ABSTRACT
Objectives: It is known that ligand-dependent activation of Toll-like receptors (TLRs), which are constitutively expressed in mouse and rat Sertoli cells, leads to increased inflammatory cytokines via a common signaling pathway. This study aimed to investigate the role of TLRs signaling in microwave radiation-mediated up regulation of inflammatory cytokines in Sertoli cells.
Methods: Sertoli cells isolated from 3-week-old Wistar rats were radiated with 30 mW/cm2 or 100 mW/cm2 S-band microwave for 5 min. TLR2-5 and pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) expressions were detected by Real-time PCR, Western blot or ELISA, p-P38, p-ERK1/2, p-JNK and p-NF-κB p65 levels were assessed by Western blot. MAPK and NF-κB inhibitors were used to evaluate the roles of MAPK and NF-κB signaling pathways in microwave-induced inflammatory cytokines. Then, 100 m W/cm2 microwave radiated spermatogenic cells were co-cultured with normal Sertoli cells, DNase, RNase and HSP inhibitor were used to investigate whether endogenous TLR agonists were produced in the microwave-irradiated spermatogenic cells.
Results: microwave radiation treatment enhanced the levels of TLR2-5 and other key factors of TLRs signaling, IL-1β, IL-6, and TNF-α, p-P38, p-ERK1/2, p-JNK and p-NF-κB p65. In addition, pretreatment with MAPK inhibitors attenuated the effects of microwave radiation on IL-1β, IL-6 and TNF-α. Furthermore, DNase treatment of microwave-radiated spermatogenic cells co-cultured with Sertoli cells decreased the expression of IL-6 and TNF-α.
Conclusions: These results suggest that microwave radiation induced upregulation of inflammatory cytokines in Sertoli cells is triggered by endogenous TLR agonists released by damaged spermatogenic cells and mediated by TLRs-MAPKs signaling pathways.
Keywords: Microwave Radiation; Sertoli Cells; TLR; Endogenous Ligand
Introduction
Microwaves are electromagnetic rays with a frequency from 300 MHz to 300 GHz. Since their wide application in daily life and national defense, their adverse effects on various biological systems have been reported, especially on sensitive testis [1]. Studies have found that microwave radiation induces degeneration, apoptosis, and necrosis of spermatogenic cells and destroys spermatogenesis and male fertility [2,3], although the detailed mechanisms have not been completely understood. Pro-inflammatory cytokines as paracrine signals regulate spermatogenesis and steroidogenesis in Sertoli cells [4]. Under normal physiological conditions, these cytokines, such as TNF-α, IL-1, and IL-6, play important roles in testis development and function [5]. By contrast, under inflammatory conditions, their levels in Sertoli cells of the testis are increased, leading to changes in the immunosuppression microenvironment, apoptosis of spermatozoa [6-7], and peroxidation of lipid in the spermatozoal membrane [8], all of which will eventually lead to impaired spermatogenesis [4,9]. We have previously shown that microwave radiation, as an environmental stimulus, increases pro-inflammatory cytokines expression in rat Sertoli cells and disrupts spermatogenesis [10]. However, the underlying molecular mechanisms are still poorly understood. Cytokines production in Sertoli cells is closely related to Toll-like receptor (TLR) signaling [11,12]. TLRs are the specific pathogen-recognition receptors (PRRs) to sense pathogens, such as protozoa, bacteria, fungi and viruses [13-15].
Activation of TLRs triggers common intracellular signaling pathways and activates NF-kB and MAPKs (p38, JINK, ERK1/2), which further induces the production of pro-inflammatory cytokines such as IL-6, IL-1β, IL-12 and TNF-α [12]. However, excessive production of inflammatory factors could result in tissue damages due to TLRs over-activation [16]. Furthermore, studies have implied the presence of endogenous activators of TLRs. For example, molecules released from stressed or damaged cells can act as activators of TLRs in the absence of pathogens [17]. Therefore, although Sertoli cells are seldom reached by blood circulation in vivo and barely contact with exogenous pathogens owing to the bloodtestis barrier, there might be activators of TLRs around Sertoli cells. Apoptotic spermatogenic cells can trigger the expression of proinflammatory cytokines [18], and residual cytoplasmic components formed during sperm metamorphosis can provoke inflammation via TLR expressed in Sertoli cells [19], implying the presence of endogenous TLR agonists in the apoptotic spermatogenic cells and their residual cytoplasm. Microwave radiation increases the expression of TLRs. Studies have shown that low-level microwave radiation significantly increases TLR4 and MAPK levels in lymphocytes [20] while ultraviolet (UV) light stimulation increases NF-κB expression in a TLR2- and TLR4-dependent manner in Langerhans cells [21], indicating that TLRs regulate cell responses to microwave and UV light. TLRs are conservatively expressed in Sertoli cells from rodents, and their activation elevates cytokines expression and initiates testicular immune reactions [22-24]. Moreover, microwave radiation activates TLRs signaling in testis [25]. However, whether microwave radiation could activate TLRs signaling in Sertoli cells and subsequently elevate the level of proinflammatory cytokines and interrupt spermatogenesis is not known yet. Furthermore, microwave radiation is known to cause spermatogenic cell apoptosis.
