ABSTRACT
Since the SARS-CoV-2 infection has been understood, many clinical trials have started to test the effect of several molecules on the virus. Cannabinoids (extracted from high-CBD Cannabis sativa) are expected to have an impact on inhibition of SARSCoV- 2 infection. There are more than 113 constituents, and the major compounds are tetrahydrocannabinol (THC < 0.1%), Cannabidiol (CBD), Flavonoids (Quercetin, Luteolin) and Terpenoids. In this study we demonstrated that 10μg/mL of high-CBD crude extracts significantly inhibit SARS-CoV-2 pseudo virus entry. However, in the current concentration of cannabinoid extracts used, we saw only minor modifications of the expression of genes implied in the SARS-CoV-2 viral entry. Then, results reveal that 10μg/mL of this high CBD crude extract of Cannabis sativa is the optimal concentration to avoid cell mortality during in vitro testing performed on A549 human pulmonary cells and Epi Oral tissues. As viral entry factors are also expressed in nasal and oral epithelium, those first results showing an effect of cannabinoid in the inhibition of ACE2 expression, encourage to try the use of different pharmaceutical forms of crude extract of cannabinoids (Nasal Spray, Mouthwash) as a local barrier of SARS-CoV-2 viral entry.
Keywords: Cannabis Extract; Cannabinoids; CBD; SARS-CoV-2
Abbreviations: SARS-CoV2: Severe Acute Respiratory Syndrome Coronavirus 2; CBD: Cannabinoid Cannabidiol; ACE2: Angiotensin Converting Enzyme 2; TMPRSS2: Transmembrane Protease Serine 2
Introduction
This study aims at assessing potential effects of Cannabinoid extracts on genes implicated in SARS-CoV-2 entry in the host cells, as suggested in Wang B. et. al. 2020 Preprints [1]. Infection of SARSCoV- 2 in human cells is essentially mediated by two host proteins: at first the angiotensin converting enzyme 2 (ACE2) serves as a receptor for the viral spike protein. Then the transmembrane protease serine 2 (TMPRSS2) cleaves the viral spike protein, allowing the fusion of the virus and host cell membranes, and the delivery of viral material inside the cell [2]. Decreasing the expression levels or activity of both ACE2 and TMPRSS2 proteins appears to be an attractive target for inhibiting SARS-CoV-2 infection [2]. Such viral entry factors are also expressed in nasal epithelium [3] and oral epithelium [4], thus, it has been suggested as an entry site for viral infection. Recent study suggests an effect of Cannabinoid in the inhibition of ACE2 expression [1]. The second part of this study aims at testing crude extract compounds for the inhibition of SARS-CoV-2 entry. Since the beginning of the SARSCoV- 2 mutation, the D614G mutation has appeared on the spike protein sequence and confers enhanced infectivity [4]. This new mutation stands for > 90% of current infections (www.nextstrain. org), the D614G mutant will be used in this experimental setup. Unfortunately, as this pandemic was evolving, we were not able to test on our model the last variant Omicron mutations.
Materials and Methods
Reagents and Cell Treatment
Cannabinoid extracts were provided by green brothers Switzerland and diluted fresh in dimethyl sulfoxide (DMSO) (Sigma Aldrich) by heating up to 50°C maximum 3 minutes. Final concentration of DMSO on cells was adjusted at 0.1%.
Culture and Treatment of Epi Oral Tissues
Epi Oral tissues (Mat Tek corporation) were handled and cultivated according to manufacturer instructions. Upon reception, Epi Oral tissues were treated with 40ng/mL tumor necrosis factor alpha (Sigma Aldrich) and 20ng/mL interferon gamma (Sigma Aldrich) as done previously [1]. 24 Hours later, cannabinoid extracts resuspended in DMSO were added in the culture media for an additional 24h. Finally, tissues were washed in phosphate buffered (Gibco) saline prior to RNA extraction.
RNA Extraction and Reverse Transcriptase Quantitative PCR
RNA extraction and cDNA synthesis were, respectively, performed using the Rneasy Mini Kit (Qiagen) and Takara Kit for cDNA synthesis primed with Oligo(dt) and Random Primers according to manufacturer’s instructions. SYBR Green reagent was used for Real-time PCR on the ABI Prism 7000 sequence detection system (Applied Biosystems) according to the manufacturer’s instructions. GAPDH was used as a housekeeping gene. The results were analyzed using the 2-ΔΔCt method. Primer sequences are listed in supplementary Table 1.
Testing Cannabinoid Extracts on ACE2 and TMPRSS2 Gene Expression
The protocol is depicted in Figure 1 in accordance with the conditions described previously [1] and Mat Tek’s Epi Oral tissue manufacturer instructions.
