ABSTRACT
Last evidence has showed that ultrasound is useful in patients with coronavirus disease (COVID-19) to image damage on heart, vessels, liver, kidney, eye, and airway. Point-Of-Care Ultrasound (POCUS) is widely available and could enable clinicians to detect early signs of cardiovascular disease at the bedside to diagnose vascular disease in COVID-19 patients and to guide anticoagulant treatment during COVID-disease. This review summarizes POCUS usefulness by using a systematic literature search in major databases. Basic echography windows allow to assess patients in shock. Cardiovascular findings were found in 33%-80% of the COVID-19 patients: heart failure and pericardial effusion are common complications in COVID-19 patients; and vascular congestion sign may help distinguish COVID-19 from community-acquired pneumonia. In patients with sudden clinical worsening or elevated d-dimer levels, pulmonary and vascular echography may reveal peripheral venous and pulmonary embolism and lead to the initiation or changes in therapy. Other vascular characteristics in COVID-19 are “vascular thickening,” or “enlargement”. In conclusion, the literature supports the use of US as a safe, non-invasive method to help with COVID-19 diagnostic, monitorization, and therapy.
Keywords: Coronavirus; COVID-19; POCUS, Vascular Changes; Echography; Cardiovascular Complications
Abbreviations: COVID-19: Coronavirus-19 Disease; POCUS: Point of Care Ultrasound; VTE: Thromboembolism ; ACS: Acute Coronary Syndrome; MI: Myocardial infarction; DIC: Disseminated Intravascular Coagulation; RCT: Randomized Controlled Trial; ICU: Intensive Care Unit; IVC: Inferior Vena Cava; DVT: Deep Venous Thrombosis; MIx: Mechanical Index; EF: Ejection Fraction; PF: Pulsatility Fraction ; RUSH: Rapid Ultrasound in Shock and Hypotension; MHz: MegaHerzt; FOCUS: Focused Cardiac Ultrasound; FATE: Focus-Assessed Transthoracic Echocardiography; FALLS: Fluid Administration Limited by Lung Sonography; CCUE: Critical Care chest Ultrasonic Examination; ASE: American Society of Echocardiography; VExUS: Venous Excess Ultrasound; AKI: Acute Kidney Injury; LV: Left Ventricular; RV: Right Ventricular; JVP: Jugular Venous Pulsation; CTA: Computed Tomography Angiography; LE: Lung Embolism
Introduction
The most common complication of coronavirus disease (COVID-19) is respiratory failure, but COVID-19 is a systemic disease that involves heart, vessels, liver, kidney, and other organs [1]. Some of the reported cardiovascular complications are heart failure, pulmonary hypertension, venous thromboembolism (VTE), ischemic stroke, acute coronary syndrome (ACS)/myocardial infarction (MI), myocarditis, fluid overload and disseminated intravascular coagulation (DIC). Common findings in patients recovered from COVID-19 are lower left ventricular ejection fraction, higher left ventricle volumes or pericardial enhancement [2]. The reduction of the arteriovenous gradient across vital organs and the endothelial barrier dysfunction may cause inadequate perfusion [3,4]. Both elevated D-dimer levels [5,6] and acute venous thromboembolism are common findings in patients with COVID-19 [5,7-9]. Point-Of-Care Ultrasound (POCUS) is widely available and enables clinicians to visualize the vascular anatomy and this gives real-time information that can influence management decisions. There are several echographic markers used to measure compliance in great veins (cava, portal, hepatic, jugular, and intra-renal) [10]. The present COVID-19 pandemic has led to coordinate multidisciplinary efforts to safely treat and monitor patients who require interventional therapies. Vascular and cardiac ultrasonography findings can provide diagnostic and prognostic information to help triage the dyspneic patient and determine the need for subsequent imaging (echocardiogram, computerized tomography, nuclear magnetic resonance; etc.) [10]. The aim of the present study is to review POCUS usefulness to recognize vascular pathologies in COVID-19 patients, (grading system prototypes and the ability to predict vascular pathologies) and to guide anticoagulant treatment during COVID-disease.