Therefore, it is possible that the damaged spermatogenic cells could produce endogenous TLR agonists to provoke TLR, thereby promoting cytokine expression and strengthening/enlarging spermatogenic cell injury. In this study, we explored the effects of microwave radiation on the expression of TLR2-5, IL-1β, IL-6, and TNFα in Sertoli cells, the mechanisms underlying TLR-mediated pro-inflammatory cytokines expression, and the significances of endogenous TLR agonists released by the damaged spermatogenic cells to the TLR signaling in Sertoli cells. Our study further deepens our understanding of the mechanisms underlying microwave radiation-induced impairment of spermatogenesis and male fertility and provides insights into the preventive and therapeutic strategies for male infertility.
Materials and methods
Animals
Wistar rats from the Laboratory Animal Center of Beijing Institute of Radiation Medicine (Beijing, China) were used in the study. All animal experiments were performed following the Guidelines for the Care and Use of Laboratory Animals of the Chinese Council on Animal Care.
Isolation of Sertoli Cells
Wistar rats (3-week-old) were euthanized by cervical dislocation under anesthesia with CO2. Decapsulated testes were taken and digested twice with 0.5 mg ml-1 collagenase solution (Sigma, St. Louis, MO, USA) at room temperature for 15 min under gentle shaking. After eliminating interstitial and myoid cells, samples were further digested with 0.5 mg ml-1 hyaluronidase solution (Sigma) for 15 min with gentle shaking and pipetting. After that, cells were collected, washed with F12/DMEM (Dulbecco’s Modified Eagle Media, Gibco, Grand Island, NY, USA) three times, and cultured in F12/DMEM supplemented with sodium bicarbonate (1.2 mg ml-1), penicillin (100 U ml-1), streptomycin (100 mg ml-1), and 10% fetal calf serum (Gibco) at 32℃ for 48 h in an incubator with 5% CO2. The cells were then treated with a hypotonic solution (20 mM Tris, pH 7.4) for 1 min and repeatedly blew with a straw to remove the spermatogenic cells adhering to Sertoli cells. The purity of Sertoli cells was tested by immunostaining Wilms’ tumor nuclear protein 1 (WT1), a marker of Sertoli cells, using antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Sertoli cells with purity higher than 95% were used for microwave radiation treatment after cultured for 24 h and reseeded in plates or dishes.
Isolation of spermatogenic cells
Spermatogenic cells were isolated from 8-week-old Wistar rats. Briefly, rats were anesthetized with CO2 and euthanised by cervical dislocation. Testes were dec apsulated and incubated with 0.5 mg ml-1 collagenase solution at room temperature with a gentle shake for 15 min. Then, the seminiferous tubules were resuspended in 0.25% trypsin solution (Sigma) for 15 min and incubated with 0.5 mg ml-1 hyaluronidase (Sigma) for 5 min with gentle shaking and pipetting. The cells were washed with F12/DMEM to stop digestion and cultured in F12/DMEM containing 10% fetal bovine serum (FBS) with 5% CO2 at 32℃. After cultured for next 24 h, spermatogenic cells not attached to the culture dishes were gathered for other tests.
Microwave Radiation
Sertoli cells were divided into the sham group and the radiation group. The cells in the radiation group were exposed to the average power density of 30 mW/cm2 or 100 mW/cm2 S-band microwave radiation (S-MW) for 5 min according to the previous studies [26,27]. The cells in the sham group were treated similarly but apart from the microwave source. After exposure, Sertoli cells were gathered for subsequent experiments, including quantitative PCR, Western blot, and ELISA.
Co-Culture of Radiated Spermatogenic Cells with Normal Sertoli Cells
At 96 h of pos t-separation, Sertoli cells were treated with 0.25% trypsin and placed in six-well plates with 1 ×106 cells per well. The prepared normal spermatogenic cells were exposed to 100 mW/ cm2 S-MW microwave for 5 min and added into the normal Sertoli cells with 1 × 107 cells per well. After 24 h of co-culture, the Sertoli cells were gathered for ELISA analyses.
Quantitative Real-Time PCR (qRT-PCR)
After Sertoli cells were exposed to microwave radiation for 2 h, 6 h, 12 h, and 24 h, RNAs were isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. A total of 1 μg RNAs were reversed transcribed into cDNA using reverse transcriptase reaction mixture in a 20 μL system containing 2.5 μM random hexamers, 2 mM deoxynucleotide triphosphates, and 200 U AMV reverse transcriptase (Bio Basic Inc., Markham, ON, Canada). qRT-PCR analysis was performed in a 20 μL system containing 1 μL of cDNA, 0.5 μM forward and reverse primers, and 10 μL of 2 x Power SYBR Green PCR master mix (Applied Biosystems, CA, USA) on an ABI PRISM 7500 real-time cycler (Applied Biosystems) with a melting step at 50°C for 2 min and a hot start step at 95°C for 10 min, followed by 40 amplification cycles of 95°C for 15 s and 60°C for 1 min. The lev els of target genes were standardized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The qRT-PCR primers are shown in Table 1.
