ABSTRACT
This case report documents the performance of the Supercritical C02 processed bone allografts in implantology treatment. Data were collected from files of 37 patients (23 females, 14 males) undergoing maxillary sinus augmentation in 12 cases and alveolar ridge augmentation in 25 cases with the bone allografts.63 implants were placed. 0 implants were well osseointegrated and functioning. 3 implants failed due to non-osteointegration and were removed. Within the limitations of this study, the supercritical CO2 processed bone allograft as a grafting material was successful during maxillary sinus augmentation and alveolar ridge augmentation.
Introduction
Allogeneic bone grafts, whether fresh, frozen, or freezedried, have several advantages, including reduced surgical morbidity, shorter operating times, and greater availability and quantity compared to autogenic bone [1,2]. Histological and histomorphometric results show that allogeneic bone has osteoconductive properties like autogenic bone [3]. The supercritical CO2 processed bone allografts (Supercrit®, BIOBank, 3, rue Georges Charpak - 77127 Lieusaint – France) are derived exclusively from human femoral heads collected from living donors who have undergone hip replacement surgery in accordance with European regulations. The femoral heads are cleaned and viralinactivated using supercritical CO2 extraction process. A terminal gamma irradiation step at 25 kGy renders the packaged bone grafts completely sterile. The process has no effect on the mineral and collagen composition of the bone matrix, preserving trabecular bone tissue integrity and mechanical strength comparable to fresh bone. As a result, the treated bone allograft has osteoconductive properties [4-8]. The aim of this case series is to demonstrate that bone augmentation with the supercritical CO2 processed bone allograft is a valuable treatment option.
Materials and Methods
Patient
Between September 2018 and February 2019, thirty-seven (37) patients received the Supercrit® processed bone allografts for:
Maxillary sinus augmentation in 12 cases.
Alveolar ridge augmentation in 25 cases.
Patients’ demographic information is detailed in Table 1 below:
Table 1 Demographic characteristics of study patients. The bone density was mainly D4 in sinus lift group (75%) and D3 in Ridge augmentation cases (52%) according to Misch classification. (Table 1 below).
Graft Material
The graft material used was the BIO Bank cancellous bone allograft powder. The allografts were prepared from living donor femoral heads treated by the supercritical CO2 process through degreasing steps and a gentle chemical oxidation of the residual proteins with preserved bone architecture. Before sinus or alveolar ridge filling, the bone allograft powder packed in syringe or vial (Figure 1) was hydrated using Metronidazole 0.5% solution (B-Braun).
Surgical Technique
All the surgical procedures were performed by the same surgeon. All sinus lift procédures were performed using the open technique, with access to the anterior sinus wall. After creating a bone window, preparing the Schneider membrane, and drilling holes for implants, the bone allograft mixed with PRF was placed under the Schneider membrane, before placing the implants. The hole was then covered with a collagen membrane and sutured. The healing and regeneration time was 5-6 months (depending on the height of the alveolar process at the implantation sites). The ridge augmentation technique was done through the following steps: incision and detachment of a full-thickness flap, preparation of the graft bed by perforation (bleeding), in the case of a thin cortical layer - decortication then application of the allograft mixed with PRF. The graft was then covered with a collagen membrane, stabilized with titanium pins, the flap mobilized before suturing.
Patients Received the Following Prophylactic Médication
Amoxicillin 625 mg twice a day, started 2 days before the procedure, and continued the day of surgery and 4 days after. In cases where the level of Vit D3 in the blood test was too low (below 30 micrograms) - supplementation was carried out with doses ranging from 4,000 to 8,000 daily, until the correct level was obtained. All patients were assessed preoperatively to determine both their dental and general health status, and the following assessments were performed at the post grafting visits:
Outcome Measures : Implant survival defined as:
The implant is present, functional, and stable.
No radiolucencies areas around the implant
No persistent and/or irreversible subjective and objective clinical signs (suppuration or pain).
Any complications such as chronic pain, infection
Radiographic Analysis : Radiographic analysis was performed using cone-beam computed tomography (CBCT) and/or panoramic radiographs taken before and after grafting and at mid-term follow up. Software programs were used to calculate bone height in millimeters.
