ABSTRACT
In order to study the involvement of the reticular formation of the midbrain on the electrical activity of the hippocampus, we performed electrical, chemical stimulation and temporary switching off of the reticular formation. The results of our studies showed that both electrical and chemostimulation (carbocholine, serotonin, norepinephrine) at low exposure parameters led to the appearance of ordered rhythmic activity in the hippocampus: in the case of electrical stimulation and administration of carbocholine, frequencies dominated in the range of 6-7.5 Hz, with the introduction of serotonin 5-6 Hz, and with the introduction of norepinephrine 4-5 Hz. A further increase in the parameters of the stimulating current leads to the registration of a pronounced desynchronized activity, which combines both theta waves and fastfrequency beta oscillations. In the electrogram of the hippocampus, there is a violation of the rhythm of the oscillatory process and the dominance of high-frequency waves in the spectrogram. With an increase in the dose of injected chemicals, the rhythmic activity of the hippocampus is transformed into convulsive discharges. Analysis of the research results showed that the chemoreactive structures of the ascending reticular activating system are chemically heterogeneous. Differences in the manifestation of cholinergic, serotonergic and noradrenergic EEG activation are mainly due to the activity of the corresponding chemoreactive structures of the ponto-mesencephalic reticular formation, i.e., neurochemical mechanisms of its occurrence. The chemical heterogeneity of the ascending reticular activating system makes it possible to obtain EEG activation upon excitation of cholinergic, serotonin, and noradrenergic structures. The disappearance of the theta rhythm caused by novocaine with local blockade of the reticular formation indicates the reticular mechanism of this effect. Under conditions of temporary RF shutdown, the flow of impulses from the brain stem to the hypothalamic-pituitary-adrenal system apparently decreases, which leads to a decrease in hippocampal activity.
Keywords: Hippocampal Theta-Rhythm; Reticular Formation; Electrical and Chemical Stimulation; Temporary Shutdown
Introduction
The study of the theta rhythm generation mechanisms revealed that its formation requires a connection between the hippocampus and the medial septal region. It is known that the neurons of this area are pacemakers of theta rhythm. Septal neurons have intense projections to all areas of the hippocampus [1,2]. In the absence of connections between the neural networks of the MSO and the hippocampus, the theta rhythm in the latter is not generated [3]. The pacemaker function of the medial septal region is regulated by projections from various stem structures: serotonergic projections of the raphe nuclei suppress the theta rhythm, and glutamatergic projections of the nuclei of the reticular formation and dopaminergic projections from the ventral tegmental region increase theta rhythm. All this proves that the theta rhythm depends on the preservation of connections between many brain structures [4,5]. Reticular formation of the brain stem has ascending influences on the cerebral cortex, as well as on the structures of the diencephalon and limbic system. The nonspecific structures of the midbrain and pons are the final destination of the impulses coming from the hippocampus through the system of the medial forebrain bundle and, at the same time, one of the two main sources of afferentation for the hippocampus. Taking into account the data available in the literature that the launch of the theta rhythm and an increase in its frequency in proportion to the flow of incoming information is carried out by the ascending activating reticular formation [6,7], the aim of this study was to study changes in the electrical activity of the CA1 and CA3 fields of the dorsal hippocampus, its ventral region, the dentate fascia, and the medial septal nucleus under conditions of electrical and chemostimulation of the reticular formation.
Methods
The research was carried out on Chinchilla rabbits (25 pieces) weighing 2.5-3 kg. EEG was recorded from the dorsal (fields CA1: P3; L2; H8 and CA3: P3; L6; H7.5) and ventral hippocampus (P4; L7; P17), dentate fascia (P4; L5; H7.5) and medial nucleus septum (A-3; L0.5; H10.5) before and after stimulation (electrical and chemical) of the reticular formation (P9; L2.5; H18.2) of the brain stem. The blockade of the reticular formation was performed with a 10% solution of novocaine. To control the rabbits in the study area was injected with saline in a volume equal to the injected solutions.