Western Blot Analysis
After 6 h and 24 h of microwave radiation treatment, Sertoli cells were lysed using the lysis buffer (BioDev-Technology, Beijing, China). Protein concentration was measured using the bicinchoninic acid protein assay kits (Pierce Biotechnology, Rockford, IL, USA). The same amounts of proteins from each sample were separated by SDS-PAGE and electro transferred onto polyvinyl difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% skim milk in Tris-buffered for 2 h, probed with rabbit antibodies against TLR2 (Bioss, Biotechnology Co, Beijing, China, bs -10472R, 1:4000), TLR3 (Abcam, Cambridge, UK, ab62566, 1:4000), TLR5 (Abcam, Cambridge, UK, ab13868, 1:1000), p-P38 (CST, Danvers, UK, 4511,1:500), p-JNK (CST, Danvers, UK, 4668, 1:500), p-ERK1/2 (CST, Danvers, UK, 4370, 1:2000), and p-NF- κB p65 (CST, 3033,1:500) as well as mouse antibodies against TLR4 (Abcam, Cambridge, UK, ab22048, 1:1000) and GAPDH (Immunoway Biotechnology Co, Newark, USA, YM3029, 1;20000) at 4°C overnight. After washed twice with Tris-buffered saline containing 0. 1% Tween-20, the membranes were incubated with proper peroxidase-conjugated secondary antibodies (Zhongshan Biotechnology Co, Beijing, China) at room temperature for 1 h. The signals were visualized using an enhanced chemiluminescence detection kit (Zhongshan), detected, and analyzed using cmiasimage analysis software (Image Center, Beihang University, Beijing, China).
ELISA
Sertoli cells were reseeded in 96-well plates with 1×106 cells in 100 ml per well and exposed to 30 m W/cm2 or 100 mW/cm2 microwave for 24 h. IL-1β, IL-6, and TNF-α levels in culture media were detected using ELISA kits (eBioscience, San Diego, CA) following the manufacturer’s instructions.
Roles of MAPK and NF-κB signaling pathways in microwaveinduced up-regulation of inflammatorycytokines
To investigate the roles of MAPK and NF-κB signaling pathways in microwave-induced up-regulation of inflammatory cytokines, Sertoli cells were treated with either control, 10 mMSB02190 (inhibitor of P38, Selleck Chemicals-Technology, TX, USA), 10 mM SP600125 (inhibitor of JNK, Selleck Chemicals-Technology, TX, USA), 10 mM GDC-0994 (inhibitor of ERK1/2, Selleck Chemicals- Technology, TX, USA), or 10 mM JSH-23 (inhibitor of NF-κB, Selleck Chemicals-Technology, TX, USA) for 2 h at 32°C prior to exposure to microwave as described above. At 24 h after exposure, IL-1β, IL-6, and TNF-α levels were detected using ELISA.
Roles of endogenous TLR ligands produced from microwave irradiated spermatogenic cells in upregulation of inflammatory cytokines in microwaveinduced Sertoli cells
To investigate whether endogenous TLR agonists were produced in the microwave irradiated spermatogenic cells, Sertoli cells were treated with PBS, 1 mM DNase (Tiangen Biotechnology Co, Beijing, China), 1 mM RNase (Solarbio Biotechnology Co, Beijing, China), and 10 mM KNK437 (HSP inhibitor, Selleck Chemicals-Technology, TX, USA) for 2 h at 32°C. Then, the cells were co-incubated with 100 mW/cm2 radiated spermatogenic cells. In addition, normal Sertoli cells cultured alone (N-Sertoli) as well as normal Sertoli cells cocultured with normal spermatogenic cells (C-N-GC-Sertoli) were used as the controls. At 24 h of co-culture, IL-1β, IL-6, and TNF-α contents in the media were detected using ELISA.
Statistical Analysis
All data were shown as mean ± standard deviation (SD) and analyzed using SPSS version 17.0 statistical software (SPSS Inc., Chicago, IL, USA). The normal distribution of data was examined using the Test for Equality of Variances. Differences between two groups were analyzed using Student’s t tests and among multiple groups were analyzed using One-way ANOVA. Differences with p < 0.05 were considered significant.
Results
Microwave Radiation Increases TLR2-5 Expression in Sertoli ells
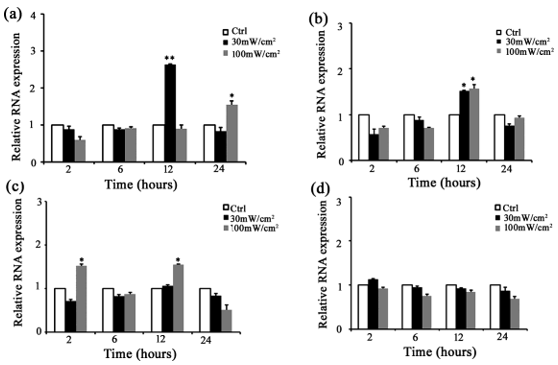
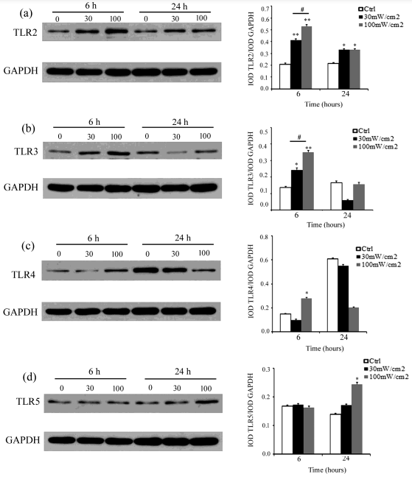
