Abstract
Background: RAS genes (KRAS, NRAS) encoded proteins participate RAS-RAFMAPK signaling pathway. Colorectal cancers (CRC) with RAS mutation are resistant to anti-EGFR monoclonal antibody therapy. The aim of the present study is to determine whether type 2 diabetes (T2DM) linked to RAS mutation of CRC.
Patients and Methods: KRAS, NRAS status was assessed in primary CRC (n=232) by ARMS-PCR (Amplication refractory mutation system).
Results: The mutation rate of KRAS in T2DM group was considerably higher than non-T2DM in overall patients (56.8% vs. 33.3%, P=0.007) and male patients (31.0% vs. 61.9%, P=0.007). Univariately, T2DM was significantly associated with KRAS mutation in overall patients (OR=2.63, 95% CI: 1.28-5.37, P=0.008) and male patients (OR=3.61, 95% CI: 1.38-9.47, P=0.009), but not in female patients. T2DM did not significantly associated with NRAS mutation status of CRC. Multivariate analysis retained the above significance. The most frequent KRAS mutations in T2DM group were codon 12, 34G>T, followed by codon 12, 35G>A. Moreover, for male patients, the NRAS mutation rate in patients with cigarette smoking habit was significantly higher than those without cigarette smoking habit (0% vs. 6.6%, P=0.042). In addition, mutation of RAS was significantly associated with pathological subtypes of CRC manifested by increased frequency of mucinous carcinoma and/or adenocarcinoma with mucinous components.
Conclusion: T2DM might influence carcinogenesis, progression and prognosis of CRC via promoting KRAS mutation and mucinous carcinoma phenotype.
Keywords: Type 2 Diabetes; Cigarette Smoking; KRAS; NRAS; Colorectal Cancer
Abbreviations: CRC: Colorectal Cancers; T2DM: Type 2 Diabetes; MPE: Molecular Pathological Epidemiology; MetS: Metabolic Syndrome; SRCC: Signet-Ring Cell Carcinoma; FPG: Fasting Plasma Glucose; TG: Triglycerides; HDL: High- Density Lipoprotein Cholesterol; FGICH: Family Gastrointestinal Cancer History; ADA: American Diabetes Association; BMI: Body Mass Index; AC: Adenocarcinoma; SD: Standard Deviation; ORs: Odds Ratio; CIs: Confidence Intervals; SHBG: Sex Hormone Binding Globulin
Introduction
Previous study has suggested a link between type 2 diabetes mellitus (T2DM) and colorectal cancer (CRC) [1,2] but the molecular link between T2DM and CRC has not fully understood. Molecular pathological epidemiology (MPE) is a new research direction that focuses on special molecular markers of tumor initiation or progression in association with exposures of interest [3,4]. Kirsten rat sarcoma viral oncogene homolog (KRAS) and NRAS are kinase in RAS-RAF-MAPK signaling pathway. CRC with KRAS or NRAS mutation showed poor response to anti-EGFR monoclonal antibody therapy [5]. KRAS mutation occurs in about 40% of CRC and showed a high mutation rate in some subtypes of CRC including adenoma-like adenocarcinoma and mucinous carcinoma [6,7]. About 90% of KRAS mutation of CRC occurs in exon2 codon 12/13 [8]. Unlike KRAS, NRAS showed a low mutation frequency in CRC [9]. T2DM is a component of metabolic syndrome (MetS), which including a set of metabolic disorders such as central obesity, hypertension, T2DM/hyperglycemia, and dyslipidemia [10]. Previous study indicated diabetes were linked to signet-ring cell carcinoma (SRCC) and colorectal adenocarcinoma (MAC) with components (mucinous, signet-ring cell, or neuroendocrine) [11]. KRAS mutation was detected at a higher frequency in mucinous carcinoma in comparison with classic colorectal adenocarcinoma (or non-mucinous adenocarcinoma) [9]. However, the association between T2DM and mutation of KRAS (or NRAS) has not been well-established so far. In the present study, we aimed to study the association between T2DM and KRAS or NRAS mutation in CRC.
Patients and Methods
Patients
The retrospective study included 232 cases (not including 11 cases excluded according exclusion criteria) of primary CRC from January 2013 to June 2021 in Zhejiang Provincial People’s Hospital. Cases without clinical data such as diabetes status were excluded from the study (n=11). The patients were divided into subgroups of KRAS-mutation (mKRAS) and KRAS-wild-type (wKRAS), as well as NRAS-mutation (mNRAS) and NRAS-wild-type (wNRAS). The basic clinical data of the patients including gender, age, weight, height, blood pressure, fasting plasma glucose (FPG)/diabetic status, fasting plasma triglycerides (TG), and fasting plasma highdensity lipoprotein cholesterol (HDL) were collected from the electronic medical records. Factors such as cigarette smoking habit, history of family gastrointestinal cancer (FGICH) were also collected. Pathological parameters including histological subtypes and histological grade of CRC were acquired from the pathological reports.
