ABSTRACT
It is very difficult to repair large bone defects, especially when they have a complex shape. We have developed a new technique to make a desired copy of rabbit bones. A rabbit distal femur was scanned by computed tomography (CT), and a rectangularshaped beta-tricalcium phosphate (β-TCP) block with 75% porosity was automatically machined using milling tools into a half-scale copy of the distal femur based on the CT data. The β-TCP block was seeded with bone morphogenetic protein-2 and bone marrow cells obtained from the femur and implanted on the periosteum of the femur. At 10 weeks after implantation, most of the β-TCP block had been replaced by bone and a complete copy of the distal femur was reconstructed. Our findings indicate that this technique will be useful in the clinical setting. We also report the representative clinical results of treatment with β-TCP graft in patients with bone defects since 1989.
Keywords: Bone Formation; BMP-2; FGF-2; Beta-TCP; Osteoblast-Osteoclast Communication
Introduction
Autologous bone is the preferred graft material for treating bone defects because it functions as an osteoconductive scaffold and also contains progenitor cells and growth factors, which promote osteogenesis [1]. However, autologous bone has several disadvantages, including limited availability, prolonged operative time, persistent pain, and donor-site morbidity [2-4]. In addition, autologous bone is difficult to obtain when the patient is a child. Allografts have commonly been used as substitutes for autogenous bone grafts in Europe and the United States but not in Japan. Significant problems associated with the use of allografts include a low bone-fusion rate and disease transmission [5,6]. Recently, tissue engineering has attracted attention as a means to solve these limitations. It relies on the three following elements: multipotent cells; growth factors or cytokines for cell differentiation; and biocompatible scaffolds to reconstruct tissue [7-9]. In the case of bone regeneration, bone marrow cells (BMCs) are often used because they are easy to obtain, are cost-effective, and have the capacity to differentiate into osteoblasts and osteoclasts. Secondly, growth factors, cytokines, or hormones can be used to differentiate BMCs into bone-specific cells such as osteoblasts and osteoclasts. Among various growth factors, bone morphogenetic protein-2 (BMP-2) is used in the clinical setting and induces the recruitment and differentiation of mesenchymal progenitor cells into mature osteoblasts, thereby producing bone matrix. BMP-2 can also directly or indirectly stimulate the differentiation of osteoclast progenitor cells and promote the function of mature osteoclasts [10-24].
Thirdly, a scaffold is required to reconstruct three-dimensional tissue. Beta-tricalcium phosphate (β-TCP) has recently received considerable attention as a bone graft substitute because of its biocompatibility and biodegradability [25-27]. β-TCP is gradually degraded during bone remodeling and is finally replaced by mature new bone. We have previously shown that the main resorption process of β-TCP is via osteoclasts [28-34], and the macropore and micropore structures within β-TCP affect not only material resorption but also bone formation [35-37]. We found that a combination of β-TCP with a superior microstructure and local BMP-2 administration accelerates bone reconstruction. In this study, we report our findings from experimental animal models and representative clinical results for the use of β-TCP implantation in patients with bone defects.
Materials
β-TCP blocks and granules with 60% or 75% porosity for clinical use and for animals were purchased from Olympus Terumo Biomaterials Co., Ltd. (Tokyo, Japan). One percent hyaluronic acid (HA) was purchased from Chugai Co., Ltd. (Tokyo, Japan). Escherichia coli-derived recombinant human BMP-2 was purchased from Osteopharma, Inc. (Osaka, Japan). Recombinant human fibroblast growth factor 2 (FGF-2) was purchased from Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan). Alendronate (ALN) solution was purchased from Teijin Pharma Co., Ltd. (Tokyo, Japan).
