter pylori adhesin SabA is controlled via phase variation and the ArsRS signal transduction system. Microbiology 154(8): 2231-2240.
- Styer CM, Hansen LM, Cooke CL, Amy M Gundersen, Sung Sook Choi, et al. (2010) Expression of the BabA adhesin during experimental infection with Helicobacter pylori. Infect Immun 78(4): 1593-1600.
- Posselt G, Backert S, Wessler S (2013) The functional interplay of Helicobacter pylori factors with gastric epithelial cells induces a multi-step process in pathogenesis. Cell Commun. Signal. 11: 77. Posselt, et al. provide a comprehensive review of the molecular aspects of Helicobacter pylori pathogenesis, focusing on the bacterium’s interaction with the host epithelial tissue.
- Suerbaum S, Josenhans C (2007) Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Micro 5(6): 441-452.
- Leja M, Axon A, Brenner H (2016) Epidemiology of Helicobacter pylori infection. Helicobacter 21(Suppl. 1): 3-7.
- Brown LM (2000) Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 22(2): 283-297.
- Gatta L, Vakil N, Vaira D, Scarpignato C (2013) Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ 347: f4587.
- G Zoumpopoulou, E Pepelassi, W Papaioannou, Marina Georgalaki, Petros A Maragkoudakis, et al. (2013) “Incidence of bacteriocins produced by food-related lactic acid bacteria active towards oral pathogens.” International Journal of Molecular Sciences 14(3): 4640-4654.
- Tominaga K, Higuchi K, Hamasaki N, Hamaguchi M, Takashima T, et al. (2002) In vivo action of novel alkyl methyl quinolone alkaloids against helicobacter pylori. J Antimicrob Chemother 50(4): 547-552.
- Chen Q, Long X, Ji Y, Liang X, Li D, et al. (2019) Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment. Pharmacol Ther 49(11): 1385-1394.
- Chey W D, Leontiadis G I, Howden C W, Moss S F (2017) ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 112(2): 212-239.
- Fallone C A, Chiba N, Van Zanten S V, Fischbach L, Gisbert J P, et al. (2016) The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151(1): e14-69.e14.
- Gisbert J P, Calvet X (2012) Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol Ther 35(2): 209-221.
- Graham D Y, Canaan Y, Maher J, Wiener G, Hulten K G, et al. (2020) Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication: a double-blind, randomized, controlled trial. Ann Intern Med 172(12): 795-802.
- Hays C, Burucoa C, Lehours P, Tran C T, Leleu A, et al. (2018) Molecular characterization of Helicobacter pylori resistance to rifamycins. Helicobacter 23(1): e12451.
- Jones N L, Koletzko S, Goodman K, Bontems P, Cadranel S, et al. (2017) Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol. Nutr 64(6): 991-1003.
- Kahlmeter G, Brown D F, Goldstein F W, Macgowan A P, Mouton J W, et al. (2006) European committee on antimicrobial susceptibility testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect 12(6): 501-503.
- Li L, Ke Y, Yu C, Li G, Yang N, et al. (2017) Antibiotic resistance of Helicobacter pylori in Chinese children: a multicenter retrospective study over 7 years. Helicobacter 22(3): e12373.
- Li H, Yang T, Tang H, Tang X, Shen Y, et al. (2019) Helicobacter pylori infection is an infectious disease and the empiric therapy paradigm should be changed. Precis Clin Med 2: 77-80.
- Liang X, Xu X, Zheng Q, Zhang W, Sun Q, et al. (2013) Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol 11: 802.e801-807.e801.
- Liu D S, Wang Y H, Zhu Z H, Zhang S H, Zhu X, et al. (2019) Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob. Resist Infect Control 8: 192.
- Liu W Z, Xie Y, Lu H, Cheng H, Zeng Z R, et al. (2018) Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 23(2): e12475.
- Liu G, Xu X, He L, Ding Z, Gu Y, et al. (2011) Primary antibiotic resistance of Helicobacter pylori isolated from Beijing children. Helicobacter 16(5): 356-362.
- Mabe K, Okuda M, Kikuchi S, Amagai K, Yoshimura R, et al. (2018) Randomized controlled trial: PPI-based triple therapy containing metronidazole versus clarithromycin as first-line treatment for Helicobacter pylori in adolescents and young adults in Japan. J Infect Chemother 24(7): 538-543.
- Malfertheiner P, Megraud F, O morain C A, Gisbert J P, Kuipers E J, et al. (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 66(1): 6-30.
- Manfredi M, Gismondi P, Maffini V, Bizzarri B, Fornaroli F, et al. (2015) Primary antimicrobial susceptibility changes in children with Helicobacter pylori infection over 13 years in northern Italy. Gastroenterol Res Pract 2015: 717349.
- Montes M, Pérez-Trallero E (2017) How long until routine Helicobacter pylori antimicrobial susceptibility testing? Lancet Infect. Dis 17(2): 130-131.
