ABSTRACT
Heparan sulphate (HS) proteoglycans are one of the most ubiquitous glycoproteins on the surface of most cells in the body. They play an important role in cell-cell communication and, as such, they mediate many biological activities including angiogenesis and cell homeostasis. They are highly sulphated and negatively charged and also play a role in blood coagulation. Due to their widely expressed nature, they are targeted by many viruses and bacteria in their mechanism of pathogenesis. This includes many sexually transmitted pathogens such as Chlamydia, Papillomavirus, Herpes Simplex Virus and other pathogens, that utilise HS binding in their adhesion to the cell surface, followed by cell entry, to enhance their survival and disease development [1-3]. The list of pathogens that utilise HS moieties in their mechanism of pathogenesis also includes Hepatitis C [4], Rabies [5] and now includes the Severe Acute Respiratory Syndrome (SARS) Corona Virus Disease 19 (COVID19) [6,7]. The role of HS binding in angiogenesis and metastasis are well documented [8,9]. The importance of the role played by HS receptors suggests that development of therapeutic products, that can work through this binding capability, can lead to effective products; not only to inhibit potential pathogen infection, as in the case of COVID19 [10] but also in the treatment of other conditions such as angiogenesis. In this article I would like to draw your attention to the discovery of a small, non-proteinaceous molecule originally isolated from the urine of children with Steroid Responsive Nephrotic Syndrome [11,12]. I have named this compound Nephronin to reflect its origin. This compound appears to be produced by the activated immune cells, most likely the T-cells. Initial studies leading to the isolation of this compound were focused on isolation of compound(s) with biological activities, that would cause this Nephrosis and with a role in immune regulation. These studies have demonstrated two major biological activities for Nephronin.
Abbreviations: HS: Heparan Sulphate; SARS: Severe Acute Respiratory Syndrome; TNF: Tumour Necrosis Factor; AD: Atopic Dermatitis; HSV: Herpes simplex virus; DRG: Dorsal Root Ganglia; RBD: Receptor-Binding Domain; HS: Heparan Sulphate; ACE: Angiotensin-Converting Enzyme; APTT: Anti Partial Thromboplastin Time
Nephronin Specifically Binds to Heparin / Heparan Sulphate
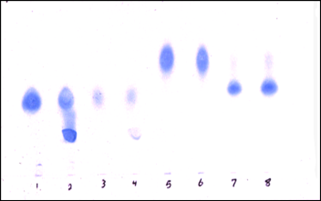
Nephronin was found to be highly negatively charged based on its tight binding to anion exchange columns (Q-column) at neutral pH. Cellulose acetate electrophoresis was used to demonstrate specific binding of Nephronin to Heparin and Heparan sulphate (Figure 1).
Figure 1: A one dimensional cellulose acetate electrophoresis, that is often used in the characterisation of glycosaminoglycans (GAGs) was used for the detection of Nephronin binding and inhibition of migration of each specific product [12].
1. Heparin.
2. Heparin + Nephronin.
3. Heparan sulphate.
4. Heparan sulphate + Nephronin.
5. Chondroitin sulphate.
6. Chondroitin Sulphate + Nephronin.
7. Dermatan Sulphate.
8. Dermatan Sulphate + Nephronin.
The Major Biological Activities Associated with this Compound
a) Nephronin is a potent inhibitor of endotoxin and endotoxin induced cytokine production. Endotoxin is the major factor in the onset of sepsis, septic shock and related diseases such as Meningitis. Currently there is no effective treatment for inhibition of stimulation and production of these cytokines. The anti-inflammatory activity of Nephronin is of paramount importance in inflammatory disease conditions.
b) They bind to receptors of heparan sulphate and in turn impede development of diseases, where heparan sulphate plays a major role in the pathogenesis of disease development. Many viruses and bacteria use heparan sulphate as anchorage in their mechanism of infection, as well as metastasising tumourogenic cells utilising heparanase. I have been able to demonstrate inhibition of Herpes virus infection of human keratinocytes by separate preparation of Nephronin.