Results and Discussion
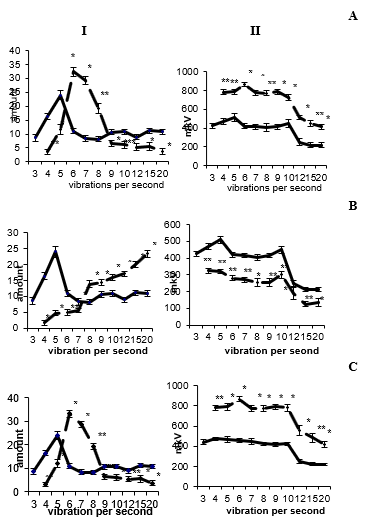
Unilateral electrical stimulation of the reticular formation at sufficiently low stimulation parameters (5-10 Hz; 50-100 μA; 0.5 ms) for 15-30 sec did not lead to visible changes in the electrical activity of the studied brain structures. Optimal for inducing electrophysical changes is stimulation with rectangular pulses with a duration of 0.5 ms, a frequency of 10-20 Hz, and a current of 100- 200 μA. From the first minutes after exposure to electric current, in the total activity of fields CA1 and CA3 of the dorsal hippocampus, its ventral region, dentate fascia and medial nucleus of the septum, an increase in the severity of the theta rhythm was observed against the background of a decrease in fluctuations in the beta range, an increase in the amplitude of activity of the ipsi- and contralateral side. Similar data were observed by us with the introduction of low doses of carbocholine - 0.5-2 μg into the reticular formation. Analysis of the frequency spectrum of the ipsi- and contralateral hippocampus revealed an increase in the rhythm of the oscillatory process, a shift of waves from the range of 3-5 vibration/s, which corresponded to the state of rest, to the frequency spectrum of 6-7.5 vibration/s. A decrease in the representation of fast-frequency beta oscillations is noted. Amplitude analysis showed a sharp increase in the amplitude of the waves of the studied brain structures (Figures 1A & 1C).
At 3-5 seconds, ordered activity transforms into convulsive discharges. Such hyperactivity appears synchronously in all the studied areas of the brain and is recorded for 3-4 hours after electrical stimulation of the reticular formation. Epileptiform activity in the hippocampus was also noted by us with an increase in the dose of carbocholine to 3 μg. A further increase in the parameters of the stimulating current (50-100 Hz, 200-300 μA) leads to the registration of a pronounced desynchronized activity, which combines both theta waves and fast-frequency beta oscillations. In the electrogram of the hippocampus, there is a violation of the rhythm of the oscillatory process and the dominance of highfrequency waves in the spectrogram. The performed frequency analysis revealed a violation of the order of oscillations, which was noted in the case of the use of high parameters of the irritating current, and an increase in the representation of fast frequencies. Amplitude analysis showed a slight decrease in the amplitude of the waves of the registered brain structures (Figure 1B).
Figure 1: Amplitude-frequency analysis of the electrical activity of the ipsilateral hippocampus before and after low-frequency (A), high-frequency (B) electrical stimulation and applications of carbocholine (C) in the reticular formation of the midbrain. I - frequency, II - amplitude analysis. On the abscissa axis - the average value of the classes of intervals in frequencies, on the ordinate axis - the frequency of repetition; The solid line is before, the dotted line is after exposure.
