ABSTRACT
After central nervous system (CNS) injury, the body loses its ability to move at different levels depending on the extension of the lesion. Monoaminergic neurotransmitters play a determining role in animal movement and neuronal regeneration, suggesting they may have a role in motor function recovery after central injury. This study aimed to determine the role of monoaminergic neurotransmitters in the recovery percentage of swimming and ambulation after CNS lesion. The study tested the role of monoaminergic neurotransmitter depletion with reserpine on the recovery percentage of swimming and ambulation behaviors following obturation of the CNS in the Mexican leech, Haementeria officinalis. After neurotransmitter depletion, in one group serotonin was replaced to evaluate its role. Every experimental assay included: control, reserpine, lesion, reserpine-lesion, and reserpine-lesionserotonin. In each group of 6 organisms, the recovery percentage of animals was determined. A total of seven assays were performed and reported as means±SE. After lesion, almost all of the injured animals lost swimming and ambulation behaviors. Seven days later, 68.42±11.11% (n=7) of the groups recovered their swimming ability, but with reserpine, only 4.71±4.71% (n=7, p=0.0002) and 94.28±5.71% recovered their ambulatory ability, but with reserpine, recuperated only 50.86±8.74% (n=7, p=0.0024). The exclusive injection of reserpine had no effect on these movement patterns. The acute administration of serotonin did not promote the recovery, 14.6±9% (n=5) and 55.8±10.95% (n=5) respectively. Monoaminergic neurotransmitters promoted the recovery percentage in swimming and ambulation abilities after CNS lesion. The restored serotonin did not contribute to the recovery percentage.
Keywords: Monoaminergic Neurotransmitter; Serotonin; Central Nervous System Lesion; Reserpine; Motor Recovery; Haementeria Officinalis Leech
Abbreviations: MN: Monoaminergic Neurotransmitter; CNS: Central Nervous System; 5HT: Serotonin; MA: Monoamine
Introduction
One of the great problems in neuroscience is the fact that central nervous system (CNS) axons are unable to regenerate and successfully reconnect with their targets in superior vertebrates. This failure contrasts with the regeneration abilities in the peripheral nervous system and among inferior vertebrates and invertebrates [1]. However, using peripheral nerve bridges, David and Aguayo [2] demonstrated the regenerative potential of CNS neurons, which is dependent on their environment. This problem of regenerative failure is also of clinical interest due to them any individuals harboring CNS injuries that in many cases, lead to various motor and/or cognitive disabilities. In the field of motor and cognitive rehabilitation, several therapies have been developed to strengthen intact connections and promote the reconnection of injured axons. However, conduct recovery still depends on neuronal regeneration and the successful reconnection of neurons with their targets. Despite the biological and clinical interest of neuronal regeneration and the functional recovery of compromised abilities due to CNS injury, the problem remains to be solved. This issue is complex, multifactorial, and neither its determining elements nor their degree of involvement have been determined. This study focused on the role of monoaminergic neurotransmitters since these molecules are highly involved in the physiological processes of animal mobility and have several effects on neuritogenesis.
Monoaminergic neurotransmitters play an important role in neuritic regeneration. Serotonin acts on neuritic axonal growth, it guides the growth cones, and participates in synaptogenesis [3,4] and many receptors may mediate its mechanism of action [5]. Studies on the somatic secretion of serotonin have posited that this neurotransmitter acts on processes that occur away from the synapsis [6,7] and may participate in neuritogenesis. Further, dopamine is one of the factors regulating neuritogenesis [8-10] and neurogenesis in the mammalian adult brain, including humans [11,12]. Likewise, octopamine (OA) is present in invertebrates such as annelids, mollusks, and arthropods, acting on neuronal and behavioral plasticity. OA has been reported to participate in neuritogenesis [13]. OA is structurally and functionally similar to adrenergic transmitters which is present in vertebrates but not in invertebrates [14,15]. All have been recognized as playing a welldescribed role in animal movement regulation [16]. Their depletion with non-specific drugs such as reserpine, or specific to each neurotransmitter, modifies different features of motor behaviors in animals [17]. An increase in blood serum serotonin leads to behavioral changes in animals in terms of mobility [18] such as aggressiveness in vertebrates and invertebrates. Dopamine is key to movement [19,20] as reflected in Parkinson´s disease, a disease compromising movement due to the degeneration of dopaminergic neurons, and that leads to a dopamine deficit in several areas of the brain [21]. Likewise, octopamine plays a role in the movement and behavior of animals [14,15,22].