Several members of the TLR family are constitutively expressed in rat Sertoli cells. Among them, TLR2, TLR3, TLR4, and TLR5 are highly expressed. Our previous study has found that microwave radiation increases TLR2 -5 expression in rat testis [25]. Therefore, we further investigated whether microwave radiation could impact TLR2-5 in Sertoli cells using both qRT-PCR and Western blot analyses. For qRT-PCR, Sertoli cells were isolated from 3-week-old rats and radiated with a microwave of 30 mW/cm2 or 100 mW/cm2 for 5 min. The total RNAs were extracted from these Sertoli cells at 2, 6, 12, and 24 h of post-exposure. qRT-PCR found that TLR2 level was approximately 3-fold higher in Sertoli cells at 12 h of postexposure to 30 mW/cm2 microwave radiation and 1. 5-fold higher at 24 h of post-exposure to 100 mW/cm2 microwave radiation than that in control (Figure 1a). TLR3 level was approximately 1.5-2-fold higher in Sertoli cells at 12 h of post-exposure to 30 mW/cm2 and 100 mW/cm2 microwave radiation than in control (Figure 1b). TLR 4 level was more than1.5-fold higher in Sertoli cells at 2 and 12 h of post-exposure to 100 mW/cm2 microwave radiation than in control (Figure 1c). TLR5 level was slightly but not significantly higher in Sertoli cells at 2 h of post-exposure to 30 mW/cm2 microwave than in control (Figure 1d). For Western bolt analysis, total proteins were extracted at 6 h and 24 h of postexposure. The results showed that TLR2-5 protein levels were the highest in Sertoli cells at 6 or 24 h of post-exposure to 30 mW/cm2 and 100 mW/cm2 microwave radiation (Figure 2), and the increase was higher in Sertoli cells exposed to 100 mW/cm2 than to 30 mW/ cm2 microwave radiation (Figure 2). These data indicated that microwave radiation significantly increased TLR2-5 expression in Sertoli cells at both mRNA and protein levels.
Figure 1: Microwave radiation up-regulated TLR2-5 mRNA in rat Sert oli cells. Shown are results of qRT-PCR measuring the mRNA levels of TLR2 (a), TLR3 (b), TLR4 (c), and TLR5 (d) in rat Sertoli cells at 2, 6, 12 and 24 h of post microwave radiation. Data are expressed mean ± SD from three experiments. *P < 0.05 and **P < 0.01 vs. the control.
Figure 2: Microwave radiation up-regulated TLR2 -5 protein in rat Sertoli cells. Shown are results of Western blot analysis measuring the protein levels of TLR2
a) TLR3
b) TLR4
c) and TLR5
d) Relative to GAPDH in Sertoli cells at 6 and 24 h of post microwave radiation. Data are expressed as mean ± SD from three experiments. *P < 0.05 and **P < 0.01 vs. the control. # P <0 .05 between the indicated groups.
Microwave Radiation Enhances the Levels of Pro- Inflammatory Cytokines in Sertoli Cells
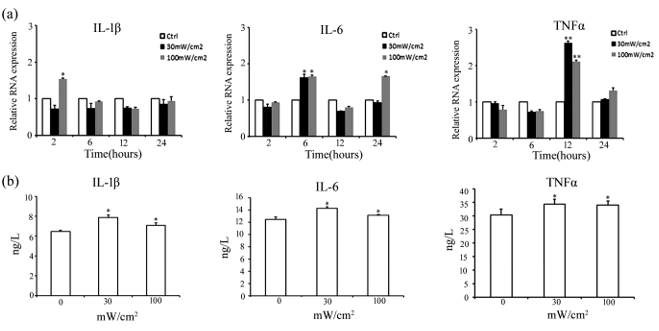
We then examined the effects of microwave radiation on the levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in Sertoli cells using both qRT-PCR and ELISA. qRT-PCR results showed that compared with the control, IL-1β level was 1.5-fold higher in Sertoli cells at 2 h of post-exposure to 100 m W/cm2 microwave radiation, IL-6 level was more than 1.5-fold higher at 6 h of post-exposure to 30 and 100 m W/cm2 microwave radiation and more than 1.5-fold higher at 24 h of post-exposure to 100 m W/ cm2 microwave radiation, and TNF-α level was more than 2-2.5-fold higher at 12 h of post-exposure to 30 and 100 mW/cm2 microwave radiation (Figure 3a). ELISA analysis of IL-1β, IL-6, and TNF-α secreted to media by Sertoli cells at 24 h of post-exposure to 30 m W/cm2 and 100 m W/cm2 microwave radiation showed that consistent with qRT-PCR results, the levels of IL-1β, IL-6, and TNF-α in media of Sertoli cells increased significantly after 30 mW/cm2 and 100 mW/cm2 radiation compared with the control, but showed no significant difference between cells exposed to 30 mW/c m2 and 100 mW/cm2 radiation (Figure 3b).
Figure 3: Microwave radiation up-regulated cytokines production in rat Sertoli cells.
a) The results of qRT-PCR analysis measuring IL-1β, IL-6, and TNF-α mRNA levels in Sertoli cells at 2, 6, 12, and 24 h of post microwave radiation.
b) The results of ELISA measuring cytokine levels in media of Sertoli cells at 24 h of post-e xposure to 30 mW/ cm2 and 100 mW/ cm2 microwave radiation. Data are expressed as mean ± SD from three e xperiments. *P < 0.05 and **P < 0.01 vs. the control.