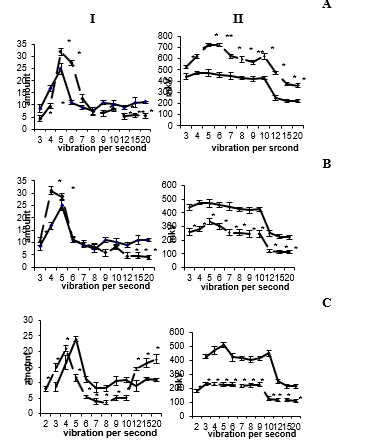
Application of 5-OT into the reticular formation at a dose of 10-30 mcg from the first minutes of administration causes the appearance of regular, synchronized activity of increased amplitude in various areas of the hippocampus, dentate fascia and medial nucleus of the septum. With an increase in the dose of the administered substance, the electrogram of the structures changes and passes from rhythmic, ordered activity to epileptiform. Restoration of the electrical activity of the studied brain structures to the background level was observed at 150-180 minutes. Spectral analysis of the EEG fields CA1 and CA3 of the dorsal hippocampus, its ventral region, dentate fascia, and medial septal nucleus revealed the dominance of waves in the range of 5-6 counts/s, a decrease in the representation of fast-frequency beta oscillations. Amplitude analysis showed an increase in the amplitude of the activity of the studied brain structures (Figure 2А). At 3-5 minutes of administration of 10-20 μg of norepinephrine into the reticular formation, in all areas of the hippocampus, dentate fascia and medial septum nucleus, synchronized, ordered, regular activity is recorded, the representation of high-frequency waves decreases and the amplitude of activity of the studied brain areas decreases somewhat. Restoration of the electrohippocampogram to the intact level is observed at 90-120 minutes.
The performed frequency analysis revealed a shift in the frequency spectrum and the dominance of waves in the range of 4-5 counts/s, a decrease in the representation of fast-frequency beta oscillations. Amplitude analysis showed a slight decrease in the amplitude of activity compared to the background indicator (Figure 2B). Temporary shutdown of the reticular formation by the introduction of a 10% novocaine solution causes significant changes in the EEG activity of the recorded brain structures: the theta rhythm disappears, although individual theta waves are present. The absence of rhythmic oscillations is preceded by the appearance of convulsive activity in the electrohippocamogram, after which irregular, low-amplitude activity is recorded, combining both slow waves and fast-frequency oscillations. The performed frequency analysis revealed a sharp decrease in the number of waves in the range from 5 to 10 counts/s and an increase in the number of delta and beta oscillations in both hemispheres of the studied brain structures. Amplitude analysis showed a significant decrease in the amplitude of the activity of the studied brain structures (Figure 2C).
Figure 2: Amplitude-frequency analysis of the electrical activity of the hippocampus (field CA3) before and after the application of 5-OT (A), NA (B), and novocaine (C) in the reticular formation of the midbrain. The rest of the designations are the same as in Figure 1.
Some scientists believe that the presence and frequency of the theta rhythm are determined by the frequency distribution of discharges in the cells of the septum (Gogolak, et al. [8]). When the reticular formation is stimulated by a current of increasing strength, the number of septum neurons that discharge in bursts increases, and the frequency of their bursts increases in parallel with the frequency of the theta rhythm. With a current strength that causes a change in the theta rhythm in the hippocampus by desynchronization, the number of rhythmically discharged cells decreases (they are inhibited or begin to give continuous high-frequency discharges without volleys), although a limited number of neurons continue to maintain high-frequency rhythmic discharges even under these conditions [8]. At a very high intensity of stimulation of the reticular formation, the high-frequency theta rhythm is replaced by desynchronization. According to some scholars, the shift theta rhythm frequencies are the result of a gradual change in the level of the input signal from the reticular formation to the septo-hippocampal system [9].
We have revealed the duality of electrical processes in the hippocampus during stimulation of the reticular formation. High-frequency stimulation of this area led to the registration of desynchronized, irregular, low-amplitude activity in various fields of the dorsal and ventral hippocampus, dentate gyrus, and medial septal nucleus. According to some scientists, these effects are determined by topographically and functionally different pathways and structures of the mesodiencephalic region [10]: the theta rhythm occurs when the dorsolateral region of the tegmentum and central gray matter are stimulated, and the transition of the theta rhythm to desynchronization with increasing current occurs when the ventromedial region is stimulated. reticular formation. The possibility of inducing desynchronization from the bulbar ventromedial tegmenta was also described by other researchers [11]. Many authors have shown that from the same point in the reticular formation it is possible to induce theta rhythm in the hippocampus at a low strength or frequency of stimulation and desynchronization with an increase in these parameters [12].