Ethics
The Ethics Committee of Zhejiang Provincial People’s Hospital reviewed and approved the study (2021KT001, KY2019012). Anonymous clinical data were used in the analysis.
Mutation Assay
Genome DNA was extracted from paraffin-embedded tissue. Mutation of KRAS (n=232) and NRAS (n=225) was assayed by ARMS-PCR (Amplication refractory mutation system) using Human Gene Mutation Detection Kit for KRAS and NRAS (Products of Beijing ACCB Biotech, Beijing, China) according manufacturer’s protocols. The KRAS Mutation Kit detects 7 hot mutations (Codon 12, 34G>T, 34G>A, 34G>C, 35G>T, 35G>A, 35G>C and Codon 13, 38G>A) of KRAS gene, which account for > 90% of known KRAS mutation. The NRAS Mutation Kit detects 9 kinds of mutations in coden 12, 13 and 61 of NRAS gene.
Diagnostic Criteria for T2DM
T2DM was diagnosed according the following criteria:
a) Patients had documented T2DM history before diagnosis of
CRC;
b) Patients did not have a T2DM history but met the T2DM
criteria of American Diabetes Association (ADA) [12]: FPG ≥
7.0 mmol/L or Hemoglobin A1C ≥ 6.5%. Medical conditions
such as dextrose and corticosteroids treatment that may
elevate blood glucose levels were excluded.
Definition of Metabolic Syndrome
MetS were determined by presence of at least 3 of the following
components according the criteria of China Diabetes Society (CDS)
[10]:
a) Central obesity [body mass index (BMI) ≥ 25.0 kg/m2
b) Dyslipidemia: hypertriglyceridemia (fasting plasma TG ≥ 1.7
mmol/L) and/or low HDL (fasting plasma HDL <0.9 mmol/L
for men or <1.0 mmol/L for women).
c) Hypertension (systolic blood pressure ≥ 140 mm Hg and/
or diastolic blood pressure ≥ 90 mm Hg and/or previously
diagnosed as hypertension and under antihypertensive drug
administration)
d) Hyperglycemia (FPG ≥ 6.10 mmol/L) or T2DM.
Pathological Subtypes
Colorectal adenocarcinomas were divided into 4 subtypes [13]:
(a) Classic adenocarcinoma
(b) Mucinous adenocarcinoma (MAC: ≥ 50% of the tumor area
was composed of extracellular mucin)
(c) Signet-ring cell carcinoma (SRCC: ≥ 50% of the tumor area was
composed of intracellular mucin)
(d) Adenocarcinoma (AC) with components (AC with presence but
< 50% components of MAC and/or SRCC).
Histological Grade
In the present study, non-mucinous CRC (including classic adenocarcinoma and adenocarcinoma with mucinous/signetring cell components) were simply divided into low grade (≥ 50% glandular formation and including well to moderately-differentiated adenocarcinoma) and high grade (<50% gland formation or poorlydifferentiated adenocarcinoma) [14]. Because mucinous carcinoma including SRCC and MAC were more aggressive than classic adenocarcinoma and not further graded [15].
Statistical Analysis
Continuous variable was expressed as mean ± standard deviation (SD) and analyzed by t-test. Categorized data were calculated as frequencies or percentage and analyzed by Chisquare test. If the theoretical frequency was less than five (T< 5) in any grid of a four-grid (or R´C) table, Fishers exact test was used for Chi-square test. Univariate or multivariate binary logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for risks of KRAS mutation in association with T2DM and other factors (non-T2DM as reference). Two-sided value of P<0.05 was considered statistically significant. Statistical analysis was performed with SPSS software version 19.0 (IBM Corp., Armonk, NY).
