ABSTRACT
Brugada syndrome is a rare non-structural cardiac pathology associated with syncope and sudden death, which affects the ion channels (Na+) of cardiomyocytes. It has a high prevalence in males, however there are still very few epidemiological studies that certify it. Currently, various studies have documented the relationship to multiple causal mutations (SCN5A gene); Despite all the advances, there are still unresolved questions that keep the investigation on this matter active. Anesthetic management is a matter of great care in brigade syndrome, its risk is directly associated with the degree of systemic absorption of the same and therefore with the plasma concentration reached. The action of local anesthetics lies in an increase in the changes of the electrocardiogram in this condition because they are sodium channel blockers, acting at the level of phase 0 of the action potential.
Keywords: Myocardium; Brugada Syndrome; Anesthesia; Action Potential; Management
Introduction
Brugada syndrome is a disease first described in 1992; with the passage of time it has become an entity of great interest due to the little knowledge obtained in the medical area [1]. It is characterized by being a non-structural cardiac pathology secondary to an alteration of the function of the myocardial ion channels, which predisposes patients to suffer potentially fatal ventricular arrhythmias, has an incidence worldwide between 5-66 cases per 10,000 people: presenting with greater frequency in the male sex [2], with an average age of diagnosis of arrhythmias around 40 years [3]. The anesthetic management in these patients is always worrying due to the adverse electrical events that support this syndrome [4], its action is aimed at avoiding the subsequent dysfunction of the cardiac sodium channel. During treatment, various conditions can lead to an arrhythmia, even in the presence of a healthy heart, which are usually benign. Patients suffering from structural cardiac alterations have an increased risk of presentation and the severity of arrhythmias is transformed into a serious setback during the perioperative period [5]. Although many of the clinical-electrocardiographic and genetic characteristics of the condition have already been defined, difficulties still persist in its diagnosis and its respective management, so the treating medical team must base itself on the above to obtain a better approach to the patient [6].
Methodology
The study design is adapted to a systematic review of the evidence present in the scientific literature on anesthetic management in brugada syndrome. The literature search took place between 2001-2022 delving into various bibliographic databases in order to obtain information and review previous studies on the subject exposed. The keywords and Boolean operators used were “myocardium”, “brugada syndrome”, “anaesthesia”, “action potential”, “management” described through DeCS (Descriptors in Health Sciences). To achieve a greater update on the subject, the articles published in the last 22 years were set as a temporary filter for the search.
Results
Brugada syndrome is defined as a primary electrical pathology or non-structural cardiac canalopathy, with an autosomal dominant genetic basis, causing approximately 4-12% of all sudden deaths in individuals who are generally asymptomatic; between 20-50% of these deaths occur in patients who do not have a demonstrable structural cardiac abnormality and because of an arrhythmia [7,8]. The heart is a muscular organ located in the chest cavity consisting of two pumps, with the ability to provide blood to all tissues of the body [9]. Electrophysiologically it consists of presenting an action potential that originates in the sinus node and spreads throughout the cardiac conduction system by means of cleft junctions. This potential corresponds to a rapid depolarization of the membrane, followed by repolarization to the membrane potential which can be recorded by an intracellular electrode. The normal resting potential varies between -85 to -95 mV. Within it 5 phases (0-4) are included, in each one the ionic input is involved (K+, Na+, Ca+) [10]. In a fast fiber, the rapid ascending phase of the action potential is called phase 0, it is due to the entry of Na+ ions into the intracellular space through voltage-gated channels, occurring at the same time with the opening of slow Ca+2 channels, causing the cardiomyocyte to depolarize and its membrane potential to rise to reach the tip (20 mvol), precise moment in which the closure of all Na+ channels happens. Followed by this occurs a brief period of early partial repolarization in which the membrane potential returns to 0 mvol, with the inactivation of Na+ channels, the output of K+ ions and entry of Cl- happens, giving rise to phase 1. Subsequently, phase 2 or better known as the plateau phase originates, which lasts approximately 0.2 seconds in the atrial muscle and 0.3 in the ventricular muscle, consisting of the entry of Ca+2 and Cl. In phase 3, the membrane is repolarized which brings the cell back to its negative potential (-85 mvol) due to the closure of the slow channels of Na+ and Ca+2 with an ascent at the output of K+.