Anti-Inflammatory Activity of Nephronin
To date, huge amounts of money have been spent in developing new therapeutic agents, including antibodies against endotoxin and some immune mediators such as tumour necrosis factor (TNF) and various interleukins, their receptors and synthetically produced antagonists of endotoxin for the treatment of various diseases, in particular autoimmune conditions such as Psoriasis, Eczema, Rheumatoid Arthritis etc. as well as infectious diseases leading to sepsis and septic shock. Unfortunately each of these agents has their own particular side-effects and they become less effective with prolonged use. In the case of septic shock, which is caused by the cytokine cascade (cytokine storm) that can often be fatal, demonstrates the pressing need to develop effective, bioavailable therapeutics. A review article entitled “Treatment of Sepsis” by Baumgartner & Calandra, [13] confirms this conclusion. Endotoxin is a structural component of Gram-negative bacterial cell wall and it is also the major contributing factor in sepsis. We have demonstrated in laboratory tests, that Nephronin inhibits endotoxin induced cytokine production. We are currently exploring options in developing suitable products as anti-inflammatory for various conditions.
Treatment of Autoimmune Conditions, Psoriasis and Eczema
Psoriasis
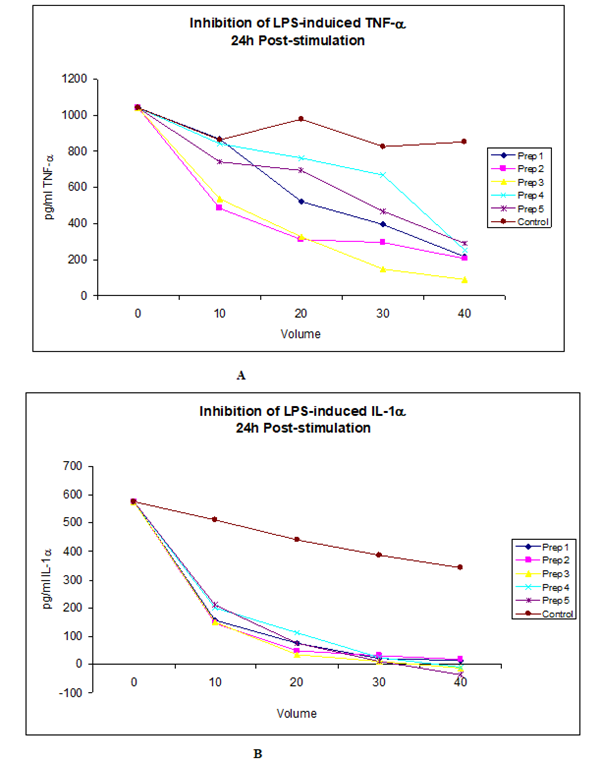
Psoriasis is a chronic inflammatory skin disease characterised by thick, raised lesions. Psoriasis presents in a number of ways, each recognised as a specific form of the condition, regardless psoriasis tends to be unsightly and almost invariably is uncomfortable for the sufferer. There is no cure for this condition and patients need to be treated on long-term therapies. There are a number of therapeutic products for the treatment and maintenance of Psoriasis, including Steroidal products for the milder types, Biologics and other nonsteroidal anti-inflammatory drugs for the more severe and systemic cases. However, all these treatments have side effects and also seem to lose efficacy with prolonged use (Figure 2).
Figure 2: The data shown above represents a Dose-dependent inhibition of LPS-induced TNF- and IL-1, two major mediators in sepsis and septic shock by four different preparations of Nephronin. It is noteworthy that when these tests were carried out, it was difficult to accurately measure the concentration of each preparation, due to contaminating salts, however the concentration of each preparation is ng/ml. Therefore when 20ul was used, the amount of active constituent (Nephronin) was estimated to be approximately 20ng. The indicated amount of each preparation was added to chambers of 24 well culture plates. 1ml of PBMC at 1.5x106 cells /ml containing 1ng/ml LPS was then added to each well. LPS was added to the cell suspension a few minutes prior to dispensing the cells into the wells of the tissue culture plates. The cells were harvested at 24 and 48 post-stimulation and the concentration of the cytokines measured by sandwich ELISA. Similar results were also obtained at 24h and 72h post-stimulation
a) Dose-dependent inhibition of LPS induced TNF-, 24h post-stimulation.
b) Dose-dependent inhibition of LPS-induced IL-1, 24h post-stimulation.