Methods
Experiment 1.1
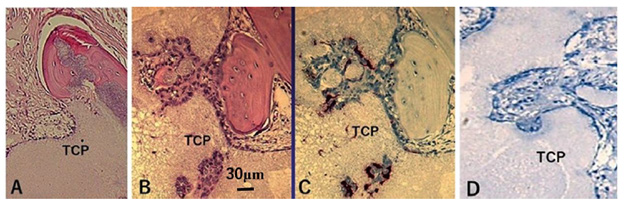
BMCs were obtained from the femora of a 6-week-old Fisher rat. The cells were cultured in osteogenic medium (β-glycerophosphate, dexamethasone, and ascorbic acid) for 2 weeks to differentiate into osteoblastic cells. After pepsin treatment, β-TCP cylinders (4.0 mm diameter × 10 mm length) with 75% porosity seeded with the harvested 1.0 × 106 cells were implanted subcutaneously in 12-week-old Fisher rats. At 10 weeks after surgery, the implanted β-TCP blocks were harvested and fixed with 4% paraformaldehyde in phosphate-buffered saline. After decalcification in 0.5 M ethylenediaminetetraacetic acid for 5–7 days, serial histological sections were prepared for hematoxylin-eosin (HE) staining (Figure 1A).
Figure 1: Histological sections of the β-TCP block with rat BMCs after subcutaneous implantation.
(A) Cultured osteoblasts seeded in the β-TCP block generated a very small amount of new bone at the periphery of the block, and most of the β-TCP was still present at 10 weeks.
(B) Whole BMCs seeded in the β-TCP block showed marked new bone formation and there was less β-TCP remaining compared with A at 5 weeks.
(C) TRAP staining of the serial histological section of B. TRAP-positive cells were present on the surface of the β-TCP and new bone.
(D) New bone formation and material resorption were strongly inhibited by ALN treatment at 5 weeks.
Experiment 1.2
The same number of BMCs as in Experiment 1.1 were prepared without differentiation. β-TCP blocks seeded with whole BMCs were implanted into 12-week-old Fisher rats in the same manner. The implanted β-TCP blocks were harvested at 5 weeks. Decalcified sections were prepared for HE and tartrate-resistant acid phosphatase (TRAP) staining (Figures 1B & 1C).
Experiment 1.3
Before cell seeding, β-TCP blocks were immersed in 1.0 × 10–3 M ALN for 3 days. Excess solution was removed with sterilized paper. ALN-treated β- TCP blocks seeded with the same number of whole BMCs as Experiment 1.2 were implanted into 12-week-old Fisher rats and then harvested after 5 weeks (Figure 1D).
Experiment 2
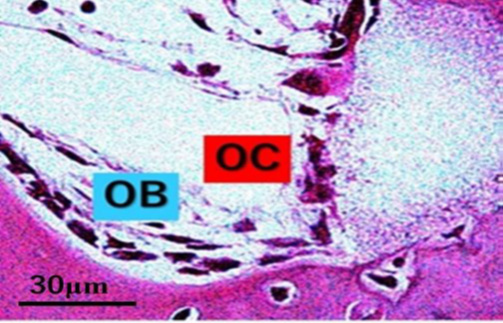
Cylindrical cancellous bone defects (4.1 mm diameter × 11 mm length) were created by drilling the lateral aspect of the rabbit distal femur, and the bone cavities were filled with β-TCP cylinders with 75% porosity (4.0 mm diameter × 10 mm length). At 2 weeks after surgery, the distal femur was harvested, and decalcified sections were prepared for HE staining (Figure 2).
Figure 2: HE staining of rabbit cancellous bone defects at 2 weeks after β-TCP implantation. Multinucleated giant cells were in contact with osteoblasts at the boundary between new bone and the remaining β-TCP. OB, osteoblasts; OC, osteoclasts.
Experiment 3.1
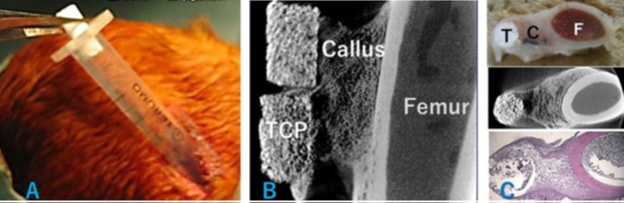
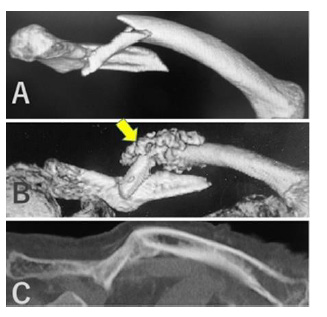
Cylindrical β-TCP blocks (4 mm diameter × 10 mm length) with 75% porosity were seeded with 12.5 μg BMP-2. Under general anesthesia, a 1-cm skin incision was made on the lateral side of the femur. Two cylindrical β-TCP blocks were implanted on the periosteum of the femur through a 1-mL syringe (Figure 3A). At 8 weeks after implantation, the distal part of the femur including the β-TCP blocks was harvested and analyzed with micro-computed tomography (CT) (Figure 3B). After decalcification, axial sections were analyzed histologically (Figure 3C).