Microwave Radiation Activates NF-κB and MAPK in Sertoli Cells
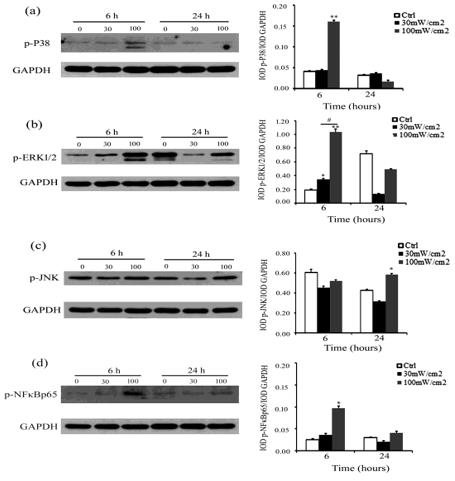
Mitogen-activated protein kinases (MAPK), including JNK, P38, and ERK, and nuclear factor-κB (NF-κB) are correlated to increased pro-inflammatory cytokines by Toll signaling [28]. To explore which Toll signaling pathway is involved in microwave radiation-induced up-regulation of pro-inflammatory cytokines in rat Sertoli cells, we analyzed the phosphorylation of MAPK and NF-κ B in Sertoli cells after exposure to 30 mW/cm2 or 100 mW/cm2 microwave radiation using Western blot analysis. As shown in Figure 4, p-p38 level was increased significantly in Sertoli cells at 6 h of post-exposure to 100 m W/cm2 microwave radiation (Figure 4a); p-ERK1/2 level was increased at 6 h of post-exposure to 30 mW/cm2 and 100 mW/ cm2 microwave radiation, and the increase was higher in cells exposed to 100 mW/cm2 than to 30 mW/cm2 microwave radiation (Figure 4b); p-JNK level was increased significantly at 24 h of post-exposure to 100 mW/cm2 microwave radiation (Figure 4c); p-NF-κB p65 level was up-regulated significantly at 6 h of postexposure to 100 m W/cm2 microwave radiation (Figure 4d). These data suggest that microwave radiation-induced production of proinflammatory cytokines in Sertoli cells could be mediated by MAPK and NF-κB signaling pathways.
Figure 4: Microwave radiation activated MAPK and NF-κ B in Sertoli cells. Shown are the results of Western blot analysis detecting the levels of activated MAPK (p-P38, p-ERK1/2, and p-JNK) and p-NF-κB p65 proteins in Sertoli cells at 6 and 24 h of post-exposure to 30 m W/ cm2 and 100 mW/ cm2 microwave radiation as well as the density of MAPK and NF-κB p65 relative to GAPDH. Data are expressed as mean ± SD from three independent experiments. *P < 0.05 and **P < 0.01 vs. the control. #P <0.05 between the indicated groups.
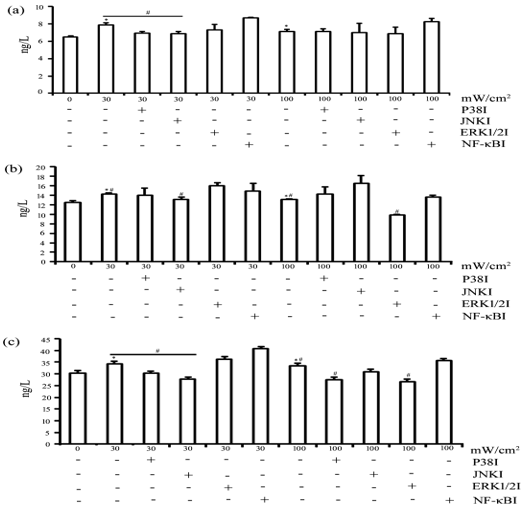
Microwave-Mediated Upregulation of Pro-Inflammatory Cytokines Mainly through MAPK Signaling in Sertoli Cells
To further investigate the roles of MAPK and NF-κB signaling pathways in microwave-induced increase of inflammatory cytokines, Sertoli cells were pretreated 10 mM SB02190, a P38 inhibitor, 10 mM SP600125, a JNK inhibitor, 10 mM GDC-0994, a ERK1/2 inhibitor, or 10 mM JSH-23, a NF-κ B inhibitor, for 2 h prior to exposure to 30 mW/cm2 and 100 mW/cm2 microwave radiation. At 24 h of post-exposure, the contents of IL-1β, IL-6 and TNF-α in the media of Sertoli cells were analyzed using ELISA. The results showed that IL-1β level was significantly increased after exposure to 30 mW/cm2 and 100 mW/cm2 microwave radiation, and the increase after exposure to 30 mW/cm2, but not 100 mW/cm2, microwave radiation was significantly attenuated by pre-treatment with P38 and JNK inhibitors (Figure 5a). IL-6 level was significantly increased after exposure to 30 mW/cm2 and 100 mW/cm2 microwave radiation, while the increase after exposure to 30 mW/cm2, but not 100 m W/cm2, microwave radiation was significantly attenuated by pretreatment with JNK inhibitors, and the increase after exposure to 100 mW/cm2 microwave radiation was significantly attenuated by pretreatment with ERK1/2 inhibitor (Figure 5b). TNF-α level was also augmented after exposure to 30 mW/cm2 and 100 mW/ cm2 microwave radiation, but these increases were attenuated by pretreatment with P38 and JNK inhibitors. In addition, the increase in TNF-α level after exposure to 100 mW/cm2 microwave radiation was significantly attenuated by pretreatment with the ERK1/2 inhibitor (Figure 5c). These findings indicated that MAPK signaling might be the major pathway mediating microwave-induced upregulation of pro-inflammatory cytokines.