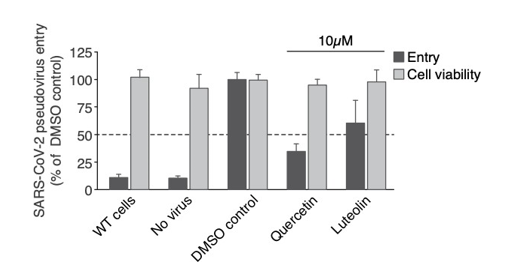
Figure 1: Viral entry inhibition test. A549 human lung alveolar basal epithelial cells overexpressing ACE2 were treated for 24h with the compounds prior to addition of pseudovirus expressing SARS-CoV-2 spike protein harboring the D614G mutation. After 6 hours the culture media was changed, and cells were incubated for 3 additional days prior to Luciferase and cell viability measurement. Viral entry was measured by the lentivirus-mediated Luciferase signal, normalised with WST8-cell viability. For all conditions the concentration of DMSO is equal to 0.1%. All conditions were performed in triplicate, error bars represent standard deviation.
SARS-CoV-2 Pseudolentivector Production
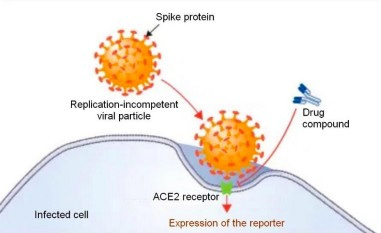
To avoid biosecurity constraints of P3 laboratory, pseudolentivectors expressing the SARS-CoV2 spike protein will be generated. these pseudolentivectors code for a reporter gene which is expressed by the infected cells (see image). Thus, upon inhibition of the viral entry, the reporter signal should decrease. Lentiviral vector stocks were generated using transient transfection of HEK 293T/17 cells with the self-inactivating vector CD511B coding for gaussia luciferase and GFP reporters, the psPAX2 plasmid encoding gag/pol, and the pCG1 - SARS-CoV-2 spike dCTer harboring the mutation D614G envelope plasmid, as described previously [5].
SARS-CoV-2 Viral Entry Assay and Cell Viability Measurements
A549 human pulmonary alveolar epithelial cells (Sigma Aldrich) stably overexpressing the human ACE2 receptor cells were routinely grown in DMEM (Gibco) with 10% foetal bovine serum (Gibco) and penicillin/streptomycin in a humidified 5% CO2 atmosphere. Cells were plated in 96-well plate tissue-culture treated (Corning) for 3h, then treated with Cannabinoid extracts. The next day the SARS-CoV-2 pseudolentivector was added in the culture media and incubated 6 hours at 37°C. Liquid is discarded, fresh culture media is added, and cells are kept at 37°C for an additional 72h. Finally, culture media is sampled and diluted at 1/6 in PBS containing 4uM Coelenterazine (Apollo Scientific) and luminescence is measured on the Paradigm reader (Molecular Devices). Cell viability is assessed with WST8 cell counting kit (Sigma Aldrich) according to manufacturer instructions, and absorbance is read on the Paradigm reader (Molecular Devices).
Protocol for Testing the Cytotoxicity of Crude Cannabinoid
A549-ACE2 cells were treated for a total of 30h with the crude extract to simulate the SARS-CoV-2 viral-entry. Treatment protocol: 24h pre-treatment + 6 hours of treatment in presence of the virus. Here no pseudo virus was used and only the cytotoxicity was measured.
Testing the Effect of Cannabinoid Extracts on SARS-CoV-2 Pseudovirus Entry in Buccal Swabs - Mouthwash
To test whether cannabinoid extracts may prevent the entry of SARS-CoV-2 via the buccal cavity, the following procedure was performed on healthy volunteers:
1. 1st buccal swab (before treatment)
2. Mouthwash containing crude extract at 10mg/mL for 1 minute
3. Mouthwash is spitted out
4. 30 minutes waiting
5. 2nd buccal swab (after treatment)
6. Cell plating
7. Two options:
a) Transduction with SARS-CoV-2 pseudo virus or
b) Treatment in vitro with crude extract at 10μg/mL (diluted from the mouth wash) for 1h, then transduction with SARSCoV- 2 pseudo virus
8. After 6h, removal of the culture media, fresh culture media is added on cells
9. After 96h luciferase signal is measured and cell viability assessed.
Testing the Effect of Cannabinoid Extracts on SARS-CoV-2 Pseudo virus Entry in Buccal Swabs - Spray & Vapotage
To test whether cannabinoid extracts may prevent the entry of SARS-CoV-2 via the buccal cavity, the procedure below was performed. 3 healthy volunteers were either treated with spray (emulsion 1% crude / 99% water) or “vapotage” (10% crude/ 90% MCT) as following:
1. 1st buccal swab (before treatment) 8:00, cell plating and pseudo-virus transduction.