- Montes M, Villalon FN, Eizaguirre F J, Delgado M, Munoz-Seca I M, et al. (2015) Helicobacter pylori infection in children. Antimicrobial resistance and treatment response. Helicobacter 20(3): 169-175.
- Egan B J, Katicic M, O connor H J, O Morain CA (2007) Treatment of Helicobacter pylori. Helicobacter 12(1): 31-37.
- Asaka M, Kato M, Takahashi S I, Fukuda Y, Sugiyama T, et al. (2010) Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 15(1): 1-20.
- Rajper S, Khan E, Ahmad Z, Alam S M Z, Akbar A, et al. (2012) Macrolide and fluoroquinolone resistance in Helicobacter pylori isolates: an experience at a tertiary care centre in Pakistan. Journal of Pakistan Medical Association 62(11): 1140-1144.
- Yi Hu, Yin Zhu, Nong-Hua Lu (2017) Primary Antibiotic Resistance of Helicobacter pylori in China. 62(5): 1146-1154.
- B Bluemel, H Goelz, B Goldmann, J Grüger, H Hamel, K Loley, et al. Antimicrobial resistance of Helicobacter pylori in Germany. Clin Microbial infect. 26(2): 235-239.
- Alba A Trespalacios-Rangél, William Otero, Azucena Arévalo-Galvis, Raúl A Poutou-Piñales, Emiko Rimbara, et al. Surveillance of Levofloxacin Resistance in Helicobacter pylori Isolates in Bogotá-Colombia. 11(7): e0160007.
- Taranum Ruba Siddiqui, Waquaruddin Ahmed, Ambreen Arif, Safia Bibi, Adnan Khan, et al. (2016) Emerging trends of antimicrobial resistance in Helocobacter phylori isolates obtained from Pakistani Patients: The need for consideration of amoxicillin and clarithromycin. 66(6): 710-716.
- Fahad Alsohaibani, Hamad Al Ashgar, Khalid Al Kahtani, Ingvar Kagevi, Musthafa Peedikayil, et al. (2015) Prospective Trial in Saudi Arabia Comparing the 14-day Standard Triple Therapy with the 10-day Sequential Therapy for Treatment of Helicobacter Pylori Infection 21(4): 220-225.
- Suat Moi Puah, Khean Lee Goh, Heng Kang Ng, Kek Heng Chua (2021) Current status of Helicobacter pylori resistance to Clarithromycin and Levofloxacin in Malaysia-findings from a molecular based study. 9: e11518.
- Kasahun GG, Demoz GT, Desta DM (2020) Primary Resistance Pattern of Helicobacter pylori to Antibiotics in Adult Population: A Systematic Review 13: 1567-1573.
- Flávia Rossi (2011) The Challenges of Antimicrobial Resistance in Brazil, Clinical Infectious Diseases. 52 (9): 1138-1143.
- Ge R, Sun X, Wang D, Zhou Q, Sun H, et al. (2011) Histidine-rich protein Hpn from Helicobacter pylori forms amyloid-like fibrils in vitro and inhibits the proliferation of gastric epithelial AGS cells. Biochim Biochys. Acta 1813(8): 1422-1427.
- Oppong P, Majumdar D, Atherton J, Bebb J (2015) Helicobacter pylori infection and peptic ulcers. Medicine 43(4): 215-222.
- Zullo A, Hassan C, Ridola L, De Francesco V (2013) Standard triple and sequential therapies for Helicobacter pylori eradication: an update. Eur J Intern Med 24(1): 16-19.
- Parsonnet J, Friedman GD, Vandersteen DP, Y Chang, J H Vogelman, et al. (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325(16): 1127-1131.
- Touati E (2010) When bacteria become mutagenic and carcinogenic: lessons from H. pylori. Mutat Res 703(1): 66-70.
- Georgopoulos SD, Papastergiou V, Karatapanis S (2013) Current options for the treatment of Helicobacter pylori. Expert Opin Pharmacol 14(2): 211-223.
- Reviews current regimens for H. Pylori eradication, including advantages and isadvantages of each and the perspectives for their rational use in clinical practice.
- Bazzoli F, Bianchi Porro G, Maconi G, Molteni M, Pozzato P, et al. (2002) Treatment of Helicobacter pylori infection. indications and regimens: an update. Digest Liver Dis 34(1): 70-83.
- Hejazi R, Amiji M (2003) Stomach-specific anti-H. pylori therapy. II. Gastric residence studies of tetracycline-loaded chitosan microspheres in gerbils. Pharm Dev Tech 8(3): 253-262.
- Erah PO, Goddard AF, Barrett DA, P N Shaw, R C Spiller (1997) The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother 39(1): 5-12.
- Vakil N (2005) Helicobacter pylori: factors affecting eradication and recurrence. Am J Gastroenterol 100(11): 2393-2394.
- Axon AT (1994) The role of acid inhibition in the treatment of Helicobacter pylori infection. Scand J Gastroenterol Suppl 201: 16-23.