Results
Baseline Characteristics
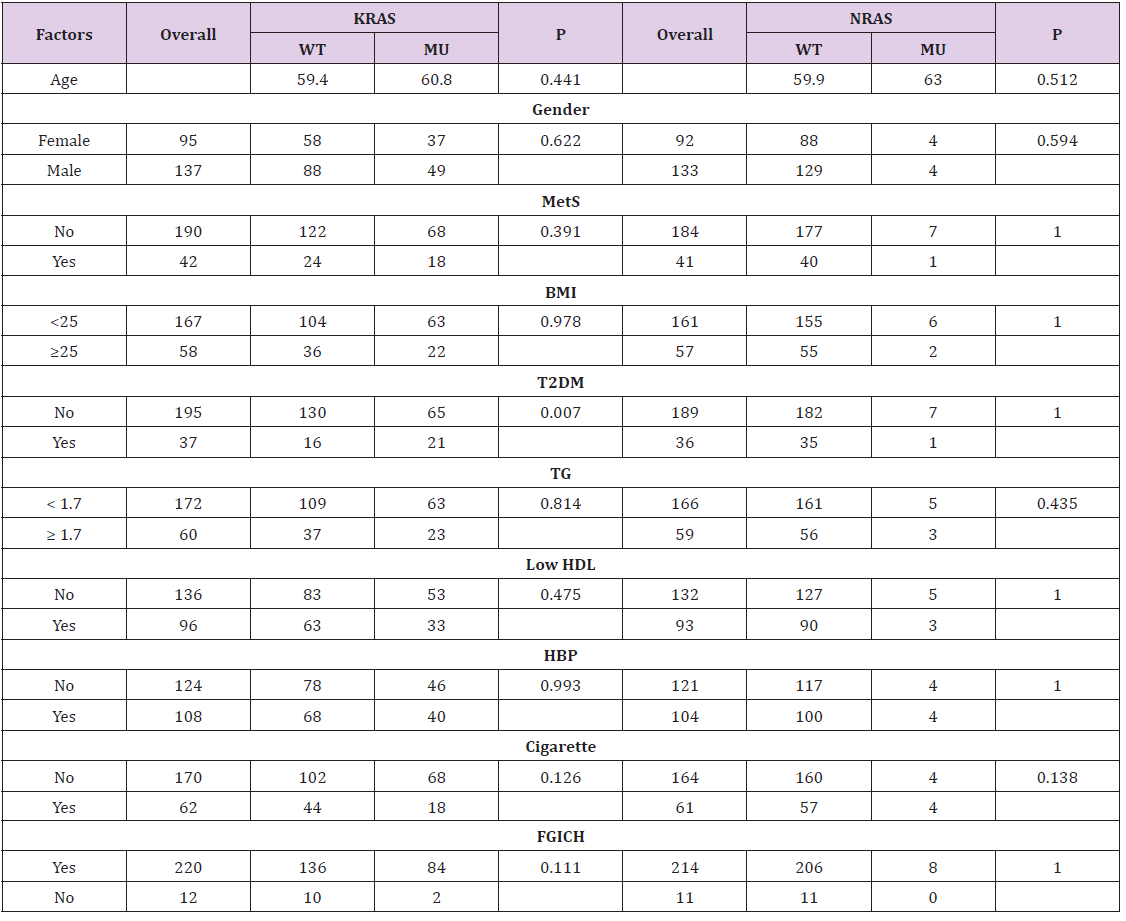
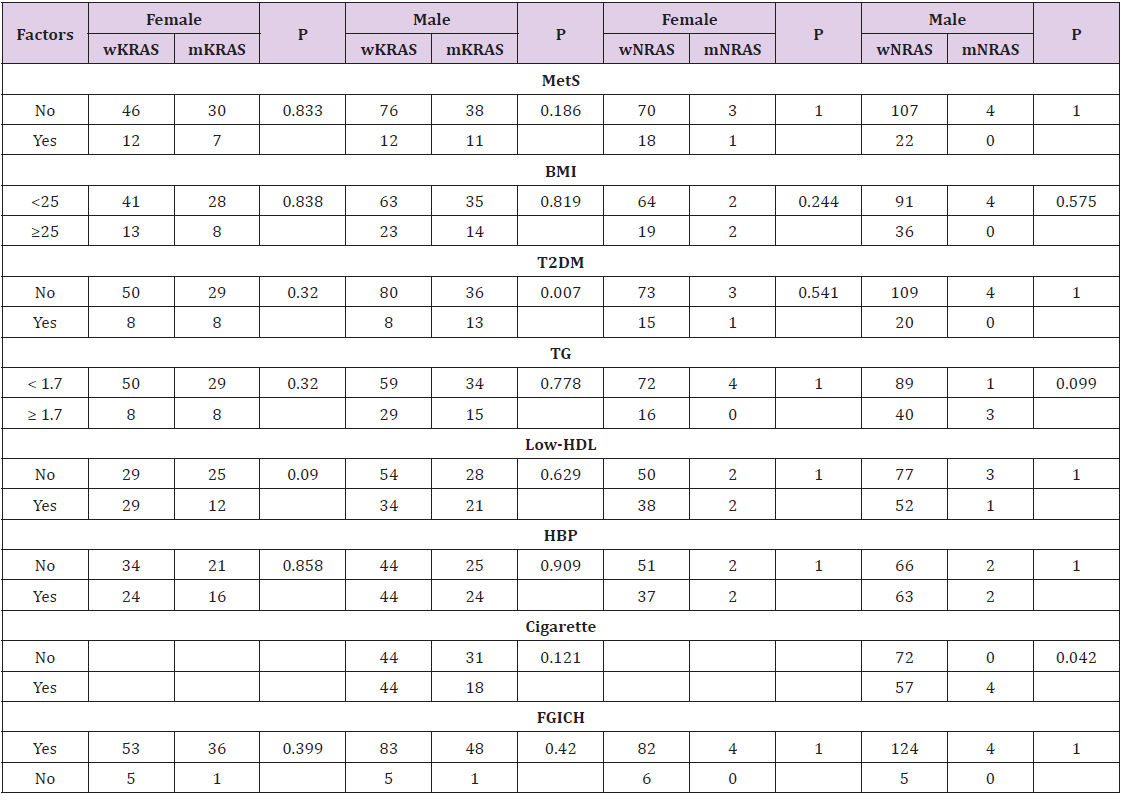
The mean age of the CRC patients was 59.9 (25-86 years). The overall T2DM rate of the CRC patients was 15.9% (37/232). Among the 37 T2DM patients, there were 25 patients with T2DM history before diagnosis of CRC, while 12 patients without T2DM history but met the criteria of ADA (FPG ≥ 7.0 mmol/L, n=10). The mutation rate of KRAS in T2DM group was considerably higher than non-T2DM in overall CRC patients (56.8% vs. 33.3%, P=0.007) (Table 1) and male patients (31.0% vs. 61.9%, P=0.007) (Table 2), but not in female patients. The KRAS mutation rate with cigarette smoking habit in male patients was slightly lower than those without cigarette smoking habit (29.0% vs. 41.3%, P=0.121) (Table 2). However, the NRAS mutation rate in male patients with cigarette smoking habit was significantly higher than those without cigarette smoking habit (0% vs. 6.6%, P=0.042) (Table 2). The results indicated cigarette smoking was positively associated with