Eczema
Atopic Eczema (Atopic Dermatitis AD) is one of the most widespread autoimmune/inflammatory skin disorders with no known cure. Global rates in children have been estimated to be 10-15%, dropping to 1-3% in the adult population who suffer from persistent AD symptoms. The disease is characterized by the presence of pruritic, erythemic commonly oedemic and vesiculated patches known as AD lesions, which are inflamed and are areas of excessive skin production. There is also a significant physiological aspect to AD and many patients suffer from severe itch, or pruritus, which may cause significant distress and disturb sleep. Patients may also experience embarrassment from the presence of the skin lesions, which can affect their sociability and personal relationships. There may be a significant effect on patient’s quality of life and incidence of depression. AD is characterized by marked intercellular oedema within the epidermis (spongiosis), which leads to formation of intra epidermal vesicles. Lymphocytic infiltration into the epidermis is common, as well as the presence of histiocytes and occasionally neutrophils and eosinophils. AD is considered a chronic and pruritic inflammatory skin disorder and is commonly associated with other allergic conditions. Genetic factors have been linked to AD pathogenesis with various genes identified, that may have roles in disease onset and progression. The immune system is also largely involved; the recent understanding is that there exists an imbalance in the Th1 and Th2 immune pathways, with a shift to the Th2 arm largely driving the increased allergic sensitisation in this disease. Diet and environmental triggers are also an important factor in the development and flaring of AD and if identified and controlled, can significantly contribute to symptomatic relief. Widely used therapies for AD include moisturisers and irritant avoidance. Other topical treatments include topical steroids and pimecrolimun as they interfere with the inflammatory pathways. Systemic treatments are also used in the case that topical therapies do not sufficiently control AD symptoms, such as biologics, cyclosporine, methotrexate and azathioprine. Many new areas of AD research are now concentrated on targeting the skin barrier, to address the disruption and breakdown of the barrier, which is an important component of the pathogenesis of AD. Immune mediators are also a large target in the development of new treatments of AD, with many anti-inflammatory compounds being developed and trialled.
There is a strong preference to reduce systemic exposure to drugs, particularly those that have side effects and a strong preference and rationale for using naturally sourced products. Despite the availability of numerous topical and systemic treatment options for atopic dermatitis and psoriasis, all are lacking with respect to participant satisfaction and durability of treatment. Many of the systemic therapies for atopic dermatitis and psoriasis have significant side effects. Most current topical therapies and many treatments under development are based on modifications of a steroid structure, on inhibiting inflammatory pathways by inhibition of calcineurin, phosphodiesterase-4 (PDE4) and other pro-inflammatory molecules or removing certain immune modulators - as in the case of biologics. This supports the development of novel immunomodulatory treatments, which are safe to use and preferably naturally sourced, for the long-term management of atopic dermatitis and psoriasis.
Characterisation of Nephronin
In an effort to elucidate the structure and nature of Nephronin molecule, I became aware that ingredients pertaining to the composition of Nephronin, when added to a biological system show the same biological activity as the isolated Nephronin from the urine of children with SRNS. Based on this finding, I have developed a number of topical and one oral product, that contain naturally sourced ingredients. These ingredients are well tolerated and there has been no report of any side effects associated with them. These products are under the brand name of ENDOR and are with the Australian Therapeutic Goods Administration (TGA) as over the counter products and are commercially available for the treatment of mild – moderate psoriasis and Eczema. ENDORTM Dermatitis Care Capsules contain multivitamins and other natural antiinflammatory ingredients, such as curcumin. There are also two topical products for use on Psoriasis and Eczema: ENDOR cream for mild-moderate conditions and ENDOR 3.5 cream for the more severe conditions. We are currently conducting a clinical trial using ENDORTM Dermatitis Care capsules and ENDORTM 3.5 cream for the treatment of moderate – severe Psoriasis and Eczema, at the St George Dermatology and Skin Cancer Centre, Sydney Australia.