- Crater JS, Carrier RL (2010) Barrier properties of gastrointestinal mucus to nanoparticle transport. Macromol Biosci 10(12): 1473-1483.
- Ensign LM, Cone R, Hanes J (2012) Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Del Rev 64(6): 557-570.
- Cammarota G, Sanguinetti M, Gallo A (2012) Review article: biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Aliment Pharm Ther 36(3): 222-230.
- Midolo PD, Turnidge JD, Munckhof WJ (1996) Is bactericidal activity of amoxicillin against Helicobacter pylori concentration dependent? Antimicrob. Agents Chemother 40(5): 1327-1328.
- Atherton JC, Cockayne A, Balsitis M, G E Kirk, C J Hawkey, et al. (1995) Detection of the intragastric sites at which Helicobacter pylori evades treatment with amoxycillin and cimetidine. Gut 36(5): 670-674.
- Bardonnet P-L, Faivre V, Boullanger P, Jean-Claude Piffaretti, Françoise Falson (2008) Pre-formulation of liposomes against Helicobacter pylori: characterization and interaction with the bacteria. Eur J Pharm Biopharm 69(3): 908-922.
- Bangham AD (1983) Liposome Letters. Academic Press, MA, USA.
- Pagliaccia C, Wang XM, Tardy F, J L Telford, J M Ruysschaert, et al. (2000) Structure and interaction of VacA of Helicobacter pylori with a lipid membrane. Eur J Biochem 267(1): 104-109.
- Papini E, Zoratti M, Cover TL (2001) In search of the Helicobacter pylori VacA mechanism of action. Toxicon 39(11): 1757-1767.
- Trampenau C, Muller KD (2003) Affinity of Helicobacter pylori to cholesterol and other steroids. Microbes Infect 5(1): 1317.
- Bardonnet PL, Faivre V, Boullanger P, Ollivon M, Falson F, et al. (2009) Glycosylated liposomes against Helicobacter pylori: behavior in acidic conditions. Biochem. Biophys. Res Commun 383(1): 48-53.
- Jain P, Jain S, Prasad KN, S K Jain, Suresh P Vyas. (2009) Polyelectrolyte coated multilayered liposomes (nanocapsules) for the treatment of Helicobacter pylori infection. Mol Pharm 6(2): 593-603.
- Stamatis G, Kyriazopoulos P, Golegou S, Basayiannis A, Skaltsas S, et al. (2003) In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J Ethnopharmacol 88(2-3): 175-179.
- Manayi A, Khanavi M, Saiednia S, Azizi E, Mahmoodpour M R, et al. (2013) Biological activity and microscopic characterization of Lythrum salicaria L. DARU J Pharm Sci 21: 61.
- Mafioleti L, da Silva Junior IF, Colodel E M, Flach A, Martins DT, et al. (2013) Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B Verl J Ethnopharmacol 150(2): 576-582.
- Moraes Tde M, Rodrigues C M, Kushima H, Bauab T M, Villegas W, et al. (2008) Hiruma-Lima C.A. Hancornia speciosa: Indications of gastroprotective, healing and anti-Helicobacter pylori actions. J Ethnopharmacol 120(2): 161-168.
- Nariman F, Eftekhar F, Habibi Z, Falsafi (2004) Anti-Helicobacter pylori activities of six Iranian plants. Helicobacter 9(2): 146-151.
- Zhang X Q, Gu H M, Li X Z, Xu Z N, Chen Y S, et al. (2013) Anti-Helicobacter pylori compounds from the ethanol extracts of Geranium wilfordii. J Ethnopharmacol 147(1): 204-207.
- Wang Y, Wang S L, Zhang J Y, Song X N, Zhang Z Y, et al. (2017) Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J Ethnopharmacol 211: 197-206.
- Lemos L M S, Martins TB, Tanajura GH, Gazoni VF, Bonaldo J, et al. (2012) Evaluation of antiulcer activity of chromanone fraction from Calophyllum brasiliesnse Camb. J Ethnopharmacol 141(1): 432-439.
- Sidahmed HMA, Azizan AHS, Mohan S, Abdulla MA, Abdelwahab SI, et al. (2013) Gastroprotective effect of desmosdumotin C isolated from Mitrella kentii against ethanol-induced gastric mucosal hemorrhage in rats: Possible involvement of glutathione, heat-shock protein-70, sulfhydryl compounds, nitric oxide, and anti-Helicobacter pylori activity. BMC Complement Altern Med 13: 183.
- Hassani A R, Ordouzadeh N, Ghaemi A, Amirmozafari N, Hamdi K, et al. (2009) In vitro inhibition of Helicobacter pylori urease with non and semi fermented Camellia sinensis. Indian J Med Microbiol 27(1): 30-34.
- Ye H, Liu Y, Li N, Yu J, Cheng H, et al. (2015) Anti-Helicobacter pylori activities of Chenopodium ambrosioides L. in vitro and in vivo. World J Gastroenterol 21(14): 4178-4183.