NRAS mutation in CRC. Factors including metabolic syndrome or its other individual components such as high BMI value (BMI ≥ 25 kg/m2), hypertension, high TG, low HDL, as well as FGICH were not significantly associated with KRAS/NRAS mutation status (P> 0.05).
Table 1: Basic characteristic according to KRAS/NRAS status of colorectal cancer.
Note: Statistics: Chi-square test; Age was expressed as mean and examined by t-test; WT: wild-type; MU: mutation-type; MetS: metabolic syndrome; TG: fasting triglyceride; T2DM: type 2 diabetes mellitus; HBP: hypertension; Cigarette: cigarette smoking habit; FGICH: family gastrointestinal cancer history.
Table 2: Basic characteristic according to KRAS/NRAS status of colorectal cancer in patients of both genders.
Note: Statistics: Chi-square test; wKRAS: wild-type KRAS; mKRAS: mutation-type KRAS; wNRAS: wild-type NRAS; mNRAS: mutation-type NRAS.
Association between T2DM and KRAS Mutation of CRC in Patients of Different Gender
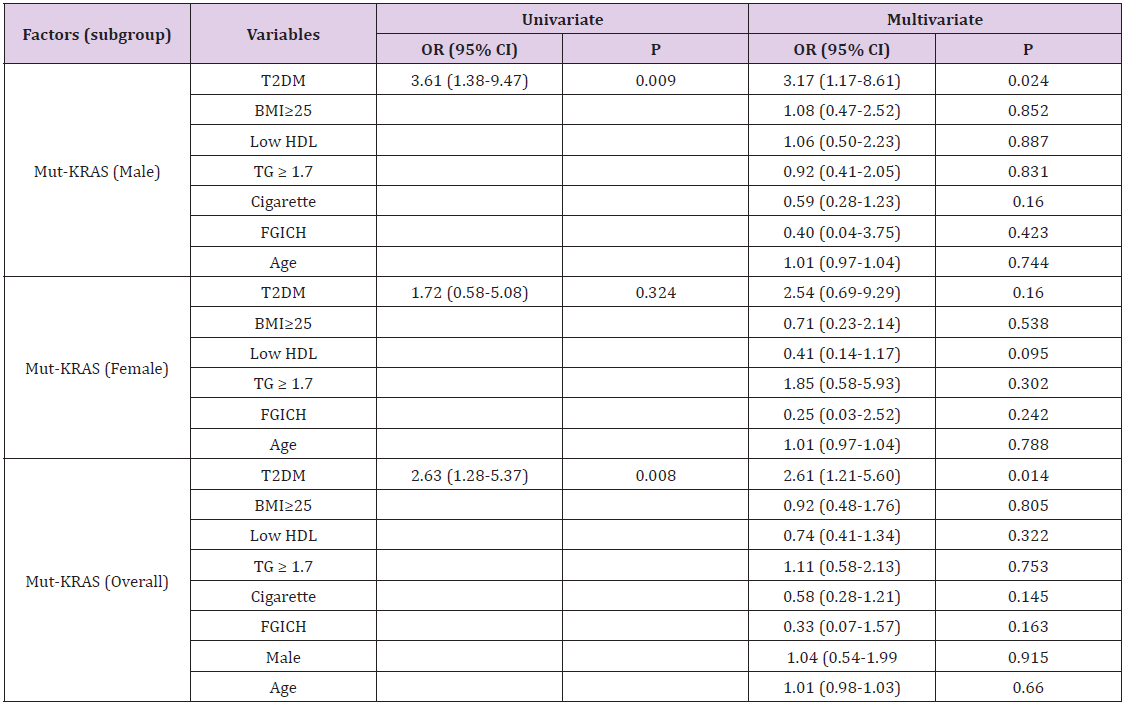