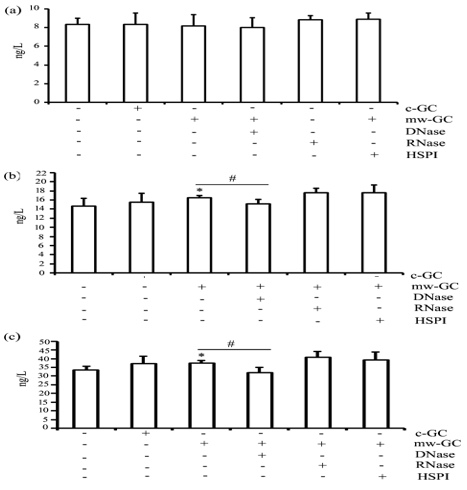
Endogenous TLR agonists released by microwaveradiated spermatogenic cells trigger pro-inflammatory cytokines expression in Sertoli cells It is well known that microwave radiation can result in apoptosis of spermatogenic cells
To investigate the presence of endogenous TLR agonists in microwave irradiated spermatogenic cells, Sertoli cells were treated with either PBS, 1 mM DNase,1 mM RNase, or 10 mM KNK437, a HSP inhibitor, for 2h, and co-cultured with either normal or 100 mW/cm2 microwave-radiated spermatogenic cells. After 24 h of co -culture, the levels of IL-1β, IL-6, and TNF-α in culture media were examined using ELISA. As shown in Fig. 6, IL-1β level was not significantly different among different groups (Figure 6a). By contras t, IL-6 and TNF-α concentrations increased significantly in the media of normal Sertoli cells co-cultured with microwave-radiated spermatogenic cells, decreased significantly in DNase-treated Sertoli cells co-cultured with microwaveradiated spermatogenic cells, and did not change in RNase and HSP inhibitor-treated Sertoli cells co-cultured with microwave-exposed spermatogenic cells (Figures 6b & 6c). These results indicated that endogenous DNA-analogue TLR agonists might be released by microwave-radiated spermatogenic cells and trigger the expression of pro-inflammatory cytokines in Sertoli cells.
Figure 5: MAPK inhibitors attenuated microwave radiation -induced cytokines expression in rat Sertoli cells. Shown are the results of ELISA measuring IL-1β (A), IL-6 (B), and TNF-α (C) levels in media of Sertoli cells at 24 h of post-exposure to 30 mW/ cm2 and 100 mW/ cm2 microwave radiation after pretreatment at 32°C for 2 h with MAPK inhibitors or NF-κB inhibitor, as indicated. All data are expressed as mean ± SD from three independent experiments. *P < 0.05 vs. the control group. # P <0 .05 between the indicated groups.
Figure 6: Analysis of endogenous TLR agonists in microwave-damaged spermatogenic cells. Shown are results of ELISA measuring the levels of IL-1β (A), IL-6 (B), and TNF-α (C) in the media of Sertoli cells pretreated with control, DNase, RNase, or HSP inhibitor for 2 h at 32°C and co-incubated with normal or 100 mW/cm2 radiated spermatogenic cells, as indicated. Data are expressed as mean ± SD from three independent experiments. *P<0.05 compared with normal Sertoli cells co-incubated with normal or 100 mW/cm2 radiated spermatogenic cells. # P <0.05 between the indicated groups.
Discussion
Microwave radiation can damage both spermatogenic cells and sperms and may eventually impair spermatogenesis and male fertility. Studies on the mechanisms underlying microwave radiation-induced impairment of spermatogenesis are mostly concentrated on spermatogenic cells rather than Sertoli cells. We have previously shown that microwave radiation increases the levels of pro-inflammatory cytokines in rat Sertoli cells and impairs spermatogenesis [10]. Recently, we showed that microwave radiation activates TLRs signaling in testis [25]. Members of the TLR family are constitutively expressed in mouse and rat Sertoli cells [22,23]. Particularly, TLR2-5 are highly expressed. Therefore, in the present study, we further explored whether microwave radiation could activate TLR signaling in Sertoli cells and the mechanisms underlying TLR-mediated pro-inflammatory cytokines expression with focuses on NF-kB and MAPKs (p38, JINK, ERK1/2) as well as whether microwave radiation could induce endogenous TLR ligands release from spermatogenic cells and subsequently elevate pro-inflammatory cytokines expression in Sertoli cells. To address these issues, the expression profile of TLRs in Sertoli cells after microwave radiation was investigated using qRT-PCR and Wes tern blot. As expected, TLR2-4 mRNA levels increased at different degrees at 2 h and/or 12 h of post-exposure to 30 mW/cm2 or 100 mW/cm2 microwave radiation, and TLR2-5 protein levels increased at 6 h and/or 24 h of post-exposure to 30 mW/cm2 and 100 mW/ cm2 microwave radiation. Moreover, the effects of microwave radiation on TLR2 and TLR3 levels were dose dependent. These results suggest that microwave radiation activates TLRs in Sertoli cells. In agreement with TLR2-5 activation, 30 m W/cm2 and 100 mW/cm2 microwave radiation also elevated IL-1β, IL-6, and TNF-α at both mRNA and protein levels.