Figure 3: An in vivo bone banking procedure in rabbits.
(A) Two cylindrical 75% porosity β-TCP blocks seeded with BMP-2 were implanted through a 1-mL syringe on the lateral side of the rabbit femur.
(B) At 8 weeks after implantation, the β-TCP blocks were harvested and analyzed by micro-CT and
(C) Decalcified sections were prepared. CT and HE-stained axial sections showed partial replacement of TCP by bone and bridging callus formation between the β-TCP blocks and femur. C, callus; F, femur, T, TCP.
Experiment 3.2
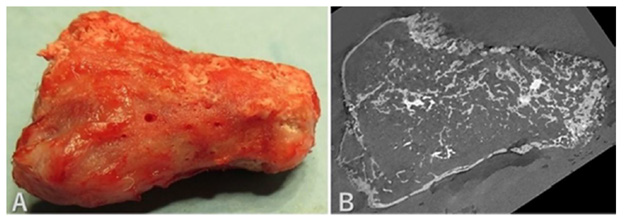
A rabbit distal femur was obtained (Figure 4A) and scanned by micro-CT. A rectangular-shaped β-TCP block with 75% porosity was machined automatically with milling tools into a half-scale copy of the distal femur based on the CT data (Figures 4B & 4C). A skin incision was made to expose the lateral aspect of the distal femur and three drill holes were made into the marrow. The β-TCP block seeded with 40 μg BMP-2 and BMCs obtained from the femur was implanted on the periosteum. At 10 weeks after implantation, the distal femur including the β-TCP block (Figure 5A) was harvested and analyzed using micro-CT (Figure 5B). The care and use of the animals in this study were in accordance with the guidelines of the Laboratory Animal Facilities of the Jikei University School of Medicine (2015-045).
Figure 4: The procedure for making a copy of the rabbit distal femur made of β-TCP.
(A) Macroscopic appearance of the rabbit distal femur.
(B) A rectangular-shaped β-TCP block with 75% porosity was automatically machined with milling tools to make a half-scale copy of the distal femur based on the CT data.
(C) The copy of the distal femur made of β-TCP.
Figure 5: Implantation of the β-TCP copy of the rabbit distal femur.
(A) The macroscopic appearance of the β-TCP copy of the femur seeded with BMP-2 and BMCs at 10 weeks after implantation.
(B) CT imaging of the distal femur showed that most of the β-TCP had been replaced by bone.
Clinical Application
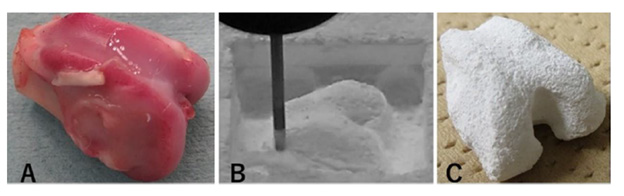
Since 1989, we have used β-TCP with 75% porosity, and since 2005, we have used β-TCP with either 75% or 60% porosity to fill bone defects in the clinical setting. In several cases, β-TCP was placed on the periosteum after fibula harvest (Figure 6). For the use of an injectable composite of β-TCP, HA, and FGF-2, all patients with fractures and dislocated bone fragments provided informed consent prior to their participation in this study, which was approved by our hospital ethics committee in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) in 2007. For the preparation of the injectable composite, 2 g β-TCP granules with 60% porosity (Figure 7A), 2 mL of 1% HA (Figure 7B), and 1 mg lyophilized FGF-2 (Figure 7C) were mixed aseptically in a dish. The mixture was transferred to 1-cm diameter cylinders (Figure 7D).