Methods
We conducted a systematic English literature search on major databases (Cochrane Central, PubMed, Embase (Elsevier), Google Scholar, and the World Health Organization database), to identify relevant systematic and narrative reviews, clinical guidelines, randomized controlled trials (RCTs), observational studies, and case series. The search terms used were “ultrasound”, “echography”, “COVID-19”, “SARS-CoV-2”, “heart”, “cardiac”, “myocardial injury”, “myocarditis”, “acute myocardial infarction”, “acute coronary syndrome”, “dysrhythmia”, “arrhythmia”, “heart failure”, “venous thromboembolism”, “coagulable”. The terms were combined to execute multiple queries, and searches were conducted by each author independently. Then, a single common reference list for evaluation it was created. Preprinted articles were also included. It was performed on April 27, 2020 and updated on July 2, 2020. The study was reviewed and approved by the local ethics committee of Hospital Clínico Universitario de Valladolid, code number PI-17- 634. Informed consent was obtained from the patients in order to publish the images for this article. Every relevant article was reviewed by at least two members of the working group, all of them with experience in clinical practice and POCUS. They all discussed which studies to include for the review by consensus, with a focus on medicine-relevant articles. The investigators assigned a priori point ranking of “2” (randomized controlled trials, systematic reviews, clinical guidelines), “1” (observational studies or narrative reviews), or “0” (all others) points to all articles that were found. One additional point was added when positive elements were present (strength of association, more than 10 patients included, scientific society guidelines) or downgraded if there were negative elements (contradictory data, uncertain imprecision, less of 10 patients, no correlation between images and text).
Results
A total of 388 articles were retrieved and assessed for relevance by the working group as mentioned previously but only 19 of them met the needed criteria. The incidence of cardiovascular findings in COVID-19 patients ranged from 7,7% to 80% [9,10], depending on the different age and severity of the disease. This range can be explained by the early use of echography [9] and for the severity of the disease and an older age in different studies [11]. COVID-19 patients median age was 38 to 66 years, depending on the country where data were extracted [9]. Most of them (68%) were men, 16% required intensive care; and the median length of stay in the ICU (Intensive Care Unit) was 12 days. The intrahospital mortality rate was 26%, after a median of 7 days from hospital admission [9,11]. Thromboembolic events were found in 7,7% – 69% [7,12], and pulmonary embolism in 33% of them [9] Other findings were cardiogenic shock due to myocarditis, acute coronary syndromes, pericardial effusion, and arrhythmia or distributive shock [13]. In COVID-19 patients, septic and cardiogenic shock are the most common types; but obstructive shock (pericardial effusion, right heart collapse, heart swing, right ventricular enlargement,” tricuspid valve regurgitation, pulmonary artery or deep vein thrombosis, etc.) and hypovolemic shock (decrease of CO, “papillary muscle kissing sign,” Inferior Vena Cava (IVC) collapse and high respiratory variability, etc.). must be excluded.
Clinical Indications for the Use of Cardiovascular Pocus in COVID-19 Patient
POCUS should be used to change clinical management or to aid procedural guidance. Nowadays it is not known whether there is any relationship between the severity of POCUS findings and a patient’s clinical course [14]. In prone position, POCUS is useful tool to diagnose popliteal thromboembolism or myocardial disfunction. Cardiovascular POCUS indications include diagnosis of pre-existing cardiovascular disease [8,15], identification of changes in cardiac function and detection of cardiovascular complications associated with COVID-19 [16], which may be present in more than a quarter of patients with critical disease [17,18]. The most frequent cardiovascular complications are myocarditis or pericardial effusion, a hypercoagulable state which leads to deep venous thrombosis (DVT) and pulmonary embolism with associated right ventricular failure and acute pulmonary hypertension and left ventricular systolic dysfunction [9,17,19]. Ultrasound is a useful tool for guiding peripheral or central venous access and for rapid assessment of circulatory status, identifying the types of shock, monitoring during respiratory and hemodynamic management, and guiding the treatment of CoV pneumonia patients, which is especially feasible, convenient, and advantageous in critically ill patients [20,21].