Statistical Analysis : Statistical analyses were carried out in IBM SPSS Statistics 26 (SPSS Inc. Chicago, USA). All included cases were reviewed, and the summary statistics were analyzed as means (standard deviations) for continuous variables and percentages for categorial variables.
Results
Overall Results
Thirty-seven (37) patients received the bone allografts for:
Maxillary sinus augmentation in 12 cases.
Alveolar ridge augmentation in 25 cases.
In total, 63 implants were inserted, 15 after sinus lift and 48 during ridge augmentation surgery. No complications were recorded during surgery. All the implants displayed primary stability. Radiologic results showed mean marginal bone height of 15.8 mm (range: 10 to 25 mm) postoperatively for the ridge augmentation group and mean 11.6 mm (range: 8 to 16 mm) postoperatively for maxillary sinus lift group. A total of 3 implants failed due to non-osteointegration and were removed: 1 implant failed at position 16, probably caused by too short healing time; 2 implants failed at positions 47 - 45 following huge inflammation around both implants due to undefined reasons.
Clinical Cases
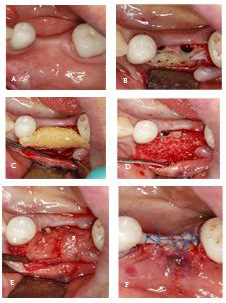
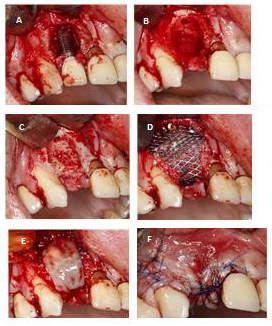
Figure 2:
A) Clinical situation before surgery.
B) Implantation 35,36, bone formation.
C) Cortico-cancellous bone BIO Bank mixed with PRF.
D) Graft in a proper position.
E) Covering the graft by PRF membrane.
F) Suturing.
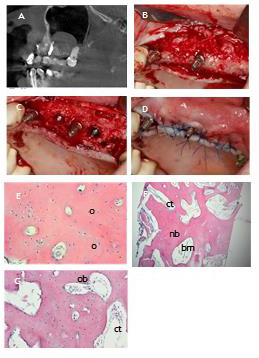
Case 1: A 57-year-old female patient presented at the Implantology and Dental Centre for the replacement of her teeth 35,36, lost a few years earlier due to caries. The CBCT (Galileos, Sirona) examination showed a defect in the mandibular process at the level of 35, 36 on the buccal side. The procedure was performed under local anesthesia with Ubistesin (3M) 4% forte. After preparation, the full-thickness flap, 2 implants 11.5 mm long and 4 mm in diameter were placed. Due to the slight exposure of the implant at position 36 and the thin bone plate from the atrium side, it was decided to improve the anatomical conditions by widening the process in this area using cortico-cancellous bone allograft. After perforation of the compact layer (cortical bone), the bone allograft mixed with PRF preparation was placed. Thanks to this, among others, a more stable form of transplant. The graft was then covered with 3 layers of PRF membrane (PRF fraction A) and sutured tightly with mattress and single sutures (Figure 2). Healing was uneventful. A follow-up CBCT examination was performed after 5 months. Full reconstruction of the graft was found, and the expected expansion of the alveolar bone was achieved. During the procedure of exposing the implants, an overgrowth of the newly formed bone tissue over the implants was evidenced (Figure 3).