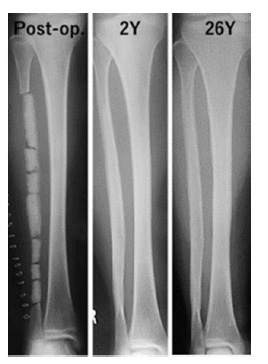
Figure 6: Radiographs of a 13-year-old girl with scoliosis (Case 1). An 18-cm-long section of the fibula was obtained for spinal fusion, and β-TCP blocks with 75% porosity were placed on the remaining periosteum. Complete resorption of β-TCP and cortical bone formation were observed at 2 years after surgery. At 26 years after implantation, the fibula still retained its original shape.
Figure 7: An injectable composite.
(A) β-TCP granules with 60% porosity,
(B) 1% hyaluronic acid, and
(C) lyophilized recombinant human FGF-2 were mixed and
(D) Transferred to a 1-cm diameter cylinder for osteochondral grafts.
Results
Animal Experiments
Experiment 1: At 10 weeks after implantation of the β-TCP blocks with osteoblastic cells alone, a very small amount of new bone was found at the periphery of the blocks and most of the β-TCP remained (Figure 1A). In contrast, β-TCP blocks seeded with whole rat BMCs induced marked bone formation at 5 weeks after subcutaneous implantation (Figure 1B). Sequential histological sections showed that TRAP-positive cells were present on the surface of β-TCP and new bone (Figure 1C). New bone formation and β-TCP resorption were strongly inhibited in the ALN-treated β-TCP blocks (Figure 1D).
Experiment 2: HE staining of rabbit cancellous bone defects at 2 weeks after implantation with β-TCP showed that multinucleated giant cells were in contact with osteoblasts at the boundary between the new bone and remaining β-TCP (Figure 2).
Experiment 3.1: Two cylindrical 75% porosity β-TCP blocks seeded with BMP-2 were implanted through a 1-mL syringe on the lateral side of the rabbit femur (Figure 3A). At 8 weeks after implantation, β-TCP was partially replaced by bone and a bridging callus had formed between β-TCP and the femur (Figures 3B & 3C).
Experiment 3.2: A rectangular-shaped β-TCP block was machined by milling tools into a half-scale copy of the distal femur based on the CT data (Figure 4). At 10 weeks after implantation of the β-TCP block seeded with BMP-2 and BMCs on the periosteum of the femur, most of the β-TCP was replaced by bone and a complete half-scale copy of the distal femur was reconstructed (Figure 5).
Clinical Application
Case 1: A 13-year-old girl with scoliosis. An 18-cm-long fibula graft was obtained for spinal fusion, and cylindrical β-TCP blocks with 75% porosity were placed on the remaining periosteum. At 2 years after implantation, the fibula had been completely reconstructed and still retained its original shape at 26 years (Figure 6).
Case 2: An 88-year-old woman with clavicular fracture and severe aortic valve stenosis (Figure 8A). Under local anesthesia, a composite of β-TCP granules, HA, and FGF-2 (Figure 7) was injected on the fractured fragments. At 3 weeks after surgery, the composite remained at its original position (Figure 8B). At 24 weeks after surgery, marked new bone formation was found and complete bone union and β-TCP resorption were obtained (Figure 8C).
Figure 8: Radiographs of an 88-year-old woman (Case 2).
(A) Clavicular fracture.
(B) The injected composite remained in its original position at 3 weeks after injection and
(C) Complete bone union and material resorption were obtained at 24 weeks. The arrow indicates the injected composite.