- O Gara EA, Hill DJ, Maslin DJ (2000) Activities of Garlic Oil, Garlic Powder, and Their Diallyl Constituents against Helicobacter pylori. Appl Environ Microbiol 66(5): 2269-2273.
- Nontakham J, Charoenram N, Upamai W, Taweechotipatr M, Suksamrarn S, et al. (2014) Anti-Helicobacter pylori xanthones of Garcinia fusca. Arch Pharm Res 37: 972-977.
- Jung J, Bae KH, Jeong CS (2013) Anti-Helicobacter pylori and Antiulcerogenic Activities of the Root Cortex of Paeonia suffruticosa. Biol Pharm Bull 36(10): 1535-1539.
- Garcia-Alonso G, Monroy-Noyola A, Contreras-Arellano A, Mariscal-Durand JF, Galvez-Molina Y, et al. (2016) Preclinical evaluation of anti-Helicobacter spp. activity of Hippocratea celastroides Kunth and its acute and sub-acute toxicity. BMC Complement Altern Med 16: 445.
- Tian A, Xu T, Liu K, Zou Q, Yan X, et al. (2014) Anti-Helicobacter pylori effect of total alkaloids of sophora alopecuroides in vivo. Chin Med J 127(13): 2484-2491.
- Pastene E, Speisky H, García A, Moreno J, Troncoso M, et al. (2010) In Vitro and in Vivo Effects of Apple Peel Polyphenols against Helicobacter pylori. J Agric Food Chem 58(12): 7172-7179.
- Kim JM, Zheng HM, Lee BY, Lee WK, Lee DH, et al. (2013) Anti-Helicobacter pylori Properties of Gut Gard. Prev Nutr Food Sci 18(2): 104-110.
- Lin Y, Kwon Y, Labbe R, Shetty K (2005) Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl Environ Microbiol 71: 8558-8564.
- Shikov AN, Pozharitskaya ON, Makarov VG, Kvetnaya AS (2008) Antibacterial activity of Chamomilla recutita oil extract against Helicobacter pylori. Phytother Res 22(2): 252-253.
- Twort FW (1915) An investigation on the nature of ultramicroscopic viruses. Lancet 186(4814): 1241-1243.
- D Herelle F (2007) On an invisible microbe antagonistic toward dysenteric bacilli: brief note by Mr. F. D’Herelle, presented by Mr. Roux 1917 Res Microbiol 158(7): 553-554.
- Abedon ST (2009) Kinetics of phage-mediated biocontrol of bacteria. Foodborne Pathog Dis 6(7): 807-815.
- Carlton RM (1999) Phage therapy: past history and future prospects. Arch Immunol Ther Exp (Warsz) 47(5): 267-274.
- Stratton CW (2003) Dead bugs don’t mutate: susceptibility issues in the emergence of bacterial resistance. Emerg Infect Dis 9(1): 10-16.
- Abedon ST, Thomas-Abedon C (2010) Phage therapy pharmacology. Curr Pharm Biotechnol 11(1): 28-47.
- Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, et al. (2010) Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11(1): 69-86.
- Skurnik M, Pajunen M, Kiljunen S (2007) Biotechnological challenges of phage therapy. Biotechnol Lett 29: 995-1003.
- Kuwahara H, Miyamoto Y, Akaike T, Kubota T, Sawa T, et al. (2000) Helicobacter pylori Urease Suppresses Bactericidal Activity of Peroxynitrite via Carbon Dioxide Production. Infect Immun 68(8): 4378-4383.
- Kutateladze M, Adamia R (2010) Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol 28(12): 591-595.
- Carlton RM (1999) Phage therapy: past history and future prospects. Arch Immunol Ther Exp (Warsz) 47(5): 267-274.
- Alisky J, Iczkowski K, Rapoport A, Troitsky N (1998) Bacteriophages show promise as antimicrobial agents. J Infect 36(1): 5-15.
- Górski A, Borysowski J, Miedzybrodzki R, Weber Dabrowska B (2007) Bacteriophages in medicine. In: McGrath S, van Sinderen D (Eds.)., Bacteriophage: Genetics and Microbiology. Norfolk, UK: Caister Academic Press, pp. 125-158.
- Skurnik M, Strauch E (2006) Phage therapy: facts and fiction. Int J Med Microbiol 296(1): 5-14.
- Skurnik M, Pajunen M, Kiljunen S (2007) Biotechnological challenges of phage therapy. Biotechnol Lett 29: 995-1003.
- Gupta R, Prasad Y (2011) Efficacy of polyvalent bacteriophage p-27/HP to control multidrug resistant Staphylococcus aureus associated with human infections. Curr Microbiol 62: 255-260.
- Munita J M, Arias CA (2018) Mechanisms of Antibiotic Resistance. World J Gastroenterol 24: 3204.