Infection Control Strategies for Ultrasound
Pocket-carried wireless ultrasound devices should preferentially be chosen, as they can be easily protected with a probe cover and clean, maneuver, and do not have a cooling fan. If hand-held devices are not available, portable machines dedicated to COVID-19 patients exclusively can be used, although maximum care for sterilization is necessary [10,20,22]. Machines with touch screens are preferable to machines with keyboards or buttons. Single-use gel packets [23] and telemedicine may be useful in evaluation of patients these patients [14].
Ultrasound Protocols
POCUS should be used by the most experienced operator involved in patient care, to minimize scanning time [9], conservation of Personal Protection Equipment, and to reduce missed findings [10]. Either convex or linear transducers can be used, according to the patient’s body size and a single–focal point modality, setting focal point on the structure to explore. Other recommendations are: to avoid saturation phenomena as far as possible; good quality control gain settings; no cosmetic filters and to save data. The mechanical index (MIx) must be kept low (start from 0.7 and reduce it further as the visual findings allowed it), as high MIx sustained for long periods may result in lung damage. Harmonic imaging, contrast, doppler, and spatial compound filters should be avoided, alongside saturation phenomena [11,12]. The basic echocardiography views to assess are apical four chambers, parasternal long axis, parasternal short axis and subxiphoid four-chamber view, subxiphoid inferior vena cava (IVC) long and short axis view. The parameters that should be measured are the diameter of IVC, EF (ejection fraction), the velocity-time integral of the left ventricular outflow during the continuous and dynamic evaluation of patients’ volume state and fluid responsiveness, left ventricular systolic function, and left ventricular output effect [14]. The IVC diameter is measured in its intra-hepatic portion at 2 cm of the junction with the hepatic veins using a longitudinal view from a subxiphoid position and moving the probe to the right side of the body until an adequate view was achieved. The maximal diameter during the respiratory cycle was measured. The Caval Index (IVC expiratory diameter – IVC inspiratory diameter)/ IVC expiratory diameter × 100) that is measured by M-mode scan can be misleading in pulmonary diseases usually present in COVID-19 such as tension pneumothorax, massive pulmonary embolism, and pericardial tamponade [24].
Vascular ultrasound assessment included popliteal vein Doppler (in prone position); hepatic vein Doppler, portal vein Doppler, intra-renal venous Doppler, and inferior vena cava (IVC) ultrasound (in supine position). Severe venous congestion was defined as the presence of severe flow abnormalities in multiple Doppler patterns with a dilated IVC (≥2 cm) [24]. For the hepatic vein Doppler, a systolic phase was of lesser amplitude than the diastolic phase but toward the liver was considered mild while the presence of a reversed systolic phase was considered severe [25]. For the portal vein Doppler, a pulsatility fraction (PF) of 30–49% was considered mild while a PF>50% was considered severe [17]. There are different specific ultrasound protocols that had been used in COVID-19 patients [26]. However, all of them present several limitations in COVID-19 disease because it is difficult to place the critical patients in left side decubitus [27]. The choice of the protocol depends on the clinical setting. RUSH protocol (Rapid Ultrasound in Shock and Hypotension) [28] facilitates the determination of the type of shock (hypovolemic, distributive, obstructive or cardiogenic) and is performed with a phased array transducer (3.5-5 MHz) for thoracoabdominal scanning and a linear transducer (7.5-10 MHz) for venous examinations. Other protocols described are the focused cardiac ultrasound (FOCUS) [29] for quick evaluation of emergency or critical patients, the focus-assessed transthoracic echocardiography (FATE) [30] advanced FATE protocol, fluid administration limited by lung sonography (FALLS) protocol [31], critical care chest ultrasonic examination (CCUE) protocol [32], American Society of Echocardiography (ASE)-POCUS protocol [10], which includes basic cardiac views, subcostal views for inferior vena cava and pericardial fluid, and chest views, and VExUS (Venous Excess Ultrasound) grading system of the severity of venous congestion to predict the occurrence of acute kidney injury (AKI) [33].