Discussion
Recently, tissue engineering has attracted considerable attention. It relies on the use of multipotent cells, growth factors or cytokines for cell differentiation, and biocompatible scaffolds to reconstruct tissue [7-9]. β-TCP is reported to have excellent osteoconduction and resorbability when used to fill a bone defect. We have more than 30 years of experience with the application of β-TCP in the clinical setting [25-27]. Regarding our 13-year-old case, β-TCP blocks with 75% porosity were implanted on the periosteum after harvesting 18 cm of fibula. The original shape of the fibula was completely reconstructed without any stimulants at 2 years after implantation. This fibula can be reused for bone grafting. We have also previously shown that microporous and macroporous structures within β-TCP facilitate the osteoclastic resorption of β-TCP and new bone formation [35]. In addition, local BMP-2 administration further accelerates the osteoclastic resorption of β-TCP and new bone formation [34,35]. A combination of β-TCP with superior microstructure and local BMP-2 administration could accelerate bone reconstruction. Autologous BMCs are often used for bone regeneration because they are easy to obtain, costeffective, and do not induce immunological responses. Many researchers expect BMCs to be used as a source of osteoprogenitor cells. However, BMCs include not only mesenchymal stem cells but also hematopoietic stem cells. Thus, they have the capacity to be differentiated into osteoblasts, osteoclasts, macrophages, chondrocytes, adipocytes, and other cells.
The results obtained from our animal BMC experiments showed that implanted β-TCP blocks seeded with whole BMCs demonstrated much faster and greater bone formation compared with blocks seeded with osteoblasts only. In addition, this bone formation was inhibited by ALN, suggesting that osteoblasts alone are not sufficient for bone formation and that osteoclastmediated resorption may play an important role in this process. After implantation of β-TCP in rabbit cancellous bone defects, osteoblasts lining the new bone were found to be in contact with TRAP-positive cells on the remaining β-TCP in many places. These findings suggest that osteoblast–osteoclast interactions may be related to new bone formation, and a coupling-like phenomenon could occur in the β-TCP-implanted area. This hypothesis was supported by recent studies indicating that osteoblast-osteoclast communication was important in bone formation and was promoted by parathyroid hormone [38,39]. Clinically, it is well known that intermittent parathyroid hormone administration increases bone mineral density. Thus, the in vivo condition is superior in terms of cell participation in bone formation. Based on the above results, we attempted large bone regeneration with a minimal surgical procedure. Cylindrical β-TCP blocks seeded with BMP-2 were implanted on the periosteum of the femur through a 1-mL syringe. We found that β-TCP was partially replaced by bone and that a bridging callus had formed between β-TCP and the femur.
It appeared that BMP-2 diffusing from the β-TCP blocks induced the formation of new bridging bone. These results suggest that this approach could be used to fill large bone defects. Next, in order to reconstruct a complex-shaped large bone, we used CT scanning to obtain three-dimensional imaging data from a rabbit distal femur. Then, a rectangular β-TCP block was machined with milling tools into a half-scale copy of the distal femur based on the micro-CT data. This β-TCP block seeded with BMP-2 and BMCs from the femur was implanted on the periosteum. At 10 weeks after implantation, most of the β-TCP had been replaced by bone and a complete copy of the half-scale distal femur had been reconstructed. This technique will be useful in the clinical setting. Unfortunately, BMP-2 has not been approved for use in Japan, and furthermore, it is expensive and a concern for carcinogenesis. To solve these problems, we have recently developed a composite to repair fractures with bone defects, especially in elderly patients. We previously reported a paste-like composite of β-TCP granules and HA or collagen that is suitable for treating defects of any shape [28,29]. This composite supplemented with 200 μg FGF-2 induced cortical bone regeneration and repaired 5-mm cortical bone defects in rabbit tibiae by 12 weeks [29]. Based on the animal results, we have applied this technique to elderly patients with long bone fractures with bone defects and found that this composite induced marked callus formation around the injected sites [40]. In the present study, we reported the case of an 88-year-old woman with a clavicular fracture and a dislocated third fragment. She had severe aortic valve stenosis; thus, we were unable to perform open reduction under general anesthesia.
After receiving informed consent, we injected the patient with the composite to bridge the broken bone fragments under local anesthesia. Surgical time was only 10 min. This approach resulted in marked callus formation, complete bone union, and resorption of β-TCP at 24 weeks after surgery. This composite can fill irregularly shaped defects and facilitate callus formation and may be useful for the treatment of other cortical bone defects such as long-bone fractures with displaced fragments with minimal surgical invasion. We speculate that osteoprogenitor cells in the periosteum, bone marrow, and muscle responded to FGF-2 diffusing from the composite, resulting in marked callus formation. Although this composite promoted callus formation, it did not shorten the time required for callus formation. If BMP-2 is used as an alternative to FGF-2, quicker callus formation may be expected. In conclusion, it is important to understand that bone regeneration is a complex, well-orchestrated physiological process that is regulated mainly by communication between osteoblasts and osteoclasts. A combination of BMCs, BMP-2, and β-TCP could be used to repair large bone defects of any shape.