To further determine whether there was any sex-specific difference for the association between T2DM and KRAS mutation, we examined the relationship of KRAS mutation and T2DM in CRC patients of both gender. Univariately, T2DM significantly associated with KRAS mutation in overall patients (OR=2.63, 95% CI: 1.28-5.37, P=0.008) and male patients (OR=3.61, 95% CI: 1.38-9.47, P=0.009), but not in female patients. Multivariate logistic analysis including other potential confounders such as age, high BMI ( ≥25), low HDL, high TG (≥ 1.7), cigarette smoking habit, and family gastrointestinal carcinoma history, retained the above significance (Table 3). The results further demonstrated T2DM was an independent risk factor for KRAS mutation in CRC patients, especially in male patients (Table 4).
Table 3: Association between type 2 diabetes and mutation of KRAS and KRAS/NRAS of colorectal cancer in overall patient or both sex.
Note: Statistics: binomial logistic regression.
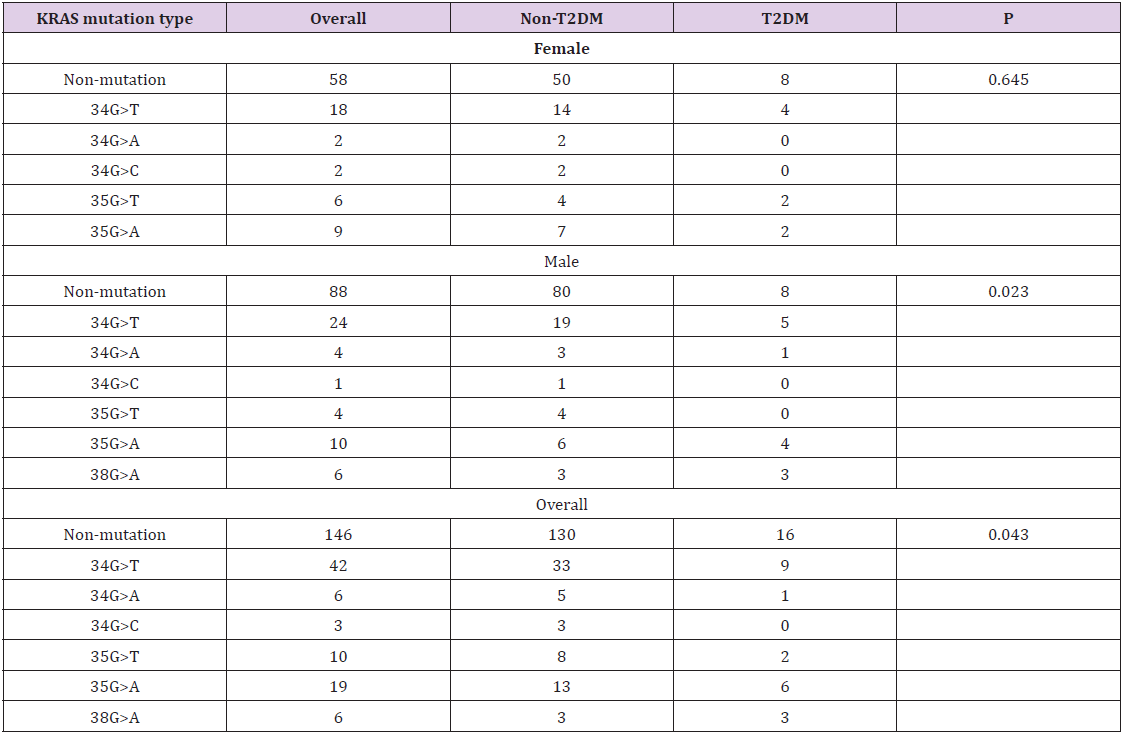
Table 4: Association between type 2 diabetes and KRAS mutation features of colorectal cancer.