Cardiovascular Ultrasound Findings in COVID-19
Point-of-care echocardiography may demonstrate a hyperdynamic cardiac function of the left ventricular (LV), with or without decrease of peripheral vascular resistance, which is often seen in the early stage following the systemic inflammatory response; acute stress-induced cardiomyopathy (Takotsubo) characterized by transitory LV segmental contraction abnormalities, most often, apical ballooning; right ventricular (RV) dilatation and acute pulmonary hypertension, mainly caused by “internal factors” (alveolar and pulmonary capillary damage caused by inflammation, hypoxia, and hypercapnia) and/or “external factors” (fluid overload, and unsuitable mechanical ventilation); diffuse myocardial inhibition at ending stage, often caused by severe hypoxia, and long term of anoxia and inflammation [34,35]. The most common types of shock in COVID-19 patients are septic and cardiogenic shock (enlargement of the heart, segmental or diffuse contraction abnormalities, IVC dilation, B lines in the lungs and pleural effusion, etc.) [36]; however, the obstructive and hypovolemic shock must be excluded [37]. The vascular changes found in COVID-19 patients were: “vascular thickening,” “vascular enlargement,” “vascular congestion”, and thrombosis phenomenon [38]. Other authors have found pulmonary embolism [39] and systemic venous hypertension resulting from fluid overload and right ventricular failure [40].
Discussion
Evidence suggests that the earlier we treat, the better patients improve with treatment [41]. Four cardiovascular settings have been described in the COVID-19 pandemic: cardiovascular patients without COVID-19; differential diagnostic between COVID-19 and a new cardiovascular disease, COVID-19 in chronic cardiovascular patients [15], and cardiovascular changes in COVID-19 patient [16,18]. Some COVID-19 patients suffer from right ventricular failure or pulmonary hypertension, and fluid overload [35]. A reduction of the arteriovenous gradient across vital organs may hamper adequate perfusion [2]. This phenomenon may be worsened with the development of interstitial edema after the prolonged elevation of capillary hydrostatic pressure in the context of endothelial barrier dysfunction [42]. Several markers of the high pressures associated with this congestive process have been proposed including the assessment of large veins (cava, internal jugular) as well as detecting abnormal venous waveforms suggestive that the limit of the systemic venous compliance in the portal vein, hepatic veins, and intra-renal veins [8]. Assessment of the inferior vena cava (IVC) and/or jugular venous pulsation (JVP) plays an important role in hemodynamic assessment of critically ill patients to enhance the physical exam and in the assessment of fluid status. Deep vein thrombosis risk increases with any critically ill bed-bound patient; and several studies described a prothrombotic state in COVID-19 [9]. Vascular endothelial dysfunction contributes to the pathophysiology of microcirculatory changes in SARSCoV- 2 infection, which may explain reports of cerebrovascular complications in younger patients, myocardial ischemia, and thromboembolic complications [42].
The development of interstitial edema in encapsulated organs such as the kidney and the brain may result in a rapid elevation in interstitial pressure, which decreases organ blood flow [42,43]. The high number of arterial and, in particular, venous thromboembolic events (VTE) diagnosed within 24 h of admission and the high rate of positive VTE imaging tests among the few COVID-19 patients tested suggest that there is an urgent need to improve specific VTE diagnostic strategies and investigate the efficacy and safety of thromboprophylaxis in ambulatory COVID-19 patients [7,10]. Some of these changes are shown in Figures 1-3. POCUS may enable clinicians to detect significant systemic venous hypertension and venous thrombosis. However, each of the markers has some pitfalls and limitations. Hepatic vein Doppler is strongly influenced by tricuspid regurgitation [44]. Pulsatile portal vein flow and IVC dilatation have been reported in healthy athletic volunteers which raise the possibility of false-positive cases [19]. Intra-renal venous Doppler is technically difficult to perform, therefore with a greater chance of providing incorrect results, especially in patients with poor ultrasound penetration or with less-sensitive Doppler devices, so it is necessary to combine two or more vessels to diagnose [45]. Few published studies have commented on incident VTE in patients with COVID-19. As reported in a retrospective study, 20 out of 81 ICU patients with severe covid-19 developed an incident VTE (25%). Of note, none of the patients had received VTE prophylaxis [12]. In a study of 25 patients with COVID-19 from Wuhan, who were suspected of having lung embolism (LE) and underwent computed tomography angiography (CTA), 10 (40%) had evidence of acute LE on imaging (https://papers.ssrn.com/sol3/papers. cfm?abstract_id=3548771); however, the study did not provide information related to use of VTE prophylaxis or the reason for performing CTA. In a study of 184 patients with severe COVID-19 from 3 academic medical centers in the Netherlands, the authors reported that 31% (95%CI 20-41) of patients developed incident VTE. All patients received pharmacological prophylaxis, although under-dosing was observed in 2 of the 3 participating centers [5]. These findings require validation in additional studies.