All of the above underscore the importance of monoaminergic neurotransmitters in animal motor functions and in neuritic regeneration, suggesting that monoamines overall or independently, may play a relevant role in motor recovery after CNS injury. In the study of neuronal regeneration and functional recovery, invertebrate animals have been used due to their spontaneous neuronal regeneration ability and their recovery of motor function following CNS injury. After CNS injury in the leech, this animal spontaneously recovers motor function [23]. Many behaviors such as swimming, flexion, shortening, drag, feeding, and heartbeat have been described as well as the neuronal circuits associated with these neurotransmitters [17,24]. The addition of serotonin to their environmental water, triggers swimming in animals depleted of this neurotransmitter after 5, 7-dihydroxytryptamine administration [25]. Likewise, in culture, adding serotonin to specific leech neuron cultures induces neuritic regeneration, as in AL1 cells that increase their number of primary neurites, their length, and their ramification; however, in other cells such as Retzius cells, serotonin has an inhibitory effect [26].
The study´s aim was to determine the role of monoaminergic neurotransmitters on the percentage of animals that recovered their swimming and ambulatory functions after CNS injury. The CNS cord was injured, and monoamine depletion was tested with intra-corporeal reserpine injection in the Mexican leech, Haementeria officinalis; this leech, as Hirudo medicinalis, the European medicinal leech, spontaneously recovers motor function after induced injury. Behavioral recovery was evaluated, and the recovery percentage was quantified. Serotonin was also replaced to establish the degree of its effects. In this work we find that these neurotransmitters promote the recovery percentage in swimming and ambulation abilities after CNS lesion. Acutely administered serotonin did not contribute to the recovery percentage.
Material and Methods
Maintenance of the Organisms
Invertebrate organisms of the Phylum Annelida were used: Haementeria officinalis is a Mexican leech from our Neuronal Regeneration Laboratory (NRL) animal colony. The procurement and maintenance of Haementeria officinalis samples has been previously described [26]. These organisms were originally collected in the lakes and dams of the central Mexican plateau by a researcher authorized by the local “Direction of Agricultural Development”. The organisms weighed between 0.5 and 0.8g and were placed in individual 1L containers with water (E-pura, Mexico), labeled, and maintained at laboratory temperatures and conditions. All procedures were approved by the Institutional Committee for the Care and Use of Laboratory Animals (INRCICUAL No. 15/11).
Study Groups and Experiment Series
Seven experimental or repetition series were performed. At each repetition, 5 study groups were created (control, injury, reserpine, injury-reserpine, injury-reserpine-serotonin) with 6 organisms each, to determine the recovery percentage in each group. The “control” animals were neither treated nor injured, the CNS was injured in the “injury” group, in the “reserpine” group, the drug was injected intracorporeally, the drug was injected and the CNS injured in the “reserpine-injury” group, and in the “reserpineinjury- serotonin” group, the animals were injected, their CNS was injured, and serotonin was replaced.
Swimming and Ambulation Motor Behaviors
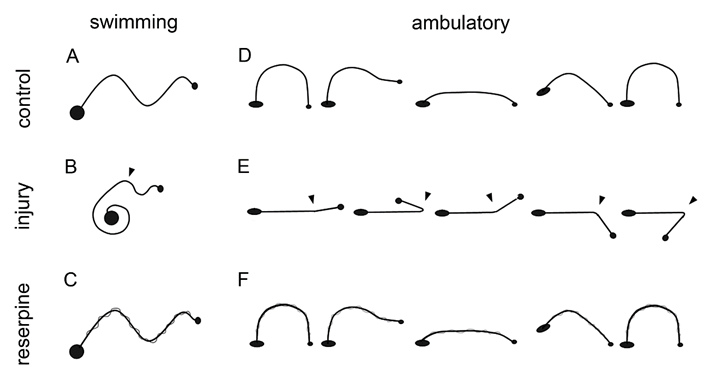
Swimming in control, uninjured, non-injected animals, follows a continuous, sine-wave pattern originating at the head and ending at the tail (Figure 1A), and the animal flattens its body in a dorsoventral axis. After injury, the animal becomes immobile for 1 to 2 days, and subsequently begins to move and swim creating incomplete sine-waves that begin at the head and end at the injury level. The area posterior to the injury does not form a sinus-wave (Figure 1B) and does not flatten. This type of swimming was not considered normal. After several days, the animal recovered and swam forming complete waves from head to tail. Reserpine injection did not affect the animal´s sine-wave swim pattern, but the body´s lateral edges became undulated, like the edges of an oak leaf (Figure 1C). The usual ambulation pattern of the healthy animal on a solid substrate includes release of the anterior sucker, body extension, adhesion of the frontal sucker to the substrate, release of the posterior sucker, body contraction, and adhesion of the posterior sucker to the substrate (Figure 1D). After injury, the animal remains immobile for 1 to 2 days, and subsequently begins to move; injured animals can release the frontal sucker and extend the anterior part of their body, but they cannot adhere the frontal sucker, moving the anterior part of their body above the injury, asynchronically from the rest of the body; over a few days, they can do so, but they are unable to release the posterior sucker (Figure 1E). These movements were not considered normal ambulation. Recovery of normal ambulation was established once the animal could adhere and release both suckers in a synchronous movement that allowed displacement on the substrate. Reserpine injection has no effect on the normal movement pattern (Figure 1F).