Heparan Sulphate Binding and Potential Inhibition of Viral Infection
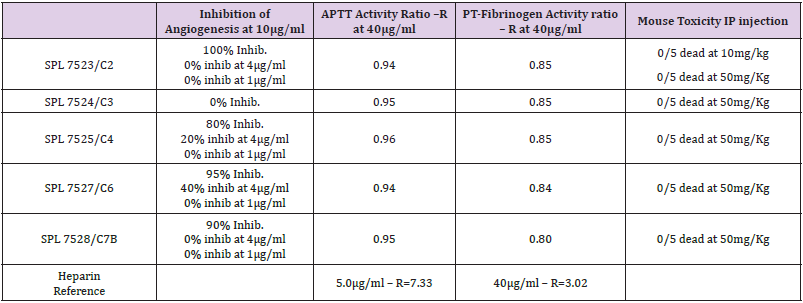
Many pathogens utilise heparan sulphate as their binding to the cell surface for entry into the cell. Herpes virus has a prerequisite first step binding to the cell surface heparan sulphate prior to entry into the cell (Table 1).
Table 1: Column 2 shows the effect of different preparations of Nephronin for the inhibition of angiogenesis. Column 3 and 4, headed as APTT activity and PT-Fibrinogen activity show the data for coagulation studies as compared with anti-coagulation of heparin. Column 5, title Mouse Toxicity, shows the data for toxicity studies when preparations of Nephronin dissolved in sterile water were injected IP into mouse.
Successful Inhibition of Hsv-1 Caused Destruction of Epidermal Cells After Axonal Transmission in Human In Vitro Model
Following Experimental Study Was Conducted by Dr Zorka Mikloska, Phd
Background: Herpes Simplex Virus (HSV) infection is one of the commonest infections of mankind and results in asymptomatic shedding of virus in urogenital skin or mucosa, mild oral recurrences (cold sores) or painful episodes of genital herpes. Genital herpes is the most important sexually transmitted disease of the Western world (after AIDS) and can lead to neonatal death and other complications. HSV establishes latency (stays inactive) after infection of the spinal cord and can be transmitted to the skin (or mucosa) via axons. The transmission of virus from axons to epidermal cells is a potential site for immune control, possibly via cytokines, chemokines and/or neutralizing antibodies [14].
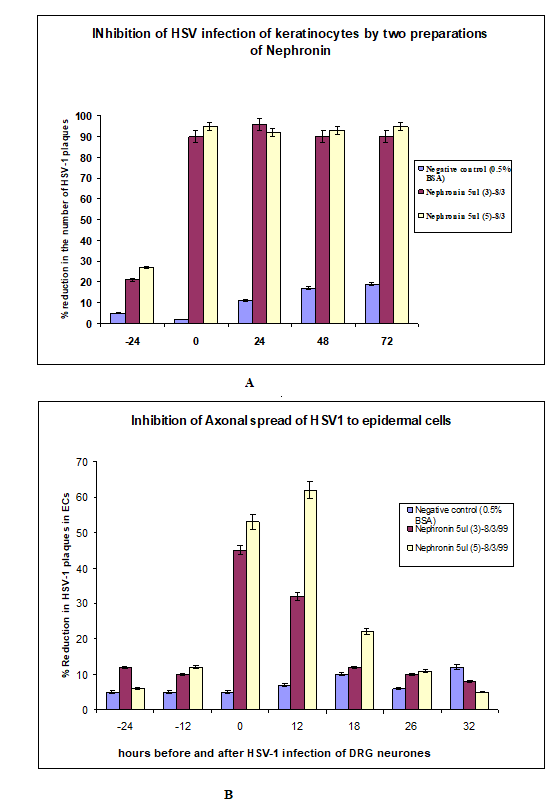
Figure 3: The data above shows the effectiveness of Nephronin in the inhibition of herpes virus in the infection of human skin cells. The data demonstrating the axonal inhibition of herpes virus by Nephronin is for the first time pointing to a possible cure for this disease.