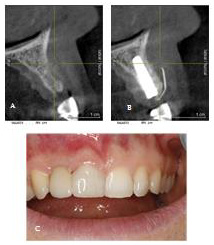
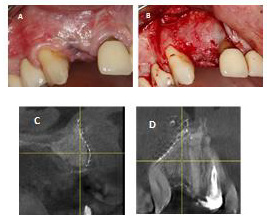
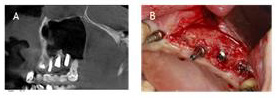
Case 2: A 47-year-old Woman presented at the Implantology and Dental Centre with the loss of tooth 12, due to an injury a few months earlier. Clinical examination revealed a defect in the maxillary bone at the level of tooth 12, especially on the labial side. The CBCT (Galileos, Sirona) examination was performed for a detailed analysis, which confirmed a significant bone loss. The CBCT examination in the sagittal plane shows the exposure of the implant, which does not threaten its good stabilization.The procedure was performed under local anesthesia with Ubistesin 4% forte (3M)/Articaini hydrochloridum 40 mg + Epinephrini hydrochloridum 0,012 mg/ml). After preparation of the full thickness flap, a Conelog (Camlog) implant 13 mm long and 3.3 mm in diameter was inserted in position 12 and tightened with a force of 35 Ncm. As predicted earlier, its exposure was found in the upper part. When making the implant hole, it was possible to partially preserve the bone plate in the paraventricular area. The bone defect with the exposed implant was covered with pellets of cortico-cancellous bone allograph, mixed with centrifuged blood in the form of PRF. The labial graft was secured with the iGen (Megagen) titanium membrane, which was stabilized by screwing to the inserted implant. The flap was then sewn with single and mattress sutures (Figure 4). After 2 weeks, the sutures were removed, and the wound was completely healed. After 5 months, the titanium membrane was removed and a control CBCT was performed, which showed complete remodeling of the graft. After another 6 weeks, the target prosthetic reconstruction was made of an all-ceramic crown (Figure 5). During the follow-up visits at 1 month, 3 months and every 6 months, the PD measurement was 0 - 0.5 mm. Three years after the procedure, a control CBCT of approx. 12/5.5 x 5cm/(Axeos, Sirona) confirmed the reconstruction of the graft. High resolution image obtained thanks to the limitation of the imaging field allowed to accurately visualize the structure of the newly formed bone. The patient has control visits every 6 months and regular hygienisation procedures.
Figure 4:
A. A, B, C, D) Before and during surgery-implants exposure.
B. E, F) Cortico-cancellous particles BIO Bank regeneration with titanium mesh.
Figure 5:
A) Initial Situation.
B) 5 months post op.
C) Full ceramic crown12.
Case 3: A 50-year-old man presented
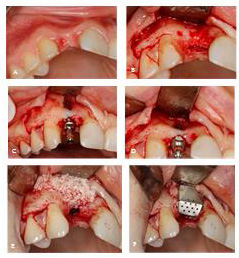
Case 3: A 50-year-old man presented to the Implantology and Dental Centre with inflammation around his implant in position 12 implanted 2 years earlier in another clinic. The patient confirmed that the inflammation occurred about 1 year after his surgery and was treated unsuccessfully by pocket cleaning and antibiotics. Our clinical examination followed by CBCT confirmed the diagnosis of large bone loss around implant 12 with pocket depth of 9-11 mm and instable implant (Figure 6A).
The Following Treatment Plan was Executed: Explantation of implant 12 followed by cleaning the bone from granulation tissue and regeneration with the cortico-cancellous bone allograft mixed with PRF and covered with titanium mesh (Figures 6B-6F). The Antibiotherapy was Amoxicillin 625mg 2 x 1 tab per day, 7 days. After 7 months, the titanium mesh was removed and the clinical and radiological examination showedvery good bone regeneration, adequate bone volume and quality (Figure 7A-7D).
Case 4: A 56-year-old female presented to the Implantology and Dental Centre with mobility and periodic pain in the jaw on the left side.The clinical examination confirmed the mobility of teeth 22,23,24,25,26, second and third degree, periodontal pockets, 4 - 9 mm deep, when probing the effusion of purulent blood. The CBCT confirmed extensive changes around the roots of the teeth 22,23,24,25,26 and 90% shading of the left maxillary sinus (Figure 8A).
Figure 6:
A) Bone loss around implant.
B) Situation after explanation.
C) Bone perforation.
D) Cortico-cancellous bone mixed with PRF and covered with titanium mesh.
E) Mesh covered with PRF membrane.
F) Suturing.
Figure 7:
A) Situation after 7 months. Small exposition of titanium mesh,
B) New bone 7 months after regeneration.
C) C and D) CBCT scans after 7 months.
Figure 8:
A) Chronic inflammation on left sinus lift,
B) Cortico-cancellous particles of BIO Bank graft generation.