Note: Statistics: Chi-square test; T2DM: type 2 diabetes; wKRAS: wild-type KRAS; mKRAS: mutant KRAS
Association between Mutation of KRAS or KRAS/NRAS and Pathological Features of CRC
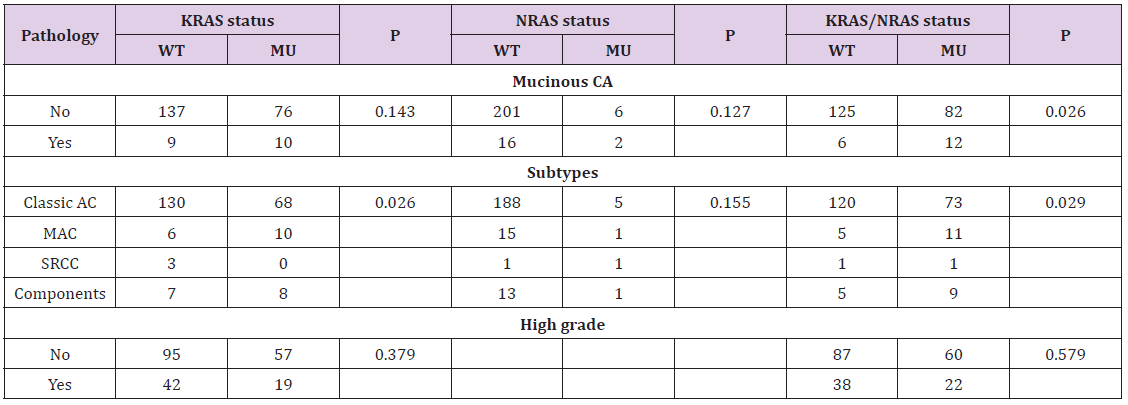
To study the association between mutation of KRAS or KRAS/ NRAS and pathological features of CRC, we examined the relationship between mutation of KRAS or KRAS/NRAS and pathological features including pathological subtypes and histological grade of CRC. As showed in Table 5, the rates of mucinous adenocarcinoma and adenocarcinoma with components (mucinous or signet-ring cell) in KRAS mutation group were 11.6% (10/86), 9.3% (8/86), which is higher than corresponding rates of KRAS wild-type group [4.1% (6/146), 4.8% (7/146), P=0.026]. Meanwhile, the rate of mucinous carcinoma in KRAS/NRAS-mutation group was significantly higher than KRAS/NRAS-wild-type group [12.8% (12/94) vs. 4.6% (6/131), P=0.026]. However, KRAS mutation was not significantly associated with histological grade in the present study (P> 0.05). The results indicated RAS mutation to be associated with mucinous histological phenotype of CRC which might lead to adverse clinical outcome.
Table 5: Association between KRAS/NRAS status and pathological features of colorectal cancer.
Note: Statistics: Chi-square test; WT: wild-type; MU: mutation-type; CA: carcinoma; Classic AC: classic adenocarcinoma; MAC: mucinous adenocarcinoma; Components: adenocarcinoma with mucinous and/or signet-ring cell components.
Discussion
There are a few reports about the association between diabetes and KRAS mutation so far and the results are inconclusive [16,17]. A recent study showed a tendency of positive association between abnormal HbA1C [ ≥48 mmol/mol (6.5%), the cut-off for diagnosis of T2DM] and KRAS mutation of CRC, although the association was not statistically significant (OR=1.85, P=0.1, n=170) [16]. Our results indicated T2DM predicted risk of mutation of KRAS, especially for men, while other assessed factors such as MetS, high BMI, low HDL, hypertension and hypertriglycemia did not significantly associated with status of KRAS mutation. The reason for the difference between above reports and our present results may partly due to the different criteria for T2DM. It is interesting that the association between T2DM and KRAS mutation of CRC was stronger in men in comparison with women. Previous studies have revealed a significant association between T2DM and CRC in men, but only a weak or unremarkable association with women [1,18]. Colon cancer risk is increased in prediabetic men, but not in women [19].
The present results demonstrated T2DM was significantly associated with mutation of KRAS in men, but only a weaker association between T2DM and KRAS mutation in women. The reason for the gender difference of diabetic impact may partly attributed to the different levels of sex hormone or its related factors such as adiponectin and sex hormone binding globulin (SHBG) [20]. Adiponectin is a kind of hormones secreted by adipose tissue and exerts an insulin-sensitizing effect [21]. Adiponectin was considered to play a role in colorectal carcinogenesis in men [22]. Low levels of adiponectin are associated with obesity, T2DM and increased risk of KRAS-mutant CRC [23]. Previous study demonstrated adiponectin level in male is significantly lower than female [24]. Moreover, lower levels of adiponectin and SHBG were likely to be correlated with family T2DM history-associated risk of CRC in male [25]. Further study is needed to clarify the molecular mechanism for the gender impact on association among T2DM and KRAS mutation, biological behaviors of CRC.