- Romero-Calle D, Guimarães Benevides R, Góes-Neto A, Billington C (2019) Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 8(3): 138.
- Capparelli R, Nocerino N, Lanzetta R, Silipo A, Amoresano A, et al. (2010) Bacteriophage-Resistant Staphylococcus aureus Mutant Confers Broad Immunity against Staphylococcal Infection in Mice. PLoS ONE 5: e11720.
- Principi N, Silvestri E, Esposito S (2019) Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front Pharmacol 10: 513.
- Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8: 317-327.
- Nobrega FL, Costa AR, Santos JF, Siliakus MF, Van Lent JWM, et al. (2016) Genetically manipulated phages with improved pH resistance for oral administration in veterinary medicine. Sci Re 6.
- Vinner GK, Rezaie-Yazdi Z, Leppanen M, Stapley AGF, Leaper MC, et al. (2019) Microencapsulation of Salmonella-specific bacteriophage felix o1 using spray-drying in a ph-responsive formulation and direct compression tableting of powders into a solid oral dosage form. Pharmaceuticals 12: 43.
- Papastergiou V, Georgopoulos SD, Karatapanis S (2014) Treatment of Helicobacter pylori infection: Meeting the challenge of antimicrobial resistance. World J Gastroenterol 20(29): 9898-9911.
- Goderska K, Agudo Pena S, Alarcon T (2018) Helicobacter pylori treatment: Antibiotics or probiotics. Appl Microbiol Biotechnol 102(1): 1-7.
- Abdel-Haliem M E, Askora A (2013) Isolation and characterization of bacteriophages of Helicobacter pylori isolated from Egypt. Future Virology 8(8): 821-826.
- Uchiyama J, Takeuchi H, Kato S, Takemura-Uchiyama I, Ujihara, et al. (2012) Complete genome sequences of two Helicobacter pylori bacteriophages isolated from Japanese patients. J Virol 86(20): 11400-11401.
- Abdel-Haliem M E, Askora A (2013) Isolation and characterization of bacteriophages of Helicobacter pylori isolated from Egypt. Future Virology 8(8): 821-826.
- Tagliaferri TL, Jansen M, Horz H P (2019) Fighting pathogenic bacteria on two fronts: Phages and antibiotics as combined strategy. Frontiers in cellular and infection microbiology 9: 22.
- Comeau A M, Tétart F, Trojet S N, Prère MF, Krisch HM, et al. (2007) Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2: e799.
- Torres-Barceló C, Hochberg ME (2016) Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol 24(4): 249-256.
- O Flynn G, Ross R, Fitzgerald G, Coffey A (2004) Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157: H7. Appl Environ Microb 70(6): 3417-3424.
- Golkar Z, Bagasra O, Pace DG (2014) Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J Infect Dev Ctries 8(02): 129-136.
- Atterbury RJ (2009) Bacteriophage biocontrol in animals and meat products. Microb Biotechnol 2(6): 601-612.
- Ma Y, Pacan JC, Wang Q, Xu Y, Huang X, et al. (2008) Microencapsulation of bacteriophage felix O1 into chitosan-alginate microspheres for oral delivery. Appl Environ Microb 74(15): 4799-4805.
- Johnson RP, Gyles CL, Huff WE, Ojha S, Huff GR, et al. (2008) Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim Health Res Rev 9(02): 201-215.
- Brüssow H (2005) Phage therapy: The Escherichia coli experience. Microbiology 151(7): 2133-2140.
- Smith HW, Huggins MB, Shaw KM (1987) Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J Gen Microbiol 133(5): 1127-1135.
- Gholizadeh P, Köse Ş, Dao S, Ganbarov K, Tanomand A, et al. (2020) How CRISPR-Cas system could be used to combat antimicrobial resistance. Infect Drug Resist 13: 1111-1121.
- Strich J R, Chertow DS (2018) CRISPR-cas biology and its application to infectious diseases. J Clin Microbiol 57(4): e01307-01318.
- Gleditzsch D, Pausch P, Müller-Esparza H, Özcan A, Guo X, et al. (2019) PAM identification by CRISPR-Cas effector complexes: Diversified mechanisms and structures. RNA Biol 16(4): 504-517.
- Ishino Y, Krupovic M, Forterre P (2018) History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. J Bacteriol 200.
- Gajdács M (2019) The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules 24(5): 892.
- Pacios O, Blasco L, Bleriot I, Fernandez-Garcia L, Bardanca MG, et al. (2020) Strategies to combat multidrug-resistant and persistent infectious diseases. Antibiotics 9(2): 65.
- Shahbazi Dastjerdeh M, Kouhpayeh S, Sabzehei F, Khanahmad H, Salehi M, et al. (2016) Zinc finger nuclease: A new approach to overcome beta-lactam antibiotic resistance. Jundishapur J Microbiol 9(1): 1-11.
- Hosseini N, Khanahmad H, Esfahani B, Bandehpour M, Shariati L, et al. (2020) Targeting of cholera toxin A (ctxA) gene by zinc finger nuclease: Pitfalls of using gene editing tools in prokaryotes. Res Pharm Sci 15(2): 182-190.