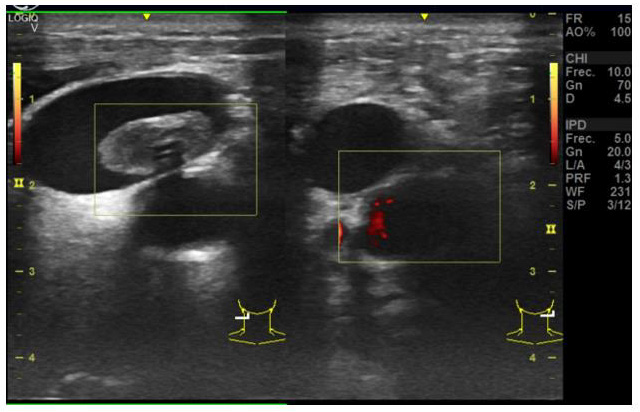
Figure 1: Cervical scan in Covid-19 patient No carotid pulse in M Mode. Massive cerebral hemorrhage diagnose was confirmed in the cerebral scan.
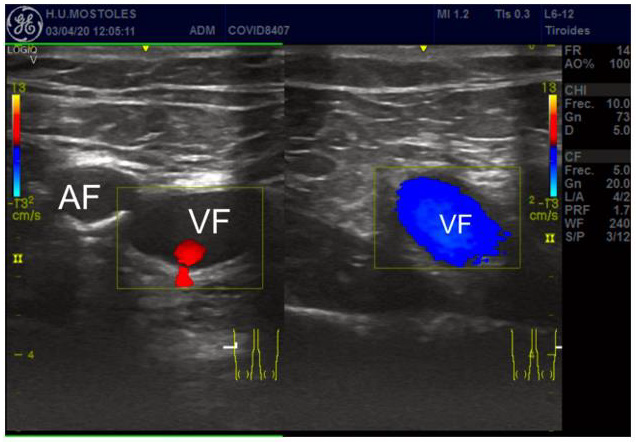
Figure 2: Popliteal vein thrombosis (B). Hyperecogenic vascular wall and thrombus (drawn as a circle). VF = Femoral Vein, AF= Femoral Arterial.
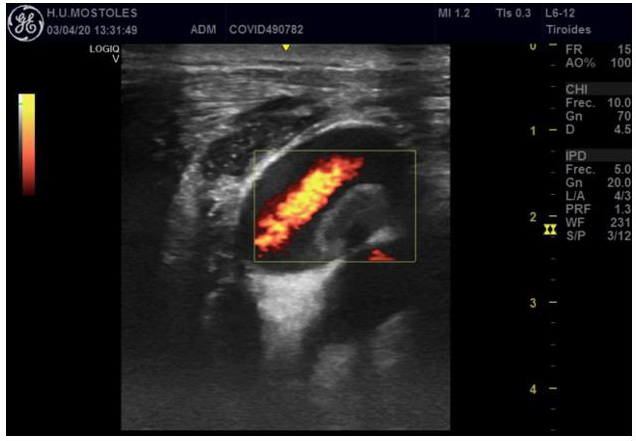
Figure 3: Jugular and carotid thrombosis. Hyperecogenic vascular wall and thrombus (inside the square).