Funding
Not applicable.
Consent for Publication
All authors have consented to the submission of this manuscript for publication.
Competing Interests
The β-TCP used in the animal experiments in the present study was provided by Olympus Terumo Biomaterials Co.
Authors’ Contributions
TT designed the experiments and clinical use. HK, NI, and SA performed the animal experiments. YK performed clinical use. MS helped to draft the manuscript.
References
- Giannoudis P V, Dinopoulos H, Tsiridis E (2005) Bone substitutes: An update. Injury 36: 20-27.
- Cockin J (1971) Autologus bone grafting; complication at the donor site. J Bone Joint Surg Br 53: 153.
- Summers B N, Eisenstein S M (1989) Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg Br 71(4): 677-680.
- Younger E M, Chapman M W (1989) Morbidity at bone graft donor sites. J Orthop Trauma 3(3): 192-195.
- Aurori B F, Weierman R J, Lowell H A, Nabel C I, Parsons J R (1985) Pseudoarthrosis after spinal fusion for scoliosis. A comparison of autogenic and allogenic bone grafts. Clin Orthop 199: 153-158.
- Karcher H L (1997) HIV transmitted by bone graft. BMJ 314: 1300.
- Langer R, Vacanti J P (1993) Tissue engineering Science 260: 920-926.
- Vacanti J P, Morse M A, Saltzman W M (1988) Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Prediatr Surg 23(1): 3-9.
- Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, et al. (2000) Tissue engineered bone regeneration. Nat Biotechnol 18(9): 959-963.
- Ji X, Chen D, Xu C, Harris S E, Mundy G R, et al. (2000) Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3-F442A. J Bone Miner Metab 18(3): 132-139.
- Hay E, Hott M, Graulet A M, Lomri A, Marie P J (1999) Effects of bone morphogenetic protein-2 on human neonatal calvaria cell differentiation. J Cell Biochem 72(1): 81-93.
- Jikko A, Harris S E, Chen D, Mendrick D L, Damsky C H (1999) Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. J Bone Miner Res 14(7): 1075-1083.
- Chaudhari A, Ron E, Rethman M P (1997) Recombinant human bone morphogenetic protein-2 stimulates differentiation in primary cultures of fetal rat calvarial osteoblasts. Mol Cell Biochem 167: 31-39.
- Jones A L, Bucholz R W, Bosse M J, Mirza S K, Lyon T R, et al. (2006) Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am 88(7): 1431-1441.
- Boden S D, Kang J, Sandhu H, Heller J G (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: Volvo Award in clinical studies. Spine 27(23): 2662-2673.
- Garrison K R, Shemilt I, Donell S, Ryder J J, Mugford M, et al. (2010) Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev 16(6): CD006950.
- Itoh K, N Udagawa, T Katagiri, S Iemura, N Ueno, et al. (2001) Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappa B ligand Endocrinology 142(8): 3656-3662.
- Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, et al. (1995) Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res 10(11): 1681-1690.
- Kaneko H, T Arakawa, H Mano, T Kaneda, A Ogasawara, et al. (2000) Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 27(4): 479-486.
- Okamoto M, Murai J, Yoshikawa H, Tsumaki N (2006) Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res 21(7): 1022-1033.
- Jensen E D, Pham L, Billington C J, Espe K, Carlson A E, et al. (2010) Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J Cell Biochem 109(4): 672-682.
- Termaat M F, Den Boer F C, Bakker F C, Patka P, Haarman H J (2005) Bone morphogenetic proteins, development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am 87(6): 1367-1378.
- Wozney J M, Rosen V, Celeste A J, Mitsock L M, Whitters M J, et al. (1988) Novel regulators of bone formation: Molecular clones and activities Science 242: 1528-1534.
- Urist M R, Nilsson O, Rasmussen J, Hirota W, Lovell T, et al. (1987) Bone regeneration under the influence of a bone morphogenetic protein (BMP) beta tricalcium phosphate (TCP) composite in skull trephine defects in dogs. Clin Orthop Relat Res 214: 295-304.