Among them, TNF-α concentration increased the most, in agreement with other reports showing the strongest adverse effect on spermatogenic cells [4,29]. These results further verify that microwave radiation activates TLRs and elevates inflammatory cytokines in Sertoli cells. TLR-mediated inflammatory cytokines signaling is classified as MyD88- dependent and TRIF-dependent pathways [12,30]. Of these two, Myd88-dependent is more important for inducing inflammatory cytokines expression. By binding to the TIR domain of all TLRs, MyD88 activates AP-1 transcription factors via a series of signal transductions, including MAP kinases (p38, JNK, and ERK1/2) and nuclear translocation of transcription factor NF-κB (NF-Kb) to induce the production of TNF-α, IL-12, IL-1β, and IL-6 [12]. In addition, activation of TLR3 and TLR4 induce IFN-β via TRIF, a TIR domain-containing adaptor, which further enhances the production of either TNF-α or IL-1β via activating NF-κB, or IFN-α and IFN-β via activating IRF3 [30].
In the present study, we examined activation of p-p38, p-ERK1/2, p-JNK, and p-NF-κB p65 using Western blot analysis. We demonstrated that microwave exposure increased the levels of p-p38, p-ERK1/2, p-JNK, and p-NF-κB p65 in a dose-dependent manner, confirming that microwave radiation indeed activates MyD88-dependent signaling pathways in Sertoli cells. Further pre-treatment of Sertoli cells with MAPK inhibitors SB02190, SP600125, and GDC-0994 as well as NF-κB inhibitor JSH-23 attenuated microwave radiation-induced production of IL- 1β, IL-6, and TNF-α, indicating that microwave radiation-mediated upregulation of pro-inflammatory cytokines in Sertoli cells is mainly regulated by MAPK signaling pathway. Many endogenous ligands of TLRs are host-cell original [31,32]. These endogenous molecules may be expressed or released in response to tissue damage [33]. To date, more than 20 endogenous TLR ligands have been proposed and classified as released intracellular proteins, extracellular matrix (ECM) components, oxidatively modified lipids, dsRNA, chromosomal DNA, and HSPs [34]. Endogenous TLR ligands from apoptotic or necrotic cells may induce inflammatory responses without microbial challenge via different TLRs [17,33]. Apoptotic cells can activate TLRs of macrophage and microglia and stimulate inflammation [17]. Sertoli cells are the only somatic cells within semini ferous tubules, which is similar to the macrophage and microglia with immunocompetence. Likewise, Sertoli cells seldom reach blood circulation and barely contact exogenous pathogens due to the blood-testis barrier. Therefore, it is worth of exploring the sources of endogenous agonists of TLRs in Sertoli cells. Studies have shown that apoptotic spermatogenic cells and residual cytoplasm are the endogenous TLR agonists [18,19].
In this study, we found that microwave-radiated spermatogenic cells co-cultured with Sertoli cells could release TLR agonists to activate TLRs in Sertoli cells, thereby elevating the levels of pro-inflammatory cytokines IL-6 and TNF-α. More importantly, the elevation of pro-inflammatory cytokines is blocked by DNase treatment, implicating that DNA analog might be the endogenous TLR agonists released from the microwave-radiated spermatogenic cells to trigger the production of pro-inflammatory cytokines in Sertoli cells. Previous studies have shown that endogenous molecules, intracellular proteins, ECM components, oxidatively modified lipids, and HSPs could stimulate TLR2 or TLR4 signaling [35,36], dsRNA could activate TLR3 signaling [17], while DNA-containing immune complexes mainly stimulate TLR9 or TLR7 [37, 38]. TLR7 and TLR9 are abundantly expressed in rat testis [23]. Therefore, it is worthy of exploring which TLR is the receptor mediating endogenous ligands signaling and the mechanisms underlying the release of endogenous TLR ligands by damaged spermatogenic cells to trigger inflammation signaling in Sertoli cells.
In conclusion, our study revealed that microwave radiation up-regulated TLR2-5 expression and increased pro-inflammation cytokines production in Sertoli cells via MAPKs signaling pathway, and small DNA molecules released from the microwave-radiated spermatogenic cells could function as the endogenous ligands to trigger inflammation signaling in Sertoli cells. These results deepen our understanding of the mechanisms underlying microwave radiation-induced impairment of spermatogenesis and provide novel clews to develop preventive and therapeutic strategies for the treatment of abnormal spermatogenesis and male infertility caused by microwave radiation.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No.81302397).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Chauhan P, Verma HN, Sisodia R, Kesari KK (2017) Microwave radiation (2.45GHz)-induced oxidative stress: Whole-body exposure effect on histopathology of Wistar rats. Electromagn Biol Med 36(1): 20-30.
- Chen HY, Wang SM, Peng RY, Gao YB, Wang LF, et al. (2011) Long-term microwave radiation affects male reproduction in rats. Zhonghua Nan Ke Xue 17(3): 214-218.
- Wang SM, Peng RY, Gao YB, Ma JJ, Chen HY, et al. (2006) Pathological study of testicular injury induced by high power microwave radiation in rats. Zhonghua Nan Ke Xue 12(6): 486-489.
- Guazzone VA, Jacobo P, Theas MS, Lustig L (2009) Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Tech 72(8): 620-628.
- Siu MK, Lee WM, Cheng CY (2003) The interplay of collagen IV, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology 144(1): 371-387.
- Suescun MO, Rival C, Theas MS, Calandra RS, Lustig L (2003) Involvement of Tumor Necrosis Factor-a in the Pathogenesis of Autoimmune Orchitis in Rats. BOR 68(6): 2114-2121.