Figure 1: Diagram of the healthy, injured, and reserpine-injected leeches´ swimming and ambulation behaviors. A, when swimming, they form a continuous sinewave from the head (small circle) to the tail (large circle). B, in injured leeches, the swimming sinewave originates from the head and reaches the injured site, while the remaining body remains contracted. D, when ambulating, the release the anterior sucker, extend the body, adhere the sucker to the substrate, release the posterior sucker, contract the body, and adhere the posterior sucker. E, during ambulation, the anterior portion of the animal moves from the injury site but independently from the rest of the body. C, F, Reserpine injection has no effect on these behaviors.
CNS Injury
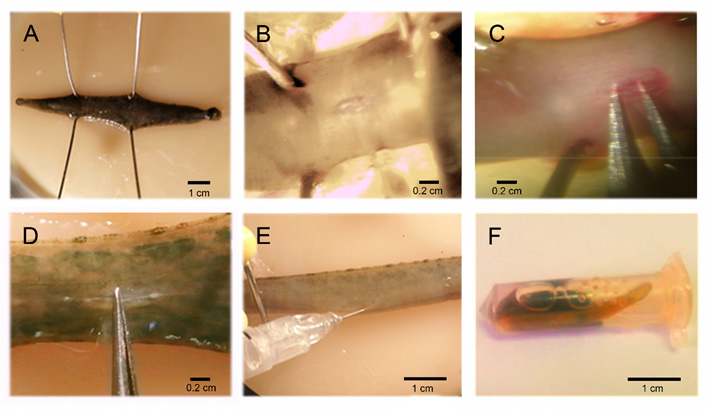
The animals were anesthetized with 9% ethanol, for 20 minutes. They were fixated with 4 pins on a wax dish, and a ventral incision was made at the level of the 7th and 8th ganglia; with microdissection forceps, the connecting nerve between the ganglia were exposed (Figures 2A & 2C), and with another forceps, its central part was obturated causing an injury 300 micrometers in length (Figure 2D). Obturation was performed with the same forceps in all cases, there were no irregularities on contact surfaces, the edges were blunt, and the blade measured 300 micrometers in width. Injury was caused by pressing on the forceps 10 consecutive times, the position of the forceps´ blades was inverted, and pressure was applied 10 more times, the forceps was again inverted, and again, pressure was applied 10 more times. This same procedure was followed in all of the injured groups.
Figure 2: CNS injury procedure, reserpine injection, and serotonin replacement. A, the anesthetized animals were fixated with 4 pins on a wax dish, and a ventral incision was made on the connecting nerve (B). The connecting nerve was exposed with forceps (C), and obturated between the 7th and 8th ganglia (D) causing an injury that compromised axonal inter-ganglionic continuity. E, Reserpine injection (Sigma, 100ug/g weight). The animals were anesthetized with 9% ethanol and placed in a wax dish, the injection was applied to the side of the connecting nerve between the roots of the 9th and 10th ganglia (F). Acute reserpine replacement. This was conducted by placing the animals in a 2 ml container with 500 μl of serotonin solution (2mM. 1hr/day. RT).
Reserpine Injection
The animals´ CNS were depleted of monoamines with reserpine [10]. Reserpine (100 μg/g of the animal´s humid weight, Sigma) was injected with an insulin syringe in organisms anesthetized with 9% ethanol and extended in a wax dish. The 9th and 10th ganglia were located, and the injection was applied between the peripheral nerves of these ganglia, to the side of the connecting nerve (Figure 2E). This procedure was used in the organisms in the groups treated with reserpine. The injury-reserpine group included animals injected with reserpine and injured 24 hours after injection.