- Gaj T, Gersbach CA, Barbas CF, ZFN, TALEN, et al. (2013) CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31(7): 397-405.
- Goltermann L, Nielsen PE (2020) PNA Antisense Targeting in Bacteria: Determination of Antibacterial Activity (MIC) of PNA-Peptide Conjugates. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, pp: 231-239.
- Edson JA, Kwon YJ (2014) RNAi for silencing drug resistance in microbes toward development of nanoantibiotics. J Control Release 189: 150-157.
- Sünderhauf D, Pursey E, Klümper U, Westra E, Gaze W, et al. (2019) AMR gene removal by conjugative delivery of CRISPR-Cas9. Access Microbiol 1: 213.
- Gomaa AA, Klumpe HE, Luo ML, Selle K, Barrangou R, et al. (2014) Programmable removal of bacterial strains by use of genome- targeting CRISPR-cas systems. mBio 5(1).
- Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, et al. (2014) Exploiting CRISPR-cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol 32: 1146-1150.
- Wang Y, Wang S, Chen W, Song L, Zhang Y, et al. (2018) CRISPRCas9 and CRISPR-assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl Environ Microbiol 84(23).
- Arslan N, Yılmaz O, Demiray-Gürbüz E (2017) Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol 23(16): 2854-2869.
- Backert S, Neddermann M, Maubach G, Naumann M (2016) Pathogenesis of Helicobacter pylori infection. Helicobacter 21(Suppl 1): 19-25.
- Bagchi D, Bhattacharya G, Stohs SJ (1996) Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res 24(6): 439-450.
- Cruchet S, Obregon MC, Salazar G, Diaz E, Gotteland M, et al. (2003) Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 19(9): 716-721.
- Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, et al. (2011) Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog 7(12): e10024.
- Dang Y, Reinhardt JD, Zhou X, Zhang G (2014) The effect of probiotic supplementation on Helicobacter pylori eradication therapy: a metaanalysis. PLoS One 9(11): 1-15.
- Kao CY, Sheu BS, Wu JJ (2016) Helicobacter pylori infection: an overview of bacterial virulence factors and pathogenesis. Biom J 39(1): 14-23.
- Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, et al. (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298(6): G851-G859.
- Magistà AM, Ierardi E, Castellaneta S, Miniello VL, Lionetti E, et al. (2005) Helicobacter pylori status and symptom assessment two years after eradication in pediatric patients from a high prevalence area. J Pediatrics Gastroenterol Nutr 40(3): 312-318.
- P D Cotter, C Hill, R P Ross (2005) “Bacteriocins: developing innate immunity for food.” Nature Reviews Microbiology 3(10): 777-788.
- J M Rodr´ıguez, M I Mart´ınez, J Kok (2002) “Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria.” Critical Reviews in Food Science and Nutrition 42(2): 91-121.
- MDR Lopez-Cuellar, A I R Hern´andez, N Chavarr´ıaHernandez (2016) “LAB bacteriocin applications in the last decade,” ´ Biotechnology & Biotechnological Equipment 30(6): 1-12.
- K E Sutyak, R A Anderson, S E Dover (2008) “Spermicidal activity of the safe natural antimicrobial peptide subtilosin”. Infectious Diseases in Obstetrics and Gynecology 540758.
- M B Najjar, D Kashtanov, M L Chikindas (2009) “Natural antimicrobials ε-poly-L-lysine and nisin A for control of oral microflora”. Probiotics and Antimicrobial Proteins 1(2): 143-147.
- L Masdea, E M Kulik, I Hauser-Gerspach, A M Ramseier, A Filippi, et al. (2012) “Antimicrobial activity of Streptococcus salivarius K12 on bacteria involved in oral malodour.” Archives of Oral Biology 57(8): 1041-1047.
- E M Balciunas, F A C Martinez, S D Todorov, B D G D M Franco, A Converti, et al. (2013) “Novel biotechnological applications of bacteriocins: a review.” Food Control 32(1): 134-142.
- Ajnikanth PS, Mishra B (2007) Preparation and in vitro characterization of Gellan based floating beads of acetohydroxamic acid for eradication of H. pylori. Acta Pharm 57(4): 413-427.
- El Nujumi AM, Dorrian CA, Chittajallu RS, Neithercut WD, McColl KE, et al. (1991) Effect of inhibition of Helicobacter pylori urease activity of acetohydroxamic acid on serum gastrin in duodenal ulcer subjects. Gut 32(8): 866-870.
- Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, et al. (2009) Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci U S A 106(46): 19268-19273.
- Zhang X, Zhang H, Wu Z, Wang Z, Niu H, et al. (2008) Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur J Pharm Biopharm 68(3): 526-534.
- Van der Lubben IM, Verhoef JC, Borchard G, Junginger HE (2001) Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur J Pharm Sci 14(3): 201-207.