2. Treatment every 2 hours from 8:00 to 22:00.
3. No treatment during the night.
4. The next day, treatment every 2 hours from 8:00 to 15:00.
5. 2nd buccal swab (after treatment), cell plating and pseudovirus transduction.
Results
The Effect of Cannabinoid Extracts on SARS-Pseudo Virus Entry
We have generated a A549 human lung alveolar basal epithelial cell line that constitutively overexpresses ACE2 to facilitate the entry of the SARS-CoV-2 spike pseudovirus. Both Quercetin and Luteolin exhibited inhibition of viral entry by 65% and 40%, respectively, at 10μM concentration with no significant cytotoxicity.
Testing the Cytotoxicity of a Cannabinoid Crude Extract. Determination of the Optimal Dilution for the Cannabinoid Extract
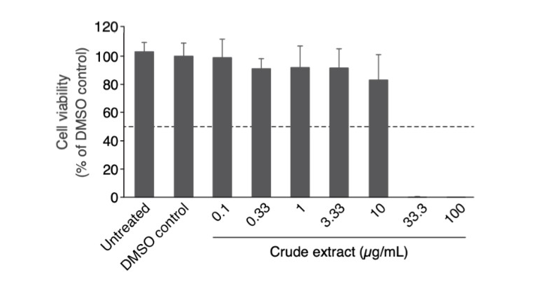
Due to the high cytotoxicity of the crude extracts on our cells, we perform a crude extract dose dependence on these A549-ACE2 cells to identify non-cytotoxic dose for further treatments. Cannabinoid crude extract did not show any toxicity at concentrations up to 10μg/mL (Figure 2). In contrast a high cytotoxicity was observed starting from 33.3μg/mL (Figure 2). Hence, we suggest using the highest non-cytotoxic concentration of 10μg/mL for further testing.
Figure 2: Cytotoxicity measurements. A549 human lung alveolar basal epithelial cells overexpressing ACE2 were treated for 30h with the crude extract received on 17th August 2020. Then the culture media was changed, and cells were incubated for 3 additional days prior to cell viability measurement with the WST8-test. Except for the “no treatment” condition, the concentration of DMSO is equal to 0.1%. All conditions were performed in triplicate, error bars represent standard deviation.
The Effect of Cannabinoid Extracts on ACE2 and TMPRSS2 Gene Expression
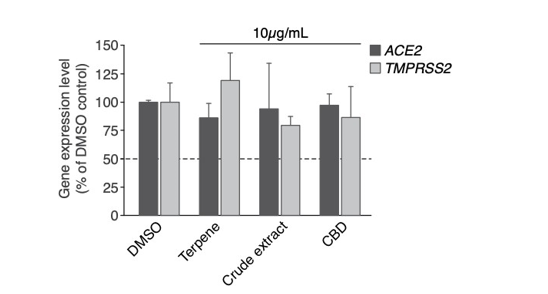
Gene expression analysis revealed no clear change in ACE2 gene expression, for all conditions: terpene, crude extract and CBD (Figure 3). Neither has any change been displayed in TMPRSS2 gene expression levels (Figure 3).
The Effect of Cannabinoid Extracts SARS-Pseudovirus Entry in Human Lung Alveolar Basal Epithelial Cells
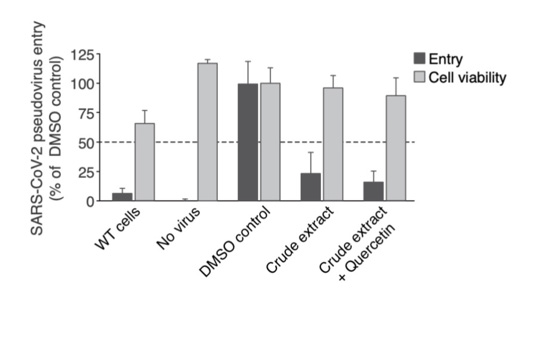
At 10μg/mL the crude extract inhibited the SARS-CoV-2 pseudo virus entry by 65% (Figure 4). Supplementation with 3.02μg Quercetin (equivalent to 10μM) did not significantly potentiate the phenotype (71% inhibition on Figure 4). Both conditions did not affect cell viability (Figure 4).