C) 2 temporary implants 23,25 for temporary restoration, with the graft coveredy by titanium mesh.
D) PRF membrane in place for better wound healing.
E) Wound suturing.
F) Composite temporary restoration.
3-Stage Treatment Plan was Implemented: During the first stage, the bone defects were removed, the bone bed cleaned and filled with cortico-cancellous bone allograft mixed with PRF, and 2 temporary implants were placed to create a temporary bridge; the graft was secured with a titanium mesh stabilized with titanium pins, the mesh covered with a PRF membrane, and the wound sutured. After wound healing, a temporary bridge (Telio) was placed, attached to 2 temporary implants to support the lack of teeth 22-26 (Figures 8B-8F). Approximately 4 weeks after the surgery, the titanium mesh was slightly exposed, with visible proper epithelial tissue formation under the mesh. Therefore, only careful hygiene was recommended, rinsing with chlorhexidine / 0.2% Chlorhexidine.
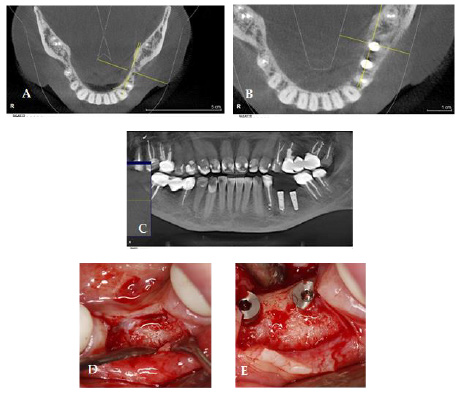
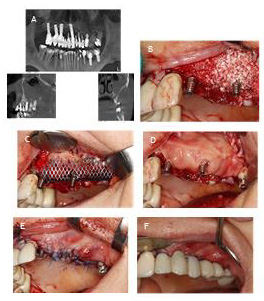
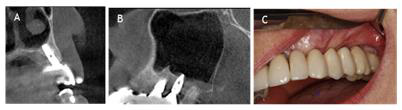
A control CBCT was performed 5.5 months after the procedure to confirm correct graft healing and the reduction of shading of the maxillary sinus by approx. 50% compared to the previous state. The second stage of treatment was then performed by removing the titanium mesh and inserting 3 implants at position 23,25,26 (Figures 9A-9D). Before placing the implants, bone fragments were collected for histological examination with a punch with a diameter of 2.5 mm, which contained both the primary alveolar bone and the newly formed bone resulting from the allograft regeneration procedure (Figures 9E-9G). Then the InKone Primo / Global D / implants were placed. Primary stabilization was achieved between 35 and 50 Ncm. Healing was uneventful. Temporary implants and the Telio bridge were allowed to fully heal. 5 months later, a control CBCT was performed to confirm complete resolution of the maxillary sinus inflammation. The radiologically correct healing of the implants and the preserved level of the regenerated bone tissue were also confirmed (Figures 10A & 10B). Implants exposure procedure performed. The Osstell measurement was 67 - 78 ISQ. Two weeks after the exposure, a prosthetic reconstruction was made of an all-ceramic zirconium bridge (Figure 11).
Figure 9:
A) A)Maxillary sinus 5 months after ridge regenarationwithout any penetration into the sinus.
B) Alveolar ridge 5 months after regeneration.
C) C,D) Implantation 23,25,26 and suturing.
D) E,F,G) Histology showing almost completely rebuilt allograft with only small particles of allograft visible. spaces filled by bone marrow and connective tissue. O:osteocusts; Ob:osteoblasts; nb:new born; ct:connective tissue; bm:bonemarrow.
Discussion
Bone allografts have been shown to be suitable alternative to autografts for bone regeneration during dental surgeries such as ridge preservation or maxillary sinus elevation. Most of the available bone allografts (Freeze dried bone allograft, demineralized freezedried bone allograft) are derived from cadaver bone treated using different methods such as physical debridement to remove soft tissue, ultrasonic washing to remove remnant cells and blood and the use of strong organic solvents for delipidation and viralinactivation [9].In this case series, the bone allografts used is exclusively derived from living donors’ femoral heads collected after hip replacement surgery and processed by supercritical CO2 extraction technology. This technology is often used in the pharmaceutical and food industries for the splitting, extraction, and decontamination of organic materials. The Supercrit® process is the combination of a degreasing step by supercritical CO2 and a gentle chemical oxidation of the residual proteins of the bone network. Preclinical studies, has demonstrated that this process applied to bone has neutral effects on the bone tissue composition, resulting in its architecture and mechanical properties preservation, particularly its high wettability, thus increases the performance [7,8,10].