- Fernandez-Urrusuno R, Romani D, Calvo P, Vila-Jato JR, Alonso MJ, et al. (1999) Development of a freeze-dried formulation of insulin-loaded chitosan nanoparticles intended for nasal administration. STP Pharma Sci 9: 429-436.
- Van der Lubben IM, Verhoef JC, van Aelst A, Borchard G, Junginger HE, et al. (2001) Chitosan microparticles for oral vaccination: Preparation, Characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials 22(7): 687-694.
- Arora S, Gupta S, Narang RK, Budhiraja RD (2011) Amoxicillin loaded chitosan– alginate polyelectrolyte complex nanoparticles as muco-penetrating delivery system for H. pylori. Sci Pharm 79(3): 673-694.
- Cone RA (1999) Mucus. In: Mucosal Immunology (2nd)., In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR (Eds.)., San Diego: Academic Press, pp. 43-64.
- Chadha R, Kashid N, Jain DV (2003) Kinetic studies of the degradation of an amino penicillin antibiotic (amoxicillin trihydrate) in aqueous solution using heat conduction microcalorimetry. J Pharm Pharmacol 55(11): 1495-1503.
- Liu Z, Lu W, Qian L, Zhang X, Zeng P, et al. (2007) In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin. J ContRel 102(1): 135-144.
- PatelJK, PatelMM (2007) Stomach specific anti-Helicobacter pylori therapy: Preparation and evaluation of amoxicillin-loaded chitosan mucoadhesive microspheres. Curr Drug Del 4(1): 41-50.
- Moon J, Y G Shula, H S Han, S Y Hong, Y S Choi, et al. (2005) A study on UV-curable adhesives for optical pick-up: I. Photoinitiator effects. International journal of adhesion and adhesives 25(4): 301-312.
- Peer D, Jeffrey M Karp, Seungpyo Hong, Omid C Farokhzad, Rimona Margalit, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology 2(12): 751.
- Hu CMJ (2011) Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proceedings of the National Academy of Sciences 108(27): 10980-10985.
- Zhang J, Weiwei Gao, Ronnie H Fang, Anjie Dong, Liangfang Zhang, et al. (2015) Synthesis of Nanogels via Cell Membrane¬Templated Polymerization. Small 11(34): 4309-4313.
- Luk BT, Ronnie H Fang, Che-Ming J Hu, Jonathan A Copp, Soracha Thamphiwatana, et al. (2016) Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics 6(7): 1004. 105.
- Makowski M, Silva Í C, Pais do Amaral C, Gonçalves S, Santos N C, et al. (2019) Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 11(11): 588.
- Medina E, Rohde M, Chhatwal G S (2003) Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect Immun 71(9): 5376-5380.
- Mikut R, Ruden S, Reischl M, Breitling F, Volkmer R, et al. (2016) Improving short antimicrobial peptides despite elusive rules for activity. Biochim. Biophys. Acta 1858(5): 1024-1033.
- Miller K P, Wang L, Benicewicz B C, Decho A W (2015) Inorganic nanoparticles engineered to attack bacteria. Chem Soc Rev 44: 7787-7807.
- Mir M, Ahmed N, Permana A D, Rodgers A M, Donnelly R F, et al. (2019) Enhancement in site-specific delivery of carvacrol against methicillin resistant Staphylococcus aureus induced skin infections using enzyme responsive nanoparticles: a proof-of-concept study. Pharmaceutics 11(11): 606.
- Mohammed Y H E, Manukumar H M, Rakesh K P, Karthik C S, Mallu P, et al. (2018) Vision for medicine: Staphylococcus aureus biofilm war and unlocking keys for anti-biofilm drug development. Microb Pathog 123: 339-347.
- Nakamura I, Fukushima S, Hayakawa T, Sekiya K, Matsumoto T, et al. (2015) The additional costs of catheter-related bloodstream infections in intensive care units. Am J Infect Control 43(10): 1046-1049.
- Nguyen T K, Selvanayagam R, Ho K K K, Chen R, Kutty S K, et al. (2016) Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem Sci 7(2): 1016-1027.
- Nostro A, Papalia T (2012) Antimicrobial activity of carvacrol: current progress and future prospectives. Recent Pat Antiinfect Drug Discov 7(1): 28-35.
- A M Fayaz, K Balaji, M Girilal, R Yadav, PT Kalaichelvan, et al. (2010) Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed Nanotechnol 6(1): 103-109.
- M Singh, S Singh, S Prasad, I Gambhir (2008) Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig J Nanomater Bio 3: 115-122.
- K Kalishwaralal, V Deepak, S Ramkumarpandian, H Nellaiah, G Sangiliyandi, et al. (2008) Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett 62(29): 4411-4413.
- İ H Boyacı (2005) A new approach for determination of enzyme kinetic constants using response surface methodology. Biochem. Eng J 25(1): 55-62.