Although the mechanism for the association between T2DM and KRAS mutation is not fully understood so far, several lines of evidence may partly contribute to the association between T2DM and KRAS mutation status of CRC. At first, high level of glucose (in T2DM condition) promotes mutagenesis in human lymphoblastoid cells [26]. Secondly, high level of glucose triggers nucleotide imbalance through O-GlcNAcylation of key enzymes and induces KRAS mutation in pancreatic cells [27]. Whether these mechanisms are involved T2DM-associated mutation of KRAS of needs further investigation. KRAS mutation was considered to be associated with aggressive pathological features and prognosis of CRC [28,29]. Oncogenic KRAS can drive invasion and metastasis in a mouse model of CRC and the TGF-beta pathway was revealed as a key mediator for mutated KRAS-driven invasiveness [30]. Our present data showed of KRAS mutation to be significantly associated with mucinous phenotype of CRC (MAC or AC with mucinous components). Recent studies suggested KRAS mutation to be associated with risk of colorectal mucinous carcinoma and gastric mucinous carcinoma [7,9,31]. Because mucinous adenocarcinoma is commonly more aggressive than classic CRC, T2DM-associated KRAS-mutation might influence progression and prognosis via promoting mucinous phenotype of CRC.
Another interesting finding of the present study is the significant association between cigarette smoking and increased frequency of NRAS mutation in CRC. Although previous studies suggested cigarette smoking might be a risk factor for KRAS mutationnegative CRC, these studies did not assess the NRAS status of CRC in the meantime [32,33]. Although our present data also showed the KRAS mutation rate of CRC in male patients with cigarette smoking habit was lower than non-smoking patients, the NRAS mutation rate in male smoking patients was significantly higher than nonsmoking patients. The results support the notion that cigarette smoking was a risk factor for NRAS mutation in CRC. This study has some limitations. It was a single center clinical research and the samples for KRAS/NRAS mutation evaluation were relative limited. The mutation rates of NRAS is very low and hardly to analyze its subgroups.
Conclusion
In summary, the present study suggested T2DM to be significantly associated with risk of KRAS mutation of CRC, especially for male patients. KRAS/NRAS mutation was associated with mucinous phenotype of CRC. Cigarette smoking might be a risk factor for NRAS mutation of CRC in men. T2DM might influence carcinogenesis, progression and prognosis of CRC by promoting KRAS mutation and mucinous phenotype.
Acknowledgement
This work was supported by Zhejiang Medical Technology Plan Project (No. 2021KY515, 2019KY024, 2017ZD003).
Statement of Conflict of Interest
None declared.
References
- Luo W, Cao Y, Liao C, Gao F (2012) Diabetes mellitus and the incidence and mortality of colorectal cancer: a meta-analysis of 24 cohort studies. Colorectal Dis 14(11): 1307-1312.
- Abudawood M (2019) Diabetes and cancer: A comprehensive review. J Res Med Sci 24: 94.
- Ogino S, Nowak JA, Hamada T, Milner DA Jr, Nishihara R (2019) Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu Rev Pathol 14: 83-103.
- Ogino S, Chan AT, Fuchs CS, Giovannucci E (2011) Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 60(3): 397-411.
- Lenz HJ, Chu E, Grothey A (2008) KRAS mutation in metastatic colorectal cancer and its impact on the use of EGFR inhibitors. Clin Adv Hematol Oncol 6(12): 1-13, 14-16.
- Gonzalez RS, Cates JM, Washington MK, Beauchamp RD, Coffey RJ, et al. (2016) Adenoma-like adenocarcinoma: a subtype of colorectal carcinoma with good prognosis, deceptive appearance on biopsy and frequent KRAS mutation. Histopathology 68(2): 183-190.
- Zhang J, Zheng J, Yang Y, Lu J, Gao J, et al. (2015) Molecular spectrum of KRAS, NRAS, BRAF and PIK3CA mutations in Chinese colorectal cancer patients: analysis of 1,110 cases. Sci Rep 5(1): 18678.
- Docs O, Fazakas F, Horvath NL, Toth L, Andras C, et al. (2015) Changes of KRAS Exon 2 Codon 12/13 Mutation Status in Recurrent Colorectal Cancer. Pathol Oncol Res 21(2): 399-404.
- Guo TA, Wu YC, Tan C, Jin YT, Sheng WQ, et al. (2019) Clinicopathologic features and prognostic value of KRAS, NRAS and BRAF mutations and DNA mismatch repair status: A single-center retrospective study of 1,834 Chinese patients with Stage I-IV colorectal cancer. Int J Cancer 145(6): 1625-1634.