Technique: In order to study the events after reactivation of HSV-1 in the neurons of Dorsal Root Ganglia (DRG), a neuronepidermal cell in vitro model consisting of autologous human foetal DRG and skin in 2 separate chambers was developed in our laboratory [15]. This allowed us to examine the events involving HSV infection of skin cells after axonal transmission, which highly resembles what actually happens in recurrent HSV infection. We previously have shown that neutralising antibodies applied to the epidermal cells of the model (or directly) at the different times after HSV-1 infection of DRG could inhibit viral destruction of these cells. In similar fashion Nephronin (in various concentrations of 5-40ml/ ml) was applied to epidermal cells in outer chamber of the model at times 24 and 12 hours before HSV-1 infection of DRG neurons in inner chamber, at the time of infection and 12, 18, 26 and 32 hours after HSV-1 infection. Epidermal cells were stained for HSV- 1 antigen 48 hours after infection and HSV-1- cytolytic plaques counted in each culture. The difference in the number of plaques between viral control (no Nephronin) and Nephronin treated cultures was calculated and expressed as %Reduction in the number of HSV-1 plaques (for detailed explanation of methods and techniques see J Virol 73:5934-44,1999) [16]. This was compared with direct HSV-1 infection of epidermal cells grown from the same tissue (Figure 3).
Results: The concentration of Nephronin that provides optimal inhibiting effect on HSV plaques and is not toxic in cell culture was tested in 2 cell lines and human foetal epidermal cell cultures and found to be 5ml/ml.Nephronin was added before, at the time of infection and after infection of epidermal cells grown in tissue culture dish. The best result was when the compound was added together with virus. In 3 experiments (with 6 replicates in each experimental group) involving our human foetal model maximal reduction in the number (and size) of HSV-1 plaques in epidermal cells was found at times 0 and 12 hours after infection of DRG and was in range of 55-67%. In directly infected epidermal cells, the reduction in number of plaques in epidermal cells was almost 100% up to 72 hours after infection.
Conclusion:
Simultaneous addition of Nephronin and HSV-1 to human foetal epidermal cells reduced the number of specific cytolytic plaques in these cells by almost 100% (as compared with viral control). Nephronin was also very efficient in our neuron-epidermal cell model. The ability of this compound to greatly reduce infection of epidermal cells after axonal transmission indicates its potential use as a topical ointment, e.g. in cream, lotion etc.
The Data Presented Below are the Results from the Above Studies: Nephronin totally inhibits infection of keratinocytes and fibroblasts by herpes virus. Our data suggests that Nephronin binds to HSV and prevents it from binding to heparan sulphate on the cells, thereby inhibiting infection by preventing the prerequisite step in HSV infection. The other aspect of HSV infection is that it can remain dormant in the nervous system with frequent episodes of infection, depending on the general health of the patient. Our studies have demonstrated that Nephronin potently inhibits axonal transmission of herpes virus, preventing the virus’s ability to reinfect the genitalia. We believe that for the first time, Nephronin offers the opportunity to develop a therapeutic drug to cure this debilitating disease. It is anticipated that a vaginal cream for the treatment and the prevention of the spread of HSV will be developed in the first instance. This will be followed by an oral medication for the inhibition of the axonal transmission of the virus, leading to a cure. Anecdotal data from people using the ENDOR Dermatitis Care capsules and the topical creams has shown effective treatment of herpes virus.