- Tanaka T, Kumagae Y, Saito M, Chazono M, Komaki H, et al. (2008) Bone formation and resorption in patients after implantation of beta-tricalcium phosphate blocks with 60% and 75% porosity in opening wedge high tibial osteotomy. J Biomed Mater Res Part B: Appl Biomater 86(2): 453-459.
- Tanaka T, Kumagae Y, Chazono M, Kitasato S, Kakuta A, et al. (2015) A novel evaluation system to monitor bone formation and beta-tricalcium phosphate resorption in opening HTO. Knee Surg Sports Traumatol Arthrosc 23(7): 2007-2011.
- Tanaka T, Komaki H, Chazono M, Kitasato S, Kakuta A, et al. (2017) Basic research and clinical application of beta-tricalcium phosphate. Morphologie 101(334): 164-172.
- Chazono M, Tanaka T, Komaki H, Fujii K (2004) Bone formation and bioresorption after implantation of injectable beta-tricalcium phosphate granules-hyaluronate complex in rabbit bone defects. J Biomed Mater Res A 70(4): 542-549.
- Komaki H, Tanaka T, Chazono M, Kikuchi T (2006) Repair of segmental bone defects in rabbit tibiae using a complex of beta-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials 27(29): 5118-5126.
- Tanaka T, Komaki H, Chazono M, Fujii K (2005) Use of a Biphasic Graft Constructed with Chondrocytes Overlying a Beta-Tricalcium Phosphate Block in the Treatment of Rabbit Osteochondral Defects. Tissue Engineering 11(1-2): 331-339.
- Ogose A, Hotta T, Hatano H, Kawashima H, Tokunaga K, et al. (2002) Histological examination of beta-tricalcium phosphate graft in human femur. J Biomed Mater Res 63(5): 601-604.
- Chazono M, Tanaka T, Kitasato S, Kikuchi T, Marumo K (2008) Electron microscopic study on bone formation and bioresorption after implantation of beta-tricalcium phosphate in rabbit models. J Orthop Sci 13(6): 550-555.
- Tanaka T, Saito M, Chazono M, Kumagae Y, Kikuchi T, et al. (2010) Effects of alendronate on bone formation and osteoclastic resorption of beta-tricalcium phosphate. J Biomed Mater Res 93(2): 469-474.
- Kitasato S, Tanaka T, Chazono M, Komaki H, Kakuta A, et al. (2020) Local application of alendronate controls bone formation and beta-TCP resorption induced by rhBMP-2. J Biomed Mater Res A 108(3): 528-536.
- Kakuta A, Tanaka T, Chazono M, Komaki H, Kitasato S, et al. (2019) Effects of micro-porosity and local BMP-2 administration on bioresorption of beta-TCP and new bone formation. Biomater Res 23: 12.
- Yamasaki N, Hirao M, Nanno K, Sugiyasu K, Tamai N, et al. (2009) A comparative assessment of synthetic ceramic bone substitutes with different composition and microstructure in rabbit femoral condyle model. J Biomed Mater Res B 91(2): 788-798.
- Davison N L, Luo X, Schoenmaker T, Everts V, Yuan H, et al. (2014) Submicron-scale surface architecture of tricalcium phosphate directs osteogenesis in vitro and in vivo. Eur Cell Mater 27: 281-297.
- Kim J, Lin C, Stavre Z, Greenblatt M, Shim J (2020) Osteoblast-osteoclast communication and bone homeostasis. Cells 9(9): 2073.
- Furuya M, Junichi Kikuta, Sayumi Fujimori, Shigeto Seno, Hiroki Maeda, et al (2018) Direct cell-cell contact between mature osteoblasts and osteoclasts dynamically controls their functions in vivo. Nat Commun 9: 300.
- Tanaka T, Kitasato S, Chazono M, Kumagae Y, Iida T, et al. (2012) Use of an injectable complex of beta-tricalcium phosphate granules, hyaluronate, and FGF-2 on repair of unstable intertrochanteric fractures. Open Biomed Eng J 6: 98-103.

 Review Article
Review Article