Serotonin Replacement
The injured animal group that was injected with reserpine and in whom serotonin was replaced, included organisms injected with reserpine, their CNS was injured 24 hr. later, and during their recovery, they were incubated daily with serotonin (serotonin chlorhydrate, Sigma), for one hour (2mM). This is the concentration at which the resting animals become active and begin to swim vigorously in the containers. The animals were individually placed in 2 mL containers, previously perforated in their upper area to allow air flow, and 500 μL of the serotonin solution were added (2mM. 1h/day. RT. Figure 2F). After incubation, the animals were washed and directly placed in their original containers.
Behavioral Registry and Statistical Analysis
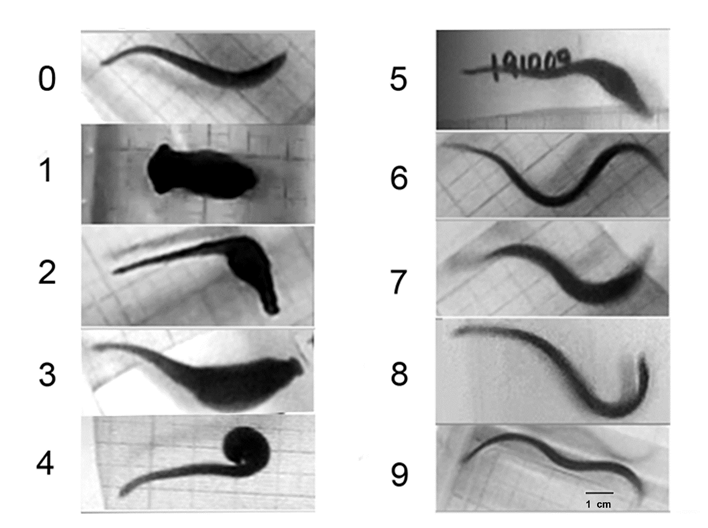
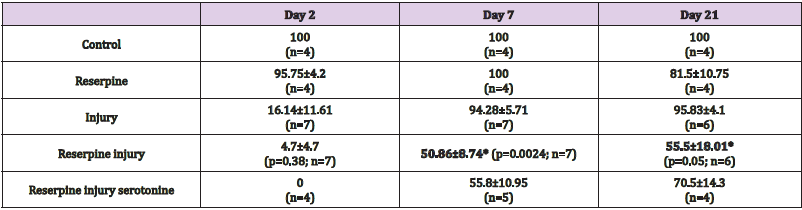
For 30 days after injury, the motor skill development of each organism was registered on video. The presence or absence of swimming and ambulation behaviors in each animal were registered. For data analysis, we recorded for each animal, whether or not they had the normal behavior of swimming or ambulatory, we obtained the percentage of recovery of motor skills in each group of 6 organisms. With the recovery percentages, we obtained statistical parameters of the 7 repetitions, and reported the percentage of recovered animals as the mean ± standard error. The level of significance was established at 95% according to the unpaired Student t test. Calculations were performed with the Sigma Plot 2001 program (Systat Sofware Inc., USA). The data tables show the recovery percentages on days 2, 7 and 21 since they are representative days of the effect of the injury and the recovery of swimming and ambulatory behaviors (Figure 3).
Figure 3: Series of images showing motor recovery after CNS injury. On day zero, before CNS injury, the animals formed a continuous sinewave from the head to the tail. After injury, the animals remained immobile for up to 24 hrs. (Day 1). The animals move asynchronically from the site of injury (day 2). When swimming, sinewaves are generated from the head to the injury site, and the rest of the body is dragged without forming sine-waves. On day 7, many of the injured animals had restored their ability to swim forming continuous waves from the head to the tail. On day 21, animals retain the ability to swim (image not shown). The behaviors of days 2, 7 and 21 were used for data analysis. Scale bar=1cm.