Considering all of the above, it can be assumed that, obviously, with an increase in the frequency and intensity of stimulation of the reticular formation, leading to the registration of desynchronized activity in the hippocampus, those sections of it that inhibit the secretion of corticosteroid hormones are activated. Different parts of the stem reticular formation take part in the implementation of neuroendocrine functions. A particularly important role is played by the reticular formation of the mesencephalic region, which has close anatomical and morphological connections with the hypothalamic-pituitary system [13]. It is known that cholinergic synapses are involved in the stimulation of the HPA axis [14], and a dose-dependent release of the hormone is noted [15]. In our experiments, the application of 0.5–2 μg of CH from the first minute led to the registration of well-defined, regular synchronized activity, which periodically alternated with epidischarges, which indicates an increase in the activity of hippocampal neurons. When hydrocortisone is applied directly to the reticular formation, the appearance of convulsive activity in the hippocampus is also noted.
In our experiments, the application of small doses of 5-OT (10–30 μg) to the RF of the midbrain led to the registration in various areas of the hippocampus, dentate fascia, and medial nucleus of the septum of regular, rhythmic, high-amplitude activity with dominance of waves in the range of 5– 6 counts / s, which, 2-5 minutes after the injection, combined short-term epidischarges recorded throughout the entire observation period (90-120 minutes). The data available in the literature testify to the activation of HPA when 5-OT is administered to various areas of the CNS [16]. Obviously, when the reticular formation is stimulated by 5-OT, the HPA system is activated, resulting in an increase in the concentration of ACTH and steroid hormones in the blood, which, by increasing the excitability of hippocampal neurons, lead to the registration of ordered, synchronized activity. Increasing the dose to 50-100 mcg leads to the registration of convulsive activity, which indicates an increase in the amount of steroid hormones in the blood, which lead to an increase in the excitability of hippocampal neurons. Similar data were obtained in electrophysiological studies [17].
Our data suggest a stimulating effect of NA on the HPA axis, leading to an increase in the secretion of ACTH and steroid hormones, which cause the theta rhythm in the hippocampus at low doses of NA and convulsive discharges with an increase in the dose of monoamine. Our assumption is confirmed by the data available in the literature on the stimulating effect of NA on the HPA axis. In addition, it has been shown that under the action of NA there is an increase in the secretion of corticoliberin, ACTH, and corticosterone [15]. Microinjection of HA into the surface plate in the region of the nuclei of the trigeminal nerve of the brainstem causes an increase in ACTH in the blood plasma [18]. Temporary shutdown of the reticular formation of the midbrain by the introduction of a 10% solution of novocaine leads to the registration of convulsive activity in the hippocampus, after which there is a decrease in the amplitude of the waves and the registration of irregular highfrequency oscillations. Similar data were obtained during bilateral coagulation of the reticular formation of the midbrain, where the disappearance of the theta rhythm and the dominance of fastfrequency oscillations in the EEG activity of the hippocampus were shown [17].