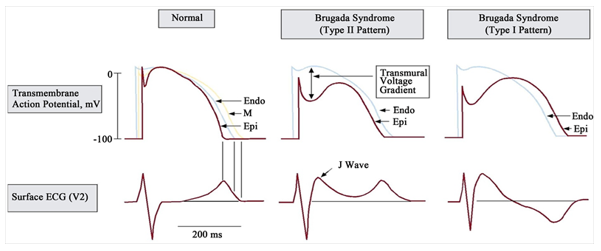
Finally the membrane recovers again the resting state of polarization or better known as phase 4, allowing a chemical balance of intra- and extracellular ions, where the Na-K-ATPase pump is responsible for pumping sodium ions to the outside of the cell through the membrane, while introducing potassium from the outside to the inside, preserving the negative electrical potential inside the cells; likewise, the Na+/Ca+2 exchanger allows the output of a Ca+2 ion by the input of 3 Na+ ions [11-13]. In the pathogenesis of brugada syndrome, the action potential shows various abnormalities and to this day the most defended theory is based on a deterioration of repolarization, which corresponds to an imbalance of positive charges by alteration of the ion flows of phase 1 or by a decrease in the entry of Ca +, elevation in the output of K+, or by decrease in the input of Na+, which leads to a loss of plateau, shortening it by 40-70% in the epicardium [14,15]. This is ultimately due to a transmural dispersion of repolarization and refractoriness, responsible for the typical electrocardiographic pattern (type I) [16].
There is a second hypothesis that is being considered, called depolarization theory secondary to the delay of conduction in the anterior epicardial region of the outflow tract of the right ventricle, which is explained by the existence of certain areas with abnormal potentials of low voltage and prolonged duration, in a situation that does not happen at the level of the anterior endocardium of the same [17]. Regarding its genetic bases, the identification of mutations that cause it has been carried out. Initially the relationship with the SCN5A gene was concluded, specifically at the locus of chromosome 3p21-2 [18], this encodes the α subunit of the sodium channel of the myocardiocyte, generating a loss of function of the sodium channel, with a result ranging from expression failures, alterations of the activation voltage to acceleration of the inactivation of the same, reducing the incoming current of the ion, which causes the clinical manifestations and the ECG characteristic of brugada syndrome. However, mutations in other genes such as SCN10A, the gene like glycerol-3-phosphate dehydrogenase-1 (GPD1L), SCN1B42, SCN3B43, RANGRF, have also isolated mutations in the genes CACNA1C, CACNB2b, CACNA2D1, KCNE347 and KCNE5, KCNJ8 [19,20] (Figure 1). A. Normal action potential of the heart. B. Action potential associated with the type I pattern. C. Action potential associated with the type II pattern. Taken from: Rodríguez- Constaín JS, López-Garzón NA, Navia-Amézquita CA, Mora-Obando DL, Dueñas-Cuellar RA. Brugada syndrome. Pathophysiological and clinical aspects and their association with infectious diseases.
Regarding the definition of the characteristic
electrocardiographic pattern and the diagnostic criteria of brugada syndrome, 3 patterns were initially highlighted:
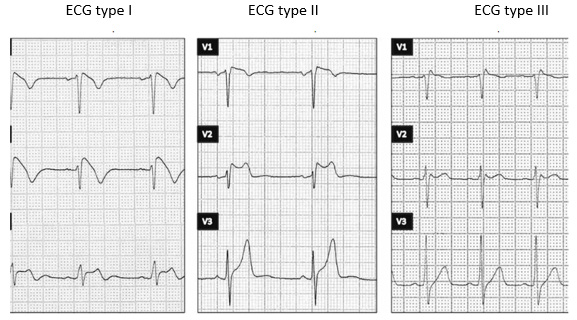
a) ECG pattern type I considered as a diagnosis of the disease, consists of a descending elevation of the ST segment ≥ 2 mm in more than one right precordial shunt (V1-V3), followed by negative T waves.
b) ECG type II pattern, with ST segment elevation ≥ 2 mm in right precordials followed by positive or isobaphasic T waves, giving the ECG a saddle-like appearance.
c) ECG pattern type III, declared as either of the two mentioned above if the ST segment elevation is ≤ 1 mm [21] (Figure 2).
Only type I is diagnostic of the syndrome. Taken from: Begoña Benitoa, Josep Brugadab, Ramón Brugadac and Pedro Brugada. Brugada syndrome.
Discussion
The introduction of anesthesia in patients with Brugada syndrome, whether general or regional, has been a challenge for the anesthesiologist. Among its considerations include reducing the triggers of ventricular arrhythmias such as adrenergic stimulation (response to surgical stress), changes in autonomic modulation, increased vagal tone, hyperkalemia, hypokalemia, combinations of glucose and insulin, hypercalcemia, hemodynamic alterations by anesthetic drugs and thermal variations, which is why the exact indication is recommended in conjunction with surveillance and monitoring. of heart rate, ECG tracing, anesthetic depth, blood pressure, neuromuscular blockade and temperature in order to avoid probables causing arrhythmogenic events. It should be noted that prior to the initiation of any procedure it is necessary to have an external defibrillator inside the operating room because it is the only effective treatment of ventricular arrhythmias in brugada syndrome [22,23]. Among the drugs commonly used in anesthesia benzodiazepines, ketorolac as an analgesic, fentanyl and meperidine have not been associated with adverse events [24].