COVID19: The recent pandemic caused by Coronavirus Disease 2019 (COVID-19) poses a serious threat to global public health and the world economy. Although COVID19 infection and threat has been reduced considerably in many developed countries, it remains a threat, particularly if new mutations lead to new, more pathogenic strain(s) that develop resistance to the current vaccines and other therapies.Similar to herpes virus, the Receptor-Binding Domain (RBD) on epithelial cells, particularly of the respiratory tract, plays an important role in the adhesion of the coronavirus to the host cell surface. The RBD on the epithelial cell surface for COVID-19 has been identified as Heparan Sulphate (HS) [17]. The coronavirus spike protein, which plays the most important role in viral attachment, fusion and entry to the host cell has a pre-requisite heparan sulphate binding step, prior to binding to Angiotensin- Converting Enzyme 2 (ACE2) receptor and entry into the cell [18].
It has been hypothesized that compounds with HS binding ability may be potential treatment agents for patients with COVID19. There is some evidence that use of anticoagulant heparin treatment is associated with decreased mortality in severe coronavirus disease [19]. Development of the ENDOR dermatitis care capsules for the treatment of Psoriasis and eczema and anecdotal observation for the treatment of herpes infection, has provided sufficient anecdotal evidence in patients infected with COVID19, for using these capsules alongside recommended treatment in patients hospitalised with COVID19 infection. Clinical trial in collaboration with Tehran University and the Department of Virology at Imam Khomeini Hospital in Iran is currently underway.
Oncology Indication: Immune surveillance results in the destruction of cells, that grow outside their normal physiological geography. Tumour cells can evade immune recognition by being able to mimic surrounding cells, including the cell surface carbohydrates and receptors. Some carbohydrates, including heparan sulphate are required for anchorage and development to tumour mass. Furthermore, cancer cells have been shown to have considerably higher levels of heparanase, an enzyme that enables cancer cells to separate from their point of origin and spread. This process is called metastasis. Tests have shown that Nephronin very strongly inhibits angiogenesis in a rate Aorta model and heparanase in an enzymatic model. The therapeutical potential for Nephronin is huge, particularly in oncology and metastasis. Earlier studies using BAF-32 cells, that are deficient in cell surface Heparan Sulphate, were used to demonstrate the interaction of Nephronin with receptors of heparan sulphate. Other studies including inhibition of Herpes Simplex virus (HSV) infection of human fibroblasts and keratinocytes, were used to indicate that Nephronin is much more potent against different / abnormal or foreign receptors of heparan sulphate.
Normal and Tumourogenic Cell Lines: The importance of heparan sulphate in cell-cell interaction is becoming clear [20]. Furthermore, recent evidence suggests that tumourogenic cell surfaces might be covered by heparan sulphate, that is different from that found on the surface of normal cells. To test the hypothesis that Nephronin might distinguish between the heparan sulphate covering the surface of normal and cancer cells, a normal fibroblast cell line (BFT-3B) and a cancer cell line (HT1080) were used. The cells were grown to single layer confluence and up to 40ng/ ml of Nephronin was added to tissue culture media. In the case of carcinogenic (HT1080) cell line, the cells contracted soon after the addition of Nephronin and at higher concentrations of Nephronin within 24 hours after the addition of test samples large vacuoles within the cells could be seen. After 48h over 90% of the cells were found to be dead. In the case of normal (BFT-3B) cell line, addition of Nephronin did not seem to have any deleterious effect on the cells. To study further the effect of Nephronin on other cell types, normal mouse muscle cell line (C2C12) and mouse neuron tumourogenic cell (B35) lines were used. Increasing amounts of Nephronin were added to the cells (up to 4ng/ml) and similar results to those above were found. The fact that preparations of Nephronin were toxic to B35 and HT1080 but not C2C12 and BFT-3B suggests the interaction of Nephronin with cell surface molecules is specific to cancer cell lines.