Results
Recovery of Swimming and Ambulation Behaviors After Injury
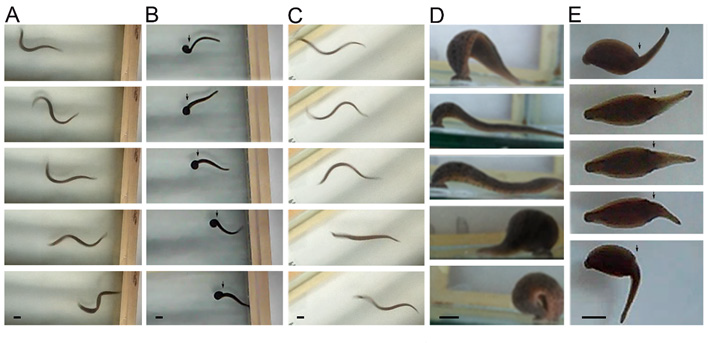
When swimming, the animals flatten their bodies dorsoventrally, and generate continuous sinus-wave shaped undulations from the head to the tail (Figure 4A). After injury, the organisms remain immobile for 24 to 48 hours at the bottom of the container, and subsequently begin their recovery; the animals can swim but using only the body portion anterior to the injury (Figure 4B). Seven days after injury, many animals recovered their swimming abilities forming sinus-wave from head to tail (Figure 4C). When ambulating at the bottom of the container, they move by adhering their suckers and contracting and extending their bodies in alternate movements (Figure 4D). During the first few days after injury, the animals are able to adhere the posterior sucker, extend their body and attempt to adhere the anterior sucker. However, they are unable to contract the body. They generate movement attempts in different directions with the anterior sucker but are unable to release the posterior sucker nor coordinate body contraction or extension (Figure 4E). They can adhere both suckers but independently from the extension and contraction of the body, as seen by the separation of movement from the area of injury.
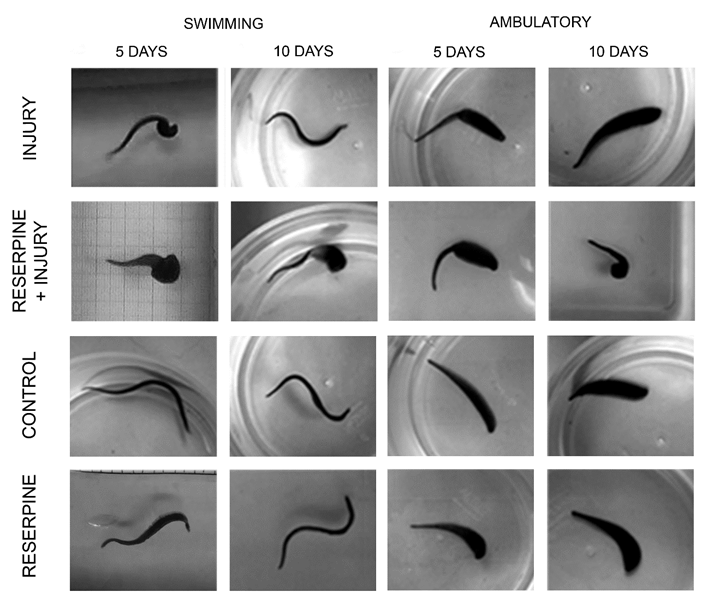
Monoamine Depletion with Reserpine Prolonged the Effect of CNS Injury
Five days after injury, these animals swim by contracting the posterior part of their body, and only generate sine-waves from the head to the injury site, but 10 days later, they can swim forming complete sine-waves (Figure 5). Likewise, on day 5, the animals still lack ambulatory synchronicity, that is recovered by day 10. Monoaminergic neurotransmitter depletion with reserpine injection, leads to persistence of swimming and ambulatory disability 10 days after injury (Figure 5). Reserpine injection did not modify the sine-wave swimming pattern nor ambulation synchronicity.
Figure 4: Series of images showing swimming and ambulation behaviors.
A) While swimming, the animals form sine-waves. The sine-wave begins at the head (right in images) and ends at the tail (left side).
B) Central nervous system (CNS) injury led to loss of the sine-wave´s continuity, originating in the head to the area of injury (arrowhead).
C) One week later, swimming abilities are recovered, forming full sine-waves from head to tail.
D) In ambulation, the posterior sucker is adhered (right in images), the animal´s body extends, and the anterior sucker adheres to the substrate; the animal´s body contracts, and the posterior sucker is released and adheres again close to the anterior sucker. This is a continuous movement that allows the animal´s displacement on the substrate.
E) Nervous system injury causes movement asynchrony in the anterior body and caudal to the injury. While the posterior sucker is adhered to the substrate, the anterior portion moves independently from the area of the injury (arrow).
Figure 5: Behavior comparisons 5 and 10 days after CNS injury. Five days after injury, swimming and ambulation remain compromised in injured animals and in those injected with reserpine and injured. After 10 days, they recover swimming fully and ambulate with synchronic movements. However, reserpine injection induced persistence of the injury´s effects. Reserpine injection did not affect the sine-wave swimming pattern nor ambulation synchrony.