Local Anesthetics
Neuraxial anesthesia has been successfully executed with local anesthetics (lidocaine, mepivacaine, bupivacaine, ropivacaine), it is commented that they can increase ECG changes in this pathology, because they are blockers of Na+ channels, working at the level of phase 0 of the action potential. The risk of local anesthetics in patients with brugada syndrome is associated with the degree of systemic absorption of these and therefore with the plasma concentration achieved [25,26]. Bupivacaine generates longer changes in depolarization than lidocaine. The R(+) isomer of bupivacaine avidly blocks sodium channels and dissociates very slowly. This transforms it into the regional anesthetic with the greatest proarrhythmic and cardiotoxic potential. There are reported cases of characteristic brugada pattern driven by the application of epidural bupivacaine. In case of placing neuraxial anesthesia, the first option should be a short-acting anesthetic such as lidocaine. If prolonged, the total dose should be reduced to the minimum possible in patients with this disease [26].
Volatile Anesthetics
The use of inhaled anesthetics is a safer methodthan intravenouss. The application of general anesthesia with nitric oxide, desfluorane, isofluorane and sevofluorane has been carried out without apparent complications. It is evident that isofluorane prolongs the QT interval on the contrary halothane shortens it. Sevofluorane does not compromise the length of the same, so it continues to be the drug of choice in this type of anesthetics [26].
Intravenous Anesthetics
Regarding this class of anesthetics, the administration of propofol in patients with brugada syndrome remains a matter of controversy; recently it has been postulated contraindicated in relation to its possible interactions with sodium and calcium channels. There are studies in which electrocardiographic patterns of the disease related to the use of propofol in prolonged infusion called propofol infusion syndrome and its chronic abuse demonstrate; just as there are several cases in which propofol has been used without incidence. Propofol affects the function of ion channels, so clinical experience does not discourage the use in bolus for anesthetic induction in patients; all done with extreme caution. It should be noted that when using neuraxial anesthesia, its combination with sedation with propofol can potentiate the syndrome and increase the risk of arrhythmogenic events in these patients. Thiopental and etomidate have been used as inducers without incident, except for some self-limiting cases of ST elevation with etomidate [25-27].
Opiates
The vast majority have not shown complications, but their brady-browning effect should be considered to avoid the appearance of complications. Those of short or ultra-short action are postulated for better use due to their rapid elimination. In the postoperative period, morphine is preferable, however several studies document that tramadol, fentanyl and sufentanil block Na+ channels with greater potency than morphine.
Neuromuscular Blockers
Succinylcholine has not exhibited adverse effects in varieties of clinical cases. Some studies have shown patients with ST elevation after administration of the relaxant [28]. Others such as rocuronium, vecuronium, atracurium, cisatracurium and mivacurium have all been used without developing adverse effects. Regarding neostigmine, the parasympathetic stimulation it causes can cause changes in TS in Brugada syndrome and its use generates controversy [29].
Conclusion
Brugada syndrome is an arrhythmogenic heart disease defined by resting ECG alterations and malignant tachyarrhythmias, so its ECG tracing alone should alarm about the possible appearance of lethal ventricular arrhythmias. It is relevant for the anesthesiologist to provide adequate anesthesia, whether regional or general, in sick patients, whose choice is directed by the properties on the autonomic nervous system. It is highly required and necessary the continuous surveillance and monitoring of heart rate, electrocardiographic imaging during the immediate pre and postoperative period (at least 24 hours after the intervention), blood pressure and anesthetic depth, temperature and neuromuscular blockade, as well as ensuring the presence of an automatic defibrillator in surgery, in order to avoid adverse reactions.
References
- Pérez Riera AR, Filho CF, Uchida AH, Zhang L, Antzelevitch C, et al. (2008) Study of the extent of the information of cardiologists from São Paulo city, Brazil, regarding a low-prevalence entity: Brugada syndrome. Ann Noninvasive Electrocardiol 13(4): 352-363.
- Littmann L, Monroe MH (2003) The Brugada numbers. Circulation 107(18): e122.
- Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, et al. (2002) Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation 106(19): 2514-2519.
- Fujiwara Y, Shibata Y, Kurokawa S, Satou Y, Komatsu T (2006) Ventricular tachycardia in a patient with Brugada syndrome during general anesthesia combined with thoracic paravertebral block. Anesthesia and Analgesia 102(5): 1590-1591.
- Francisco Alejandro López-Jiménez, Mabel Estíbaliz Mondragón-Villanueva (2008) Brugada syndrome and anesthesia. Mexican Journal of Anesthesiology 31(1).
