ABSTRACT
Abbreviations: SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronavirus Disease 2019; CVST: Cerebral Venous Sinus Thrombosis; CRAO: Central Retinal Artery Occlusion; OCT: Ocular Coherence Tomography; EUA: Emergency Use Authorization
Introduction
The Ad26.COV2.S COVID-19 is a viral vector vaccine (Janssen/Johnson & Johnson) that was issued an emergency use authorization (EUA) by the U.S. Food and Drug Administration for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This vaccine was 66.3% effective in clinical trials at preventing laboratory-confirmed COVID-19 infection (sadoff, et al. [1]). Several cases of cerebral venous sinus thrombosis (CVST) reported among recipients of this vaccine resulted in temporary national pause in vaccination with this product on April 13, 2021 (Isaac See, et al. [2-4]). On April 23, administration of this vaccine continued after its use was reaffirmed by the CDC’s Advisory Committee on Immunization Practices (ACIP) due to a complete review of the cases following post authorization (Isaac See, et al. [2-4]). In addition to CVST, other venous and arterial events were reported following the administration of this vaccine (See, et al. [1,2,6]). Central Retinal Artery Occlusion (CRAO) is a devastating condition where the retina is permanently damaged due to lack of perfusion. This condition has been reported following COVID-19 infection (Montesel, et al. [7,8]). However, there are no reported cases of CRAO following COVID-19 vaccinations. We are reporting a case of acute onset CRAO after receiving Ad26.COV2.S COVID-19 vaccine.
Case Presentation