Reserpine Injection Decreased the Recovery Percentage After Injury
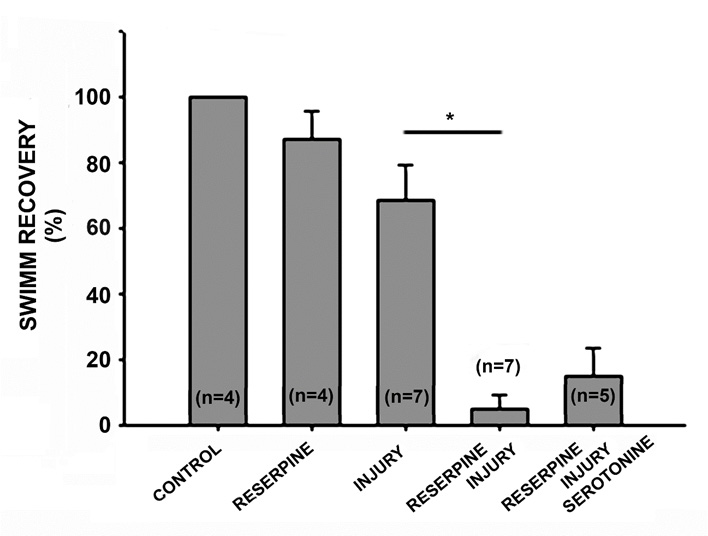
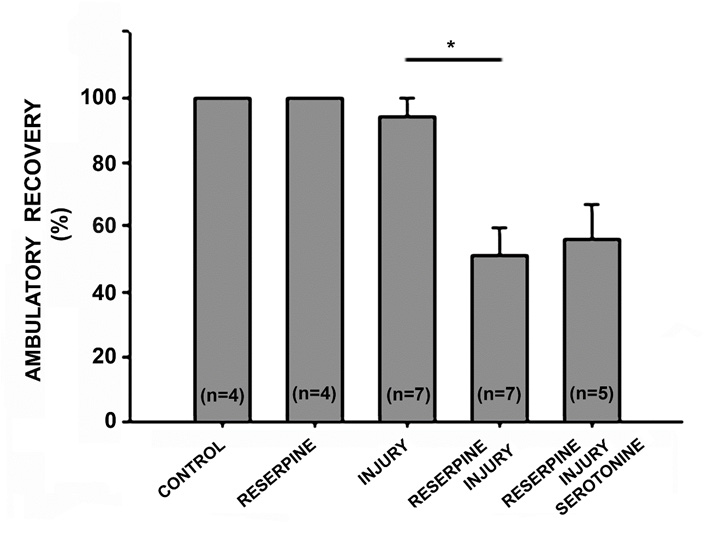
Two days after CNS injury, almost all animals were unable to swim or deambulate (Tables 1 & 2). Seven days after injury, 68.42±11.11% of injured animals can swim forming a continuous sinus-wave from the head to the tail and also recover ambulatory synchronicity (94.28±5.71%) at the bottom of the container, with alternate body extension and contraction, and adhering and releasing the suckers. The animals injected with reserpine and injured, the recovery percentage in swimming abilities decreased (4.71±4.71% p=0.0002) compared with the animals that were only injured (Table 1, Figure 6). Seven days after reserpine injection, the percentage of ambulation recovery decreased in the injured animals (50.86±8.74%, p=0.0024) in comparison with the recovery of animals that were only injured (94.28±5.71%. Table 2. Figure 7).
Figure 6: Graph of the percentage of swimming recovery 7 days after injury. The injection of reserpine did not affect swimming behavior, and all animals swam like those in the control group. Among the injured animals, 68.42% recovered the ability to swim forming continuous waves from the head to the tail. Monoaminergic neurotransmitter depletion associated with reserpine injection decreased the recovery percentage to 4.71% (p=0.0002, n=7), and the acute replacement of serotonin did not modify this recovery percentage (14.6±9%. p=0.31, n=5).
Table 1: Recovery percentage of swimming after intra-corporal injection of reserpine (100μg/g) and CNS injury. Data is presented as average values and standard errors. Bold numbers refer to the significant between-group differences: injury and reserpine-injury.
Table 2: Recovery percentage of ambulation after intra-corporal injection of reserpine (100μg/g) and CNS injury. Data is presented as average values and standard errors. Bold numbers refer to the significant between-group differences: injury and reserpine-injury.
Figure 7: Graph of the percentage of ambulation recovery 7 days after injury. Reserpine injection did not affect the animals´ ambulation behavior, and 100% of animals could move on the substrate just as the control group. Among the injured animals, 94.28% recovered the ability to ambulate continuously by adhering the suckers in an alternate manner and extending and contracting the body. Monoaminergic neurotransmitter depletion with reserpine injection decreased the recovery percentage to 50.86% (p=0.0024, n=7), and acute serotonin replacement did not modify this recovery percentage (55.8±10.95%. p=0.73, n=5).