- Carlos J Jaramillo, Luis F Perdomo, Esteban Cardona, Gabriel A Londoño (2010) Brugada syndrome in a patient with syncope. Presentation of a case and review of the literature. Rev Colomb Cardiol 17: 177-186.
- Benito B, Brugada J, Brugada R, Brugada P (2009) Brugada syndrome. Rev Esp Cardiol 62(11): 1297-1315.
- Santos LF, Correia E, Rodrigues B, Nunes L, Costa A, et al. (2010) Spontaneous fluctuations between diagnostic and nondiagnostic ECGs in Brugada syndrome screening: Portuguese family with Brugada syndrome. Ann Noninvasive Electrocardiol 15(4): 337-343.
- (2009) Ramirez-Ramirez Fco Jaffet. Cardiac physiology. Medical Journal 3(1).
- Babuty D, Argibay J, Hatem S (2008) Cardiac electrophysiology. Cardiology 3(3): 1-18.
- Arthur Guyton, Hall JE (2011) Treatise on Medical Physiology (12th)., Spain: Elsevier.
- Angarita BEV, Peña CLS, Neira MC (2016) Principles of electrophysiology, arrhythmias. Colombian College of Cardiovascular Electrophysiology 2: 790-805.
- Rodríguez-Constaín JS, López-Garzón NA, Navia-Amézquita CA, Mora-Obando DL, Dueñas-Cuellar RA (2019) Brugada syndrome. Pathophysiological and clinical aspects and their association with infectious diseases. Iatreia 32(3): 217-231.
- Asensio E, Álvarez B, Lozano E, Farías A, Brugada R, et al. (2000) ST elevation, Right bundle branch block and sudden death: Brugada syndrome. Arch Inst Cardiol Méx 70(3): 301-311.
- Campuzano Ó, Sarquella-Brugada G, Brugada R, Brugada P, Brugada J (2009) Genetic bases of malignant arrhythmias and cardiomyopathies. Rev Esp Cardiol 62(4): 422-436.
- Pérez-Riera A, Fortunato de Cano S, Fleury de Padua N, Schapachnik E (2001) Brugada syndrome: new concepts and future expectations. Rev Argent Cardiol 69: 652-662.
- Meregalli PG, Wilde AAM, Tan HL (2005) Pathophysiologic mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder or more. Cardiovasc Res 67(3): 367-378.
- García-Castro M, García C, Reguero JR, Miar A, Rubín JM, et al. (2010) Mutational spectrum of the SCN5A gene in Spanish patients with Brugada syndrome patients. Rev Esp Cardiol 63(7): 856-859.
- K Mizumaki, A Fujiki, T Tsuneda, M Sakabe, Nishida K, et al. (2004) Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol 15(6): 667-673.
- C Antzelevitch, P Brugada, M Borggrefe, Josep B, Ramon B, et al. (2005) Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 111(5): 659-670.
- Begoña Benitoa, Josep Brugadab, Ramón Brugadac, Pedro Brugada (2009) Brugada syndrome. Rev Esp Cardiol 62(11): 1297-1315.
- Kim JS, Park SY, Min SK, Kim JH, Lee SY, et al. (2004) Anaesthesia in patients with Brugada syndrome. Acta Anaesthesiol Scand 48(8): 1058-1061.
- Santambrogio LG, Mencherini S, Fuardo M, Caramella F, Braschi A (2005) The surgical patient with Brugada syndrome: a four-case clinical experience. Anesth Analg 100(5): 1263-1266.
- Pablo Arco de la Torre, María del Pilar Sáenz Pascual, Marta Aguado Sevilla (2021) Anesthetic management in a patient with Brugada syndrome, about a case. Chilean journal of anesthesia. Clinical Case 6(50).
- Fernández Suárez FE, Argüelles Tamargo L, Varela Rodríguez L, Quintela Baizán AF (2008) Brugada syndrome and local anesthetics. Rev Esp Anestesiol Reanim 55(8): 518.
- Espinosa A, Abad A, Rodríguez Bustamante V, Ripollés Melchor J, Marmanya Mezquita S, et al. (2016) Brugada Syndrome and Anesthesia: Generalities. Rev Electron Anesthesia.
- Rossenbacker T, Priori SG (2007) The Brugada syndrome. Current Opinion in Cardiology 22(3): 163-170.
- Kloesel B, Ackerman MJ, Sprung J, Narr BJ, Weingarten TN, et al. (2011) Anesthetic management of patients with Brugada syndrome: a case series and literature review. Can J Anaesth 58(9): 824-836.
- Conde R, Pereira M (2013) Anesthetic approach in patients with Brugada syndrome-Use of sugammadex in major abdominal surgery. Rev Bras Anestesiol 63(1): 159-160.

 Review Article
Review Article