It is possible but unknown that VTE remains underdiagnosed in patients with severe COVID-19. From March 19 to April 11, 2020, 26 consecutive patients with severe COVID-19 were screened for VTE. Eight patients (31%) were treated with prophylactic anticoagulation, whereas 18 patients (69%) were treated with therapeutic anticoagulation. The overall rate of VTE in patients was 69%. The percentage of VTE was higher in patients with antithrombotic prophylaxis (100% vs 56%, respectively, P = 0.03). Surprisingly, we found a high rate of thromboembolic events in COVID-19 patients treated with therapeutic anticoagulation, with 56% of VTE and 6 pulmonary embolisms [12]. This is pivotal because ARDS in patients with COVID-19 is, itself, a factor of hypoxic pulmonary vasoconstriction, pulmonary hypertension, and right ventricular failure. A further insult from LE may be unrecoverable [13]. Myocardial injury associated with the SARS-CoV-2 was a common condition in patients diagnosed with COVID-19 and associated with a higher risk of in-hospital mortality [6,35]. A study of 416 hospitalized patients with COVID-19 in Wuhan showed that 19.7% present cardiac injury, the ones older, with more comorbidities, higher leukocyte counts, levels of C-reactive protein, procalcitonin, creatinine kinase-myocardial band, myohemoglobin, high-sensitivity troponin I, N-terminal pro-B-type natriuretic peptide, aspartate aminotransferase, and creatinine.
The respiratory signs were more severe, and complications were more comun in patients with cardiac injury. [46]. A lot of the COVID-19 treatments can modify cardiac preload and cause electrocardiographic changes (anti-HIV drugs, hydroxychloroquine; ventilatory support, prone positioning, and extracorporeal membrane therapy). Therefore, POCUS could be useful, being performed on several time points helping in the clinical diagnosis, in determining early congestive failure [47], treatment monitoring during the paucisymptomatic phase of the disease and potentially playing a role in treatment decisions. POCUS can be used to monitor cardiovascular in every setting, since secondary level imaging studies (such as CT scan) are not everywhere easily accessible. Besides, angiography carries a significant risk for nosocomial spread of the virus and acute kidney injury in COVID-19 patients [43]. However, some limitations of POCUS are that image quality in some patients is inadequate and it relies on the expertise of the operator [48], and the nonthermal induction of pulmonary capillary hemorrhage of pulsed ultrasound in animal models [47].
Limitations
Circumstances surrounding the pandemic state are extremely dynamic, so recommendations change as our knowledge of the disease improves. The current literature evaluating cardiovascular complications and effects associated with COVID-19 suffers from several limitations, including significant heterogeneity in patient selection, outcomes, comparators, and study design, as well as low numbers of included patients and high risk of bias. During the current pandemic, a significant amount of literature is published in preprint form before completion of full peer review. Further data are needed concerning the mentioned cardiovascular complications and COVID-19 [49,50].
Conclusion
Ultrasound evaluation of COVID-19 patients depends on available resources, the expertise of personnel, and logistic configurations unique to each situation. POCUS may be considered the first-line ultrasound examination (whenever personnel protective equipment is available) to diagnose cardiac involvement and VTE. POCUS has lower risk of viral spread and does not produce acute kidney failure (in contrast with pulmonary angiography or scanner), especially in non-collaborator patients. Meticulous disinfection of any POCUS device is critical to reducing the risk of SARS-CoV-2 infection. Vascular findings are useful to provide both diagnostic and prognostic information and might significantly contribute to patient management. The vascular congestion sign may help distinguish COVID-19 from community-acquired pneumonia.
Declarations
Ethics Approval and Consent to Participate
This article has been approved by local Ethic Committee of Hospital Clínico Universitario de Valladolid.
Consent for Publication
All authors confirm consent for publication.
Availability of Data and Materials
Competing Interests
All authors declare no conflict of interest.
Funding
No external sources of funding.