Acute Serotonin Replacement had no Effect on the Recovery of Swimming or Ambulatory Skills
In five repetitions, acutely applied serotonin replacement, 500 μL (2mM) daily for one hour, had no effect on the percentages of swimming and ambulation skills in the injured animals treated with reserpine. In swimming skills, serotonin replacement led to a recovery of 14.6±9% (p=0.31), in comparison with the injuryreserpine group (4.71±4.71%. Table 1, Figure 5). Likewise, serotonin replacement induced an ambulatory recovery percentage of 55.8±10.95% (p=0.73) compared with the injury-reserpine group (50.86±8.74%) (Table 2, Figure 6).
Discussion
Monoaminergic neurotransmitters promote the recovery of swimming (93%) and ambulation (46%) after CNS injury. Monoamine depletion via reserpine injection decreased the percentage of swimming and ambulation recovery in animals injured by obturation of their central nervous system. Seven days after injury, they decreased from 87.25% in swimming recovery to 4.71%, and from 94.28% in ambulation recovery to 50.86%. This underscores the importance of these neurotransmitters in the process of motor recovery after CNS injury. The differences in the percentages of recovery of swim and ambulation skills on day 7, in both the animals that were only injured and in the injured group injected with reserpine, suggest that these behaviors follow different neuronal pathways. Indeed, both behaviors have been shown to be based in different groups of neurons [27]. These different circuits may possess different regeneration speeds and integration of their functional webs, leading to the differences in recovery percentages 7 days after injury. In terms of the CNS monoaminergic neurotransmitters, we question their degree of participation in functional recovery. In this study, after monoamine depletion with reserpine injection, serotonin was acutely replaced (2mM; 1hr/day). The recovery percentages in the injury-reserpine and the injury-reserpine-serotonin groups showed no significant differences in swimming or ambulatory skills, revealing that acute serotonin replacement has no effect on recovery percentages.
This lack of a positive serotonin effect is in accordance with results on its effect in some cultured neuronal types such as AE or Retzius cells [26]. In this study, serotonin either had no effect or had an inhibitory effect on the regeneration of specific neurons, whereby systemic serotonin replacement would not foster the regeneration of some neuronal types, and hence, the reestablishment or development of neuronal circuits required for swimming and ambulation motor behaviors. In the future a similar study, chronic testing of other serotonin concentrations would be of interest. Likewise, the role of dopamine and octopamine replacement on the functional recovery of motor skills in this system remains to be tested. The constitutive activity of the 2C serotonin receptor has been shown to increase the excitability of neurons caudal to a spinal injury in rats, and finally leads to recovery of some motor functions [28]. This and other experiments have established the relevance of the serotoninergic system in motor function recovery after spinal cord injury. However, clinical trials conducted in humans have shown that drugs associated with serotonin and its receptors do not promote recovery after CNS injury [29]. Currently, there are controversial results on the role of serotonin and its receptors in functional recovery [30,31] and more studies need to be done on humans and other systems.
Acknowledgements
To Gabriela Montiel and Alejandra Zea for their participation in conducting experiments and data analysis.
References
- Horner PJ, Gage FH (2000) Regenerating the damaged central nervous system. Nature 407: 963-970.
- David S Aguayo LJ (1981) Axonal Elongation into Peripheral Nervous System "Bridges" After Central Nervous System Injury in Adult Rats. Science 214(4523): 931-933.
- Koert CE, Spencer GE, Minnen J, Li KW, Geraerts WPM, et al. (2001) Functional Implications of Neurotransmitter Expression during Axonal Regeneration: Serotonin, But Not Peptides, Auto-Regulate Axon Growth of an Identified Central Neuron. J Neurosci 21(15): 5597-5606.
- Alam T, Maruyama H, Li C, Pastuhov S, Nix P, et al. (2016) Axotomy-induced HIF-serotonin signaling axis promotes axon regeneration in C. elegans. Nat commun 7: 1038.
- Homma K, Kitamura Y, Ogawa H, Oka K (2006) Serotonin induces the increase in intracellular Ca2+ that enhances neurite outgrowth in PC12 cells via activation of 5-HT3 receptors and voltage-gated calcium channels. J Neurosci Res 84(2): 316-325.
- De-Miguel FF, Trueta C (2005) Synaptic and Extrasynaptic Secretion of Serotonin. Cel Mol Neurobiol 25: 297-312.