The results of our histological examinations performed mainly after sinus lift procedures before planned implantations are particularly relevant. In these cases, the bone fragments collected with a punch (Trephine Ejection Kit by Prof. Dr Fouad Khoury) showed both the patient’s own bone (from the alveolar level) and the newly formed bone (sinus level). The results were particularly valuable because the sinus lift graft had a very limited contact with the patient’s own bone, and yet, histological examinations revealed up to 85% of new bone tissue produced within the graft. These results are consistent with the recently published data on this type of allograft [11,12].
Conclusion
The clinical, radiological, and histological results in our cases support the very high regenerative potential of the Supercritical CO2 processes allografts preparations. Although the bone allograft showed good histological result in terms of newly formed bone and residual graft material in the maxillary sinus elevation in our case series, longer term histological studies will be needed to understand better resorption modalities and times.
References
- Gomes KU, Carlini JL, Biron C, Rapoport A, Dedivitis RA (2008) Use of Allogeneic Bone Graft in Maxillary Reconstruction for Installation of Dental Implants. J. Oral Maxillofac. Surg 66: 2335-2338.
- Alessandro Viscioni, Maurizio Franco, Adolfo Paolin, Elisa Cogliati, Maura Callegari, et al. (2011) Effectiveness of fresh frozen and cryopreserved homologue iliac crest grafts used in sinus lifting: A comparative study. Cell Tissue Bank 12: 263-271.
- Xavier, S, Silva E, Kahn A, Chaushu L, Chaushu G (2015) Maxillary Sinus Grafting with Autograft Versus Fresh-Frozen Allograft: A Split-Mouth Evaluation of Bone Volume Dynamics. Int J Oral Maxillofac Implants 30: 1137-1142.
- J Fages, A Marty, C Delga, JS Condoret, D Combes, et al. (1994) Use of supercritical CO2 for bone delipidation. Biomaterials 15: 650-656.
- J Fages, B Poirier, Y Barbier, P Frayssinet, ML Joffret, et al. (1998) Viral inactivation of human bone tissue using supercritical fluid extraction. ASAIO J 44: 289-293.
- J Fages, B Poirier, Y Barbier, P Frayssinet, ML Joffret, et al. (1998) Bone allografts and supercritical processing: effects on osteointegration and viral safety. J Supercrit Fluids 13: 351-356.
- Frayssinet P, Rouquet N, Mathon D, Autefage A, Fages J (1998) Histological integration of allogeneic cancellous bone tissue treated by supercritical CO2 implanted in sheep bones. Biomaterials 19: 2247-2253.
- Mitton D, Rappeneau J, Bardonnet R (2005) Effect of a supercritical CO2 based treatment on mechanical properties of human cancellous bone. Eur J Orthop Surg Traumatol 15: 264-269.
- Eppley BL, Pietrzak WS, Blanton MW (2005) Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon, Journal of craniofacial surgery 16: 981-989.
- Vastel L, Masse C, Mesnil P, Crozier E, Padilla F, et al. (2009) Comparative ultrasound evaluation of human trabecular bone graft properties after treatment with different sterilization procedures. J Biomed Mater Res B Appl Biomater 90: 430-437
- Chalard JJ (2021) Supercritical CO2 Viral-Inactivated Allogenic Bone Graft in Maxillary Sinus Augmentation Procedures: 10-Year Retrospective Clinical and Radiographic Results. Int J Periodontics Restorative Dent 41: 433-441.
- Chalard JJ, Gregoire Edorh (2021) Long-Term Clinical and Radiographic Outcome of Extraction Socket Grafting with a Supercritical CO2 Viral-Inactivated Allogeneic Bone Graft. Biomed J Sci & Tech Res 35(5).

 Case Report
Case Report