Authors’ Contributions
All authors have been involved in the design, data collection, and final writing of the manuscript.
Acknowledgements
Not applicable.
References
- Yao, XH Yao, TY Li, ZC He, YF Ping, et al. (2020) A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi Chinese J Pathol 49(5): 411-415.
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, et al. (2020) Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 5(11): 1265-1273.
- De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL (2014) Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 5(1): 73-79.
- Saito S, Uchino S, Takinami M, Uezono S, Bellomo R, et al. (2016) Postoperative blood pressure deficit and acute kidney injury progression in vasopressor-dependent cardiovascular surgery patients. Crit Care 20: 74.
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, et al. (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191: 145-147.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229): 1054-1062.
- Cui S, Chen S, Li X, Liu X, Wang F, et al. (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 18(6): 1421-1424.
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, et al. (2020) Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol 75(18): 2352-2371.
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, et al. (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191: 9-14.
- Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, et al. (2020) ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J Am Soc Echocardiogr 33(6): 648-653.
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, et al. (2020) Global COVID-19 Thrombosis Collaborative Group, endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol 75(23): 2950-2973.
- Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, et al. (2020) High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 18(7): 1743-1746.
- Boukhris M, Hillani A, Moroni F, Annabi MS, Addad F, et al. (2020) Cardiovascular Implications of the COVID-19 Pandemic: A Global Perspective. Can J Cardiol 36(7): 1068-1080.
- Kim DJ, Jelic T, Woo MY, Heslop C, Olszynski P, et al. (2020) Just the Facts: Recommendations on point-of-care ultrasound use and machine infection control during the coronavirus disease 2019 pandemic. CJEM 22(4): 445-449.
- Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, et al. (2020) Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 69(24): 759-765.
- Davis GK, Adlan A, Majewski J, Ibrahim B (2020) SARS-CoV-2 pandemic and the cardiovascular system: What the non-cardiologist needs to know. Clin Med (Lond) 20(3): 262-265.
- Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, et al. (2020) COVID-19 and cardiovascular disease. Circulation. 141(20): 1648-1655.
- Dhawan R, Gundry RL, Brett-Major DM, Mahr C, Thiele GM, et al. (2020) COVID-19 and cardiovascular disease: What we know, what we think we know, and what we need to know. J Mol Cell Cardiol 144: 12-14.
- Vidovich MI, Fischman DL, Bates ER (2020) COVID-19 STEMI 2020: It's Not What You Know, It's How You Think. JACC Case Rep 2(9): 1289-1290.
- Peng QY, Wang XT, Zhang LN (2020) Using echocardiography to guide the treatment of novel coronavirus pneumonia. Crit Care 24(1):143.
- Johri AM, Galen B, Kirkpatrick JN, Lanspa M, Mulvagh S, et al. (2020) ASE Statement on Point-of-Care Ultrasound during the 2019 Novel Coronavirus Pandemic. J Am Soc Echocardiogr 33(6): 670-673.
- Buonsenso D, Pata D, Chiaretti A (2020) COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med 8(5): e27.
- Kampf G, Todt D, Pfaender S, Steinmann E (2020) Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 104(3): 246-251.
- Wallace DJ, Allison M, Stone MB (2010) Inferior vena cava percentage collapse during respiration is affected by the sampling location: an ultrasound study in healthy volunteers. Acad Emerg Med 17(1): 96-99.
- Mordi I, Manian U, Bagur R, Tzemos N (2019) Diagnosis and follow-up of idiopathic dilatation of inferior vena cava. Echocardiography 36(5): 831-836.
- Zhang L, Wang B, Zhou J, Kirkpatrick J, Xie M, et al. (2020) Bedside Focused Cardiac Ultrasound in COVID-19 from the Wuhan Epicenter: The Role of Cardiac Point-of-Care Ultrasound, Limited Transthoracic Echocardiography, and Critical Care Echocardiography. J Am Soc Echocardiogr 33(6): 676-682.
- Wang X, Liu D, He H, Du W, Zhang H, et al. (2015) Using critical care chest ultrasonic examination in emergency consultation: a pilot study. Ultrasound Med Bio 41(2): 401-406.