- Del-Bel E, De-Miguel FF (2018) Extrasynaptic Neurotransmission Mediated by Exocytosis and Diffusive Release of Transmitter Substances. Front Synaptic Neurosci 10: 13.
- Todd RD (1992) Neural development is regulated by classica lneurotransmitters: dopamine D2 receptor stimulation enhances neurite out growth. Biol Psychiatry 31(8): 794-807.
- Spencer GE, KlumpermanJ, Syed NI (1996) Neurotransmitters and neurodevelopment. Role of dopamine in neurite outgrowth, target selection and specific synapse formation. Cell Mol Neurobiol 16: 577-589.
- Izumi Y, Wakita S, Kanbara C, Nakai T, Akaike A, Kume T (2017) Integrin α5β1 expression on dopaminergic neurons is involved in dopaminergic neurite outgrowth on striatal neurons. Sci Rep 7: 42111.
- Höglinger UG, Rizk P, Murie MP, Duyckaerts C, Oertel WH, et al. (2004) Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7: 726-735.
- Mishra A, Singh S, Tiwari V, Parul, Shukla S (2019) Dopamine D1 receptor activation mproves adult hippocampal neurogenesis and exerts anxiolytic and antidepressant-like effect via activation of Wnt/β-catenin pathways in rat model of Parkinson's disease. Neurochem Int 122: 170-186.
- Koon AC, Ashley J, Barria R, DasGupta S, Brain R, et al. (2011) Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci 14: 190-199.
- Roeder T, Seifert M, Kähler C, Gewecke M (2003) Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch Insect Biochem Physiol 54(1): 1-13.
- Roeder T (2005) Tyramine and octopamine: ruling behavior and metabolism. Annu rev entomol 50: 447-477.
- Li Y, Jiao Q, Du X, Bi M, Han S, et al. (2018) Investigation of Behavioral Dysfunctions Induced by Monoamine Depletions in a Mouse Model of Parkinson's Disease. Front Cell Neurosci 12: 241.
- O'Gara BA, Chae H, Latham LB, Friesen WO (1991) Modification of leech behavior patterns by reserpine-induced amine depletion. J Neurosci 11(1): 96-110.
- Crisp MK, Mesce KA (2006) Beyond the central pattern generator: amine modulation of decision-making neural pathways descending from the brain of the medicinal leech. J Exp Biol 209(9): 1746-1756.
- Alves SJ, Tecuapetla F, Paixão V, Costa RM (2018) Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554: 244-248.
- Wolfram S (2007) Behavioral dopamine signals. Trends Neurosci 30(5): 203-210.
- German DC, Manaye K, Smith WK, Woodward DJ, Saper CB (1989) Midbrain dopaminergic cell loss in Parkinson's disease: computer visualization. Ann Neurol 26(4): 507-514.
- Xu L, Jiang H, Chen X, Xiong Y, Lu X, et al. (2018) How Tyramine β-Hydroxylase Controls the Production of Octopamine, Modulating the Mobility of Beetles. Int J Mol Sci 19(3): 846.
- Mladinic M, Muller KJ, Nicholls JG (2009) Central nervous system regeneration: from leech to opossum. J Physiol 587(12): 2775-2782.
- Kristan WB, Calabrese RL, Friesen WO (2005) Neuronal control of leech behavior Prog Neurobiol 76(5): 279-327.
- Glover J, Kramer A (1982) Serotonin analog selectively ablates indentified neurons in the leech embryo. Science 216(4543): 317-319.
- Vargas J, Alfaro-Rodriguez A, Pérez-Orive J (2019) Serotonin induces or inhibits neuritic regeneration of leech CNS neurons depending on neuronal identity. Braz J Med Biol Res 52(2): e7988.
- Lamb DG, Calabrese RL (2011) Neural circuits controlling behavior and autonomic functions in medicinal leeches. Neural Systems&Circuits 1: 13.
- Fouad K, Rango MM, Vavrek R, Murray KC, Sanelliy L, et al. (2010) Locomotion After Spinal Cord Injury Depends on Constitutive Activity in Serotonin Receptors. J Neurophysiol 104(6): 2975-2984.
- Leech KA, Kinnaird CR, Hornby TG (2014) Effects of Serotonergic Medications on Locomotor Performance in Humans with Incomplete Spinal Cord Injury. J Neurotraum 31(15): 1334-1342.
- Chollet F, Tardy J, Albucher J, Thalamas C, Berard E, et al. (2011) Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 10(2): 123-130.
- Dennis M, Forbes J, Graham C, Hackett M, Hankey GJ, et al. (2019) Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet 393(10168): 265-274.

 Research Article
Research Article