- Y H Xiong, J Z Liu, H Y Song, L N Ji (2004) Enhanced production of extracellular ribonuclease from Aspergillus niger by optimization of culture conditions using response surface methodology. Biochem Eng J 21(1): 27-32.
- H S Shin, HC Choi, Y Jung, SB Kim, HJ Song, et al. (2004) Chemical and size effects of nanocomposites of silver and polyvinyl pyrrolidone determined by X-ray photoemission spectroscopy. Chem Phy Lett 383(3-4): 418-422.
- Yeh Y C, Huang T H, Yang S C, Chen C C, Fang J Y, et al. (2020) Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Frontiers in chemistry 8: 286.
- Yin I X, Zhang J, Zhao I S, Mei M L, Li Q, et al. (2020) The antibacterial mechanism of silver nanoparticles and its application in dentistry. International journal of nanomedicine 15: 2555.
- Mohd Yusof H, Mohamad R, Zaidan U H, Rahman A (2019) Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. Journal of animal science and biotechnology 10(1): 1-22.
- Patil MP, Kim G D (2017) Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Applied microbiology and biotechnology 101(1): 79-92.
- S Liau, D Read, W Pugh, J Furr, A Russell, et al. (1997) Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterialaction of silver ions. Lett Appl Microbiol 25(4): 279-283.
- K Nomiya, A Yoshizawa, K Tsukagoshi, N C Kasuga, S Hirakawa, et al. (2004) Synthesis and structural characterization of silver (I), aluminium (III) and cobalt (II) complexes with 4-isopropyltropolone (hinokitiol) showing noteworthy biological activities. Action of silver (I)-oxygen bonding complexes on the antimicrobial activities. J Inorg Biochem 98(1): 46-60.
- A Gupta (1998) Silver as a biocide: will resistance become a problem? Nature Biotechnol 16(10): 888-898.
- Gad El-Rab S M F, Halawani E H, Hassan A M (2018) Formulation of Ceftriaxone Conjugated Gold Nanoparticles and their Medical Applications against Extended-Spectrum β-LactamaseProducing Bacteria and Breast Cancer J. Microbiol Biotechnol 28(9): 1563-1572.
- Halawani E H, Hassan A M, Gad El-Rab S M F (2020) Nanoformulation of biogenic Cefotaxime-Conjugated-Silver nanoparticles for enhanced antibacterial efficacy against multidrug-resistant bacteria and anticancer studies. International Journal of Nanomedicine 15: 1889-1901.
- Jaiswal S, Mishra P (2018) Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells Med. Microbiol Immunol 207(1): 39-53.
- Quinteros M A (2018) Bio-synthesized silver nanoparticles: decoding their mechanism of action in Staphylococcus aureus and Escherichia coli Int. J Biochem Cell Biol 104: 87-93.
- Ghosh S (2012) Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents Int. J Nanomed 7: 483-496.
- Li P, Li J, Wu C, Wu Q, Li J, et al. (2005) Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 16: 1912-1917.
- Paiva L, T K S Fidalgo, L P da Costa, L C Maia, L Balan, et al. (2018) Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J Dent 69: 102-119.
- Azarsina M, Kasraei S, Yousef-Mashouf R, Dehghani N, Shirinzad M, et al. (2013) The antibacterial properties of composite resin containing nanosilver against Streptococcus mutans and Lactobacillus J Contemp. Dent Pract 14: 1014-1018.
- Bapat RA, Tanay V Chaubal, Chaitanya P Joshi, Prachi R Bapat, Hira Choudhury, et al. (2018) An overview of application of silver nanoparticles for biomaterials in dentistry Mater. Sci Eng C 91: 881-898.
- Abou Baker D (2020) Plants against Helicobacter pylori to combat resistance: An ethnopharmacological review. Biotechnology Reports 26: e00470.
- Elbreki M, Ross R P, Hill C, O Mahony J, McAuliffe O, et al. (2014) Bacteriophages and their derivatives as biotherapeutic agents in disease prevention and treatment. Journal of Viruses.
- Manohar P, Madurantakam Royam M, Loh B, Bozdogan B, Nachimuthu R, et al. (2022) Synergistic Effects of Phage–Antibiotic Combinations against Citrobacter amalonaticus. ACS infectious diseases 8(1): 59-65.
- González de Aledo M, González-Bardanca M, Blasco L, Pacios O, Bleriot I, et al. (2021) CRISPR-Cas, a Revolution in the Treatment and Study of ESKAPE Infections: Pre-Clinical Studies. Antibiotics 10(7): 756.
- Cotter P D, Ross R P, Hill C (2013) Bacteriocins—a viable alternative to antibiotics? Nature Reviews Microbiology 11(2): 95-105.
- Gebreslassie Y T, Gebretnsae H G (2021) Green and cost-effective synthesis of tin oxide nanoparticles: a review on the synthesis methodologies, mechanism of formation, and their potential applications. Nanoscale research letters 16(1): 1-16.

 Review Article
Review Article