Discussion and Perspectives
As identified in Figure 3, Gene expression analysis revealed no clear change in ACE2 and TMPRSS2 gene expression for all terpene, crude extract and CBD conditions. We showed that 10μg/mL of high-CBD crude extracts (Figure 4) significantly inhibit SARS-CoV-2 pseudo virus entry which is not potentiated by the addition of 3.02 μg/mL Quercetin. We suppose that 10 μg/mL crude extracts display highly potent inhibition which may not allow us to observe additional effects by Quercetin. Different combinations of Quercetin may be performed with lower concentrations of crude extract in nasal spray or mouthwash to observe potential synergistic effects on inhibition of SARS-CoV-2 entry during future clinical trials on healthy volunteers (Figure 5).
Figure 3: ACE2 and TMPRSS2 gene expression analysis. Rt-qPCR displays gene expression levels as percentage of DMSO control. All conditions were performed in duplicate, normalised with GAPDH housekeeping gene. Error bars represent standard deviation.
Figure 4: Viral entry inhibition test. A549 human lung alveolar basal epithelial cells overexpressing ACE2 were treated for 24h with the crude extract (10μg/mL) and/or Quercetin (3.02μg/mL equivalent of 10μM) prior to addition of pseudo virus expressing SARS-CoV-2 spike protein harboring the D614G mutation. After 6 hours the culture media was changed, and cells were incubated for 3 additional days prior to Luciferase and cell viability measurement. Viral entry was measured by the lentivirus-mediated luciferase signal normalized with WST8-cell viability. For all conditions the concentration of DMSO is equal to 0.2%. All conditions were performed in triplicate, error bars represent standard deviation.
Importance
As a complement to vaccines, new therapies agents are needed to treat or prevent infections by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and its variants, which cause COVID-19. The inhibition of viral entry and thus spread is a plausible therapeutic avenue. SARS-CoV-2 uses receptor-mediated entry into a human host via the angiotensin-converting enzyme 2 (ACE2), which is expressed in lung tissue as well as the oral and nasal mucosa, kidney, intestines and gastrointestinal tract. Then the transmembrane protease serine 2 (TMPRSS2) cleaves the viral spike protein, allowing the fusion of the virus and host cell membranes, and the delivery of viral material inside the cell. Decreasing the expression levels or activity of both ACE2 and TMPRSS2 proteins appears to be an attractive target for inhibiting SARS-CoV-2 infection [2]. Such viral entry factors are also expressed in nasal epithelium [3], and oral epithelium [4], thus, it has been suggested as an entry site for viral infection. Recent study suggests an effect of Cannabinoid in the inhibition of ACE2 expression [1]. Cannabis sativa, especially those high in the anti-inflammatory cannabinoid cannabidiol (CBD), has been found to alter gene expression and inflammation and harbour anti-cancer and antiinflammatory [6]. Recent studies revealed that cannabinoids seem to have stable conformations with the binding pocket of the Mpro enzyme of SARS-CoV-2, which has a pivotal role in viral replication and transcription. They are found to be suppressive of viral entry and viral activation by downregulating the ACE2 receptor and TMPRSS2 enzymes in the host cellular system. The extracts of our most successful novel high-CBD C. sativa lines, pending further this investigation, may become a useful and safe addition to the prevention of COVID-19 as an adjunct therapy by simple gargling of the mouthwash of this extract on a daily basis.
Acknowledgment
Green Brothers (Switzerland) Neurix (Switzerland) University of Geneva.
References
- Xinchen Wang, Ryan Dhindsa, Gundula Povysil, Anthony Zoghbi, Joshua Motelow, et al. (2020) TMPRSS2 Transcriptional Inhibition as a Therapeutic Strategy for COVID-19. Preprints.
- Markus Hoffmann, Hannah Kleine-Weber, Simon Schroeder, Nadine Krüger, Tanja Herrler, et al. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181(2): 271-280.
- Waradon Sungnak, Ni Huang, Christophe Bécavin, Marijn Berg, Rachel Queen, et al. (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. NNat Med 26(5): 681-687.
- Lizhou Zhang, Cody B Jackson, Huihui Mou, Amrita Ojha, Erumbi S Rangarajan, et al. (2020) The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. BioRxiv.
- Marc Giry-Laterrière, Els Verhoeyen, Patrick Salmon (2011) Lentiviral vectors. Methods Mol Biol 737: 183-209.
- Richard B van Breemen, Ruth N Muchiri, Timothy A Bates, Jules B Weinstein, Hans C Leier, et al. (2022) Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J Nat Prod 85(1): 176-184.

 Research Article
Research Article