- Perera P, Mailhot T, Riley D, Mandavia D (2010) The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am 28(1): 29-56.
- Labovitz AJ, Noble VE, Bierig M, Goldstein SA, Jones R, et al. (2010) Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr 23(12): 1225-1230.
- Nagre AS (2019) Focus-assessed transthoracic echocardiography: Implications in perioperative and intensive care. Ann Card Anaesth 22(3): 302-308.
- Lichtenstein D (2012) Fluid administration limited by lung sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Med 6(2): 155-162.
- Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, et al. (2020) Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 12(1):16.
- Bhardwaj V, Vikneswaran G, Rola P, Raju S, Bhat RS, et al. (2020) Combination of Inferior Vena Cava Diameter, Hepatic Venous Flow, and Portal Vein Pulsatility Index: Venous Excess Ultrasound Score (VEXUS Score) in Predicting Acute Kidney Injury in Patients with Cardiorenal Syndrome: A Prospective Cohort Study. Indian J Crit Care Med 24(9): 783-789.
- Jain SS, Liu Q, Raikhelkar J, Fried J, Elias P, et al. (2020) Indications for and Findings on Transthoracic Echocardiography in COVID-19. J Am Soc Echocardiogr 33(10): 1278-1284.
- Eketunde AO, Mellacheruvu SP, Oreoluwa P (2020) A Review of Postmortem Findings in Patients With COVID-19. Cureus 12(7): e9438.
- Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, et al. (2020) Cardiovascular manifestations and treatment considerations in COVID-19. Heart 106(15): 1132-1141.
- Galluccio F, Ergonenc T, Garcia Martos A, Allam AE, Pérez-Herrero M, et al. (2020) Treatment algorithm for COVID-19: a multidisciplinary point of view. Clin Rheumatol 39(7): 2077-2084.
- Lee JH, Lee SH, Yun SJ (2019) Comparison of 2-point and 3-point point-of-care ultrasound techniques for deep vein thrombosis at the emergency department: A meta-analysis. Medicine (Baltimore) 98(22): e15791.
- Danzi GB, Loffi M, Galeazzi G, Gherbesi E (2020) Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 41(19): 1858.
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher, et al. (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7): 685-713.
- McCullough PA, Kelly RJ, Ruocco G Lerma E, Tumlin J, Wheelan KR, et al. (2021) Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med 134 (1): 16-22.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, et al. (2020) Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endothelitis in COVID-19. Lancet 395(10234): 1417-1418.
- Cheng Y, Luo R, Wang K, Zhang M, Wang Z, et al. (2020) kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97(5): 829-838.
- Chouchani M, Michaelsen J, Langenbrink L, Piatkowski M, Altiok E, et al. (2020) Quantification of tricuspid regurgitation area by 3-dimensional color Doppler echocardiography considering different clinical settings. Echocardiography 37(7): 1120-1129.
- Cruces P, Salas C, Lillo P, Salomon T, Lillo F, et al. (2014) The renal compartment: a hydraulic view. Intensive Care Med Exp 2(1): 26.
- Miller DL, Dong Z, Dou C, Raghavendran K (2018) Pulmonary Capillary Hemorrhage Induced by Different Imaging Modes of Diagnostic Ultrasound. Ultrasound Med Biol 44(5): 1012-1021.
- Yockelson SR, Heitner SB, Click S, Geleto G, Treggiari MM, et al. (2019) Right Ventricular Systolic Performance Determined by 2D Speckle-Tracking Echocardiography and Acute Kidney Injury After Cardiac Surgery. J Cardiothorac Vasc Anesth 33(3): 725-731.
- Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, et al. (2020) Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19: A Simple, Quantitative, Reproducible Method. J Ultrasound Med 39(7): 1413-1419.
- Scheinfeld, A Bilali, M Koenigsberg (2009) Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics 29(7): 2081-2098.
- Shi S, Shi M, Qin B, Shen Y, Cai T, et al, (2020) Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol 5(7): 802-810.

 Research Article
Research Article