- Pelleti er RM, Yoon SR, Akpovi CD, Silvas E, Vitale ML (2009) Defects in the regulatory clearanc e mechanisms favor the breakdown of self-tolerance during spontaneous autoimmune orchitis. Am J Physiol Regul Integr Comp Physiol 296(3): 743-762.
- Allam JP, Fronhoffs F, Fathy A, Novak N, Oltermann I, et al. (2008) High percentage of apoptotic spermatozoa in ejaculates from men with chronic genital trac t inflammation. Andrologia 40(5): 329-334.
- Lysiak JJ (2004) The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol 2: 9.
- Wu H, Wang D, Shu Z, Zhou H, Zuo H, et al. (2012) Cytokines produced by microwave-radiated Sertoli cells interfere with spermatogenesis in rat testis. Andrologia 44(1): 590-599.
- Li YP, Huang J, Huang SG, Xu YG, Xu YY, et al. (2014) The compromised inflammatory response to bacterial components after pediatric cardiac surgery is associated with cardiopulmonary bypass-suppressed Toll-like receptor signal trans duction pathways. J Crit Care 29(2): 312.e7-13.
- Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S (2007) Toll-like receptor signal transduction. Exp Mol Med 39(4): 421-438.
- Wu MH, Zhang P, Huang X (2010) Toll-like receptors in innate immunity and infectious diseases. Front Med China 4(4): 385-393.
- Akhm atova NK, Egorova NB, Kurbatova EA, Akhmatov EA (2014) Activation of innate immunity by bacterial ligands of toll-like receptors. Front Immunol 5: 89.
- Lester SN, Li K (2014) Toll-like receptors in antiviral innate immunity. J Mol Biol 426(6): 1246-1264.
- Sun B, Qi N, Shang T, Wu H, Deng T, et al. (2010) Sertoli cell-initiated testicular innate immune response through toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology 151(6): 2886-2897.
- Kariko K, Weissman D, Welsh FA (2004) Inhibition of toll-like receptor and cytokine signaling—a unifying theme in ischemic J Cereb Blood Flow Metab 24(11): 1288-1304.
- Theas MS (2018) Germ cell apoptosis and survival in testicular inflammation. Andrologia 50(11): e13083.
- Hedger MP (2011) Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation--a perspective. J Reprod Immunol 88(2): 130-141.
- Novoselova EG, Khrenov MO, Cherenkov DA, Glushkova OV, Novoselova TV, et al. (2008) The role of TLR4 receptor in the stress response of lymphocytes. Biofizika 53(3): 457-461.
- Wang X, Bi Z, Wang Y, Wang Y (2011) Increased MAPK and NF-kappaB expression of Langerhans cells is dependent on TLR2 and TLR4, and increased IRF-3 expression is partially dependent on TLR4 following UV exposure. Mol Med Rep 4(3): 541-546.
- Wu H, Wang H, Xiong W, Chen S, Tang H, et al. (2008) Expression patterns and functions of toll-like receptors in mouse sertoli cells. Endocrinology 149(9): 4402-4412.
- Palladino MA, Johnson TA, Gupta R, Chapman JL, Ojha P (2007) Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod 76(6): 958-964.
- Starace D, Galli R, Paone A, De Cesaris P, Filippini A, et al. (2008) Toll-like receptor 3 activation induces antiviral immune responses in mouse sertoli cells. Biol Reprod 79(4): 766-775.
- Wu H, Wang D, Meng Y, Ning H, Liu X, et al. (2018) Activation of TLR signalling regulates microwave radiation-mediated impairment of spermatogenesis in rat testis. Andrologia 50(1): e12828.
- Zuo H, Wang D, Peng R, Wang S, Gao Y, et al. (2009) Effects of electromagnetic radiation on Raf/MEK/ERK sig-naling pathway in rats hippocampus. Chin J Appl Physiol 25(2): 186-189.
- Yao H, Wang D, Zhu M, Peng R, Wang S, et al. (2010) Comparison of the damaging effect of three bands elec trom agnetic radiation on rat testis. Acta Labrat Ani Sci Sinica 18: 467-470.
- Kawai T, Akira S (2006) TLR signaling. Cell Death Differ 13(5): 816-825.
- Lysiak JJ (2004) The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol 2: 9.
- O'Neill LA, Bowie AG (2007) The family of five: TIR-dom ain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7(5): 353-364.
- Sloane JA, Blitz D, Margolin Z, Vartanian T (2010) A clear and present danger: endogenous ligands of Toll-like receptors. Neuromolecular Med 12(2): 149-163.
- Tsan MF, Gao B (2007) Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res 13(1): 6-14.
- Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81(1): 1-5.
- Erridge C (2010) Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol 87(6): 989-999.
- Abdollahi-Roods az S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, et al. (2008) Stim ulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Inves 118(1): 205-216.
- Tsan MF, Gao B (2004) Endogenous ligands of Toll-like receptors. J Leukoc Biol 76(3): 514-519.
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, et al. (2007) Toll-like receptor 9-dependent activation by DNA-contai ning immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8(5): 487-496.
- Moody KL, Uccellini MB, Avalos AM, Marshak-Rothstein A, Viglianti GA (2016) Toll-Like Receptor-Dependent Immune Complex Activation of B Cells and Dendritic Cells. Methods Mol Biol 1390: 249-272.

 Research Article
Research Article