Given the presence of connections between the reticular formation and the hypothalamus and participation in the control of the hypothalamic-pituitary-adrenal system, it can be assumed that as a result of its temporary shutdown, a violation occurs in the regulation of the hypothalamic-pituitary-adrenal system, resulting in a decrease in the amount of steroid hormones in the blood, which leads to decreased excitability of hippocampal neurons. In favor of this assumption, some works can be cited that show a decrease in the secretion of hormones of the adrenal cortex after the destruction of the reticular formation [18]. Summarizing the material presented in this article, we can say that various effects on the activating reticular formation lead to its excitation, which is reflected in the appearance of theta rhythm in the hippocampus. The excitability of the hippocampus can also be judged by the appearance of convulsive discharges in its activity, indicating its pathological excitation. Temporary shutdown of the activating reticular formation in the first minutes also leads to epileptiform activity in the hippocampus, after which desynchronized, lowamplitude waves are recorded. The general properties of the reticular formation and its physiological significance in the body suggest its participation in the control of endocrine functions. Impulses emanating from the reticular formation, affecting the tone of various parts of the central nervous system, change their reactivity to the action of hormones circulating in the blood, thereby determining the intensity of influences from these parts of the brain on the hypothalamic-pituitary system. In addition, the impulses emanating from the reticular formation, reaching the endocrine effectors, determine the tone and reactivity of the latter to the action of humoral and nervous stimuli.
References
- Unal G, Joshi A, Viney TJ, Kis V, Somogyi P (2015) Synaptic targets of medial septal projections in the hippocampus and extrahippocampal cortices of the mouse. J Neurosci 35: 15812-15826.
- Zutshi I, Brandon MP, Fu ML, Donegan ML, Leutgeb JK, et al. (2018) Hippocampal neural circuits respond to optogenetic pacing of theta frequencies by generating accelerated oscillation frequencies. Curr Biol 28: 1179-1188.e3.
- Colgin LL (2013) Mechanisms and functions of theta rhythms. Annu Rev Neurosci 36: 295-312.
- Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E (2015) Theta sequences are essential for internally generated hippocampal firing fields. Nat Neurosci 18(2): 282-288.
- Steriade M (1996) Reticular activating system. Sciense 272: 225-226.
- Kichigina VF, Kudina TA (2001) Sensory reaction of hippocampal neurons of the rabbit under functional you turn off the structures that control theta rhythm. J Higher Nervous Activity 51 (2): 228-235.
- Mysin IE (2020) Mechanisms of hippocampal theta rhythm. Journal of Higher Nervous Activity 70(3): 291-313.
- Gogolak G, Stumpf Ch, Petsche H, Sterc J (1968) The firing pattern of septal neurons and the form of the hippocampal theta wave. Brain Research 7(2): 201-207.
- Kamp A, Lopes da Silva FH, Storm van Leenwen W (1970) Common aspects of hippocampus electrical activities during different behavioral tasks. Brain Research 24(3): 547-548.
- Yokota T, Fujimori B (1964) Effects of brain-stem stimulation upon hippocampal electrical activity, somatomotor reflexes and automatic functions. EEG and Clin Neurophysiology 16(4): 375-382.
- Hedge GA, Smelik PG (1968) Corticotropin release: inhibition by intrahypothalamic implantation of atropine. Sciеnce 159(3817): 891-892.
- Hillhouse EW, Burden J, Jones MT (1975) The effect of various putative neurotransmitters on the release of corticotropin-releasing hormone from the hypothalamus of the rat in vitro. I. The effect of acetylcholine and noradrenaline. Neuroendocrinol 17(1): 1-11.
- Naumenko EV (1971) Central regulation of the pituitary-adrenal complex. L Nauka 190.
- Blier P, de Montigny C (1990) Electrophysiological investigation of the administration of 5-HT1A receptor agonist. J Cardiovasc Pharmacol 15(7): 42-48.
- Plotsky PM (1991) Pathways to the secretion of adrenocorticotropin: A veiw from the portal. J Neuroendocrinology 3(1): 1-9.
- Lu J, Bereiter D (1991) Microinjections of norepienephrine within the superficial laminae of trigeminal subnucleus caudalis evoke increases in plasma adenocorticotro-pine in the rat. J Brain Research 568: 152-158.
- Parmeggiani PL (1967) On the functional significance of the hippocample theta rhythm. Progr Brain Research 27: 413-441.
- Buntser IaM (1964) Endocrine system and reticular formation of the brain. Successes of modern biology 57(1): 143-158.

 Research Article
Research Article