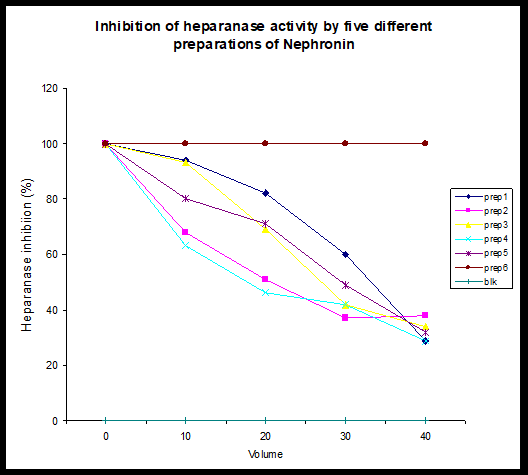
Inhibition of Heparanase: The data shows the dose dependent inhibition of heparanase activity. Given the fact that heparanase activity is shown to be of particular importance in tumour development and metastasis, the ability of Nephronin to inhibit heparanase activity might suggest that Nephronin could play an important role in the inhibition of metastasis and inhibition of tumourogenic cells to solid tumours. Dose dependent inhibition of human platelet heparanase activity by four preparations of Nephronin. Samples 1-5 refers to separate preparations of Nephronin. Preparation 6 is a control preparation. Blank (Blk) refers to a control for the enzyme reaction. At the time of study it was difficult to quantitate the active component of the preparations accurately. However it was estimated to be at 1 ng of Nephronin per 1ml of sample (Figure 4).
Angiogenesis: The anti-angiogenesis activities of preparations of Nephronin were assessed in an assay based on quantitative measurement of new vessel growth in rat aortic rings cultured in a minimally enriched medium [21-23]; the table below demonstrates the percent inhibition achieved by the chosen preparations of Nephronin. Because of the functional nature of Nephronin, their ability to inhibit coagulation was studied using Anti Partial Thromboplastin Time (APTT) and (Prothrombin Time) PT-fibrinogen assay systems were used. In each case a Coulter ACL3000 Blood Analyser was used and the data presented as described below:
For APTT: In this test the expected values for human plasma are quoted as being in the following range: - Coagulation Time 24.3 - 35.0 seconds with Coagulation Ratio 0.82 – 1.18 – these are for calibration plasma (IL 8469210).
PT-Fib: In this test the expected values for human plasma are quoted as being in the following range: - PT Coagulation Time 9.0 – 12.6 seconds with Coagulation Ratio 0.81 – 1.13, Fibrinogen 168 –392 mg/L and R= 0.92 – these are for calibration plasma (IL 8469210) [Fibrinogen values were not tabled because they relate to the constant use of calibrated plasma rather than changing samples]
The assays were performed in triplicates and the data presented is averaging of the results.
Protocol for Mouse Toxicity Tests
All tests were carried out on 6-8week old male white mice, strain BALB/c, weighing 20 to 25g, supplied by the Animal Breeding Unit of the University of N.S.W. or CSIRO Molecular Science Animal House, North Ryde, Sydney Australia. The mice were obtained at least one day prior to the test day and acclimatised in the test room (Temp 25oC), where they were held, five to a box and given commercial rat pellets and water ad lib. On the day of the test, the compound to be tested was weighed out and dissolved in sterile water to give a stock solution which when injected at the rate of 100 μl per mouse gave a dose of 50mg/Kg (i e 1mg/mouse). 100 μl of the test solution was given by intraperitoneal injection to each test mouse. Five mice were dosed at 50mg/Kg along with five control mice for each series of tests. On the day of testing, mice were initially observed at half-hourly intervals for the first three hours and symptoms recorded. Further readings were taken for the next seven days after which the surviving mice were disposed of by overanaesthetisation with carbon dioxide.
Comments
These preparations of Nephronin were found to be very potent in inhibiting heparanase activity and angiogenesis. The data presented here indicates significant therapeutic potential use of Nephronin and in turn, ENDOR Dermatitis Care Capsules for the treatment of cancer and inhibition of metastasis.
There is some anecdotal evidence that ENDOR capsules are effective in preventing relapse in breast cancer patients post operation. Work is being pursued to set a clinical trial for the evaluation of ENDOR capsules in this regard.