- Metabolic syndrome study cooperation group of chinese diabetes society. (2004) Suggestions about metabolic syndrome of Chinese diabetes society. Chin J Diab 12: 156-161.
- Sharma A, Ng H, Kumar A, Teli K, Randhawa J, et al. (2014) Colorectal cancer: Histopathologic differences in tumor characteristics between patients with and without diabetes. Clin Colorectal Cancer 13(1): 54-61.
- American Diabetes Association. (2018) 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 41: S13-S27.
- Nagtegaal I, Arends MJ, Salto-Tellez M (2019) WHO Classification of Tumours, Digestive System Tumours (5th)., IARC: Lyon, France, pp. 177-187.
- Fletcher C (2012) Diagnostic Histopathology of Tumors (4th)., Elsevier Saunders, Philadelphia, pp. 452-460.
- Bagante F, Spolverato G, Beal E, Merath K, Chen Q, et al. (2018) Impact of histological subtype on the prognosis of patients undergoing surgery for colon cancer. J Surg Oncol 117(7): 1355-1363.
- Tabuso M, Christian M, Kimani PK, Gopalakrishnan K, Arasaradnam RP (2020) KRAS Status is Associated with Metabolic Parameters in Metastatic Colorectal Cancer According to Primary Tumour Location. Pathol Oncol Res 26(4): 2537-2548.
- Yilmaz A, Mohamed N, Patterson KA, Tang Y, Shilo K, et al. (2014) Clinical and metabolic parameters in non-small cell lung carcinoma and colorectal cancer patients with and without KRAS mutations. Int J Environ Res Public Health 11(9): 8645-8660.
- Campbell PT, Deka A, Jacobs EJ, Newton CC, Hildebrand JS, et al. (2010) Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 139(4): 1138-1146.
- Onitilo AA, Berg RL, Engel JM, Glurich, Stankowski RV, et al. (2013) Increased risk of colon cancer in men in the pre-diabetes phase. PLoS One 8(8): e70426.
- Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, et al. (2013) Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol 11(4): 419-424.e1.
- Kadowaki T, Yamauchi T (2005) Adiponectin and adiponectin receptors. Endocr Rev 26(3): 439-451.
- Song M, Zhang X, Wu K, Ogino S, Fuchs CS, et al. (2013) Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer Prev Res (Phila) 6(9): 875-885.
- Inamura K, Song M, Jung S, Nishihara R, Yamauchi M, et al. (2015) Prediagnosis Plasma Adiponectin in Relation to Colorectal Cancer Risk According to KRAS Mutation Status. J Natl Cancer Inst 108(4): djv363.
- Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, et al. (2002) Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51(9): 2734-2741.
- Ma W, Song M, Kvaerner AS, Prescott J, Chan AT, et al. (2018) Sex-Specific Association between Family History of Diabetes and Risk of Colorectal Cancer: Two Prospective Cohort Studies. Cancer Prev Res (Phila) 11(9): 535-544.
- Zhang Y, Zhou J, Wang T, Cai L (2007) High level glucose increases mutagenesis in human lymphoblastoid cells. Int J Biol Sci 3(6): 375-379.
- Hu CM, Tien SC, Hsieh PK, Jeng YM, Chang MC, et al. (2019) High Glucose Triggers Nucleotide Imbalance through O-GlcNAcylation of Key Enzymes and Induces KRAS Mutation in Pancreatic Cells. Cell Metab 29(6): 1334-1349.
- Yuan Y, Liu Y, Wu Y, Zhang J, Shen C, et al. (2021) Clinical characteristics and prognostic value of the KRAS mutation in Chinese colorectal cancer patients. Int J Biol Markers 36(2): 33-39.
- Zhang M, Hu W, Hu K, Lin Y, Feng Z, et al. (2020) Association of KRAS mutation with tumor deposit status and overall survival of colorectal cancer. Cancer Causes Control 31(7): 683-689.
- Boutin AT, Liao WT, Wang M, Hwang SS, Karpinets TV, et al. (2017) Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev 31(4): 370-382.
- Hewitt LC, Saito Y, Wang T, Matsuda Y, Oosting J, et al. (2019) KRAS status is related to histological phenotype in gastric cancer: results from a large multicentre study. Gastric Cancer 22(6): 1193-1203.
- Weijenberg MP, Aardening PW, de Kok TM, de Goeij AF, van den Brandt PA (2008) Cigarette smoking and KRAS oncogene mutations in sporadic colorectal cancer: results from the Netherlands Cohort Study. Mutat Res 652(1): 54-64.
- Samadder NJ, Vierkant RA, Tillmans LS, Wang AC, Lynch CF, et al. (2012) Cigarette smoking and colorectal cancer risk by KRAS mutation status among older women. Am J Gastroenterol 107(5): 782-789.

 Research Article
Research Article