References
- Vaibhav Tiwari, Erika Maus, Ira M Sigar, Kyle H Ramsey, Deepak Shukla (2012) Role of heparan sulfate in sexually transmitted infections. Glycobiology 22(11): 1402-1412.
- Deepak Shukla, Patricia G Spear (2001) Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest 108(4): 503-510.
- Valeria Cagno, Eirini D Tseligka, Samuel T Jones, Caroline Tapparel (2019) Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias. Viruses 11(7): 596.
- Yan Xu, Pierre Martinez, Karin Séron, Guangxiang Luo, Fabrice Allain, et al. (2015) Characterization of Hepatitis C Virus Interaction with Heparan Sulfate Proteoglycans. J Virol 89(7): 3846-3858.
- Sasaki M, Anindita PD, Ito N, Sugiyama M, Carr M, et al. (2018) The Role of Heparan Sulfate Proteoglycans as an Attachment Factor for Rabies Virus Entry and Infection. J Infect Dis 217(11): 1740-1749.
- Aleksandra Milewska, Miroslaw Zarebski, Paulina Nowak, Karol Stozek, Jan Potempa, et al. (2014) Human Coronavirus NL63 Utilizes Heparan Sulfate Proteoglycans for Attachment to Target Cells 88 (22): 13221-13230.
- Kim SY, Jin W, Sood A, Montgomery DW, Grant OC, et al. (2020) Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions Antiviral Res 181.
- Li N, Spetz MR, Ho M (2020) The Role of Glypicans in Cancer Progression and Therapy. J Histochem Cytochem 68(12): 841-862.
- Del Molino Del Barrio I, Meeson A, Cooke K, Malki MI, Barron Millar B, et al. (2021) Contribution of Heparan Sulphate Binding in CCL21-Mediated Migration of Breast Cancer Cells. Cancers (Basel) 13(14): 3462.
- Carfora V, Spiniello G, Ricciolino R, Di Mauro M, Migliaccio MG, et al. (2021) Anticoagulant treatment in COVID-19: a narrative review. Journal of thrombosis and thrombolysis 51(3): 642-648.
- Kim SH, Park SJ, Han KH, Kronbichler A, Saleem MA, et al. (2016) Pathogenesis of minimal change nephrotic syndrome: an immunological concept. Korean journal of pediatrics 59(5): 205-211.
- Domanig R, Jöbstl W, Gruber S, Freudemann T (2009) One-dimensional cellulose acetate plate electrophoresis--a feasible method for analysis of dermatan sulfate and other glycosaminoglycan impurities in pharmaceutical heparin. J Pharm Biomed Anal 49(1): 151-155.
- Baumgartner JD, Calandra T (1999) Treatment of sepsis: past and future avenues. Drugs 57(2): 127-132.
- Mikloska Z, Danis VA, Adams S, Lloyd AR, Adrian DL, et al. (1998) In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions--do herpes simplex virus-infected keratinocytes contribute to their production. J Infect Dis 177(4): 827-838.
- Penfold ME, Armati P, Cunningham AL (1994) Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA 91(14): 6529-633.
- Mikloska Z, Sanna PP, Cunningham AL (1999) Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J Virol 73(7): 5934-5944.
- Tai W, He L, Zhang X, Pu J, Voronin D, et al. (2020) Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 17(6): 613-620.
- Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, et al. (2014) Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol 88(22): 13221-13230.
- Tang N, Bai H, Chen X, Gong J, Li D, et al. (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18(5): 1094-1099.
- Lin X, Perrimon N (2000) Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biol 19(4): 303-307.
- Nicosia RF, Ottinetti A (1990) Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest 63(1): 115-122.
- Nicosia RF, Ottinetti A (1990) Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol 26(2): 119-128.
- Liekens S, Neyts J, Degrève B, De Clercq E (1997) The sulfonic acid polymers PAMPS [Poly(2-Acrylamido-2-Methyl-1-Propanesulfonic Acid] and related analogues are highly potent inhibitors of angiogenesis. Oncology Research 9(4): 173-181.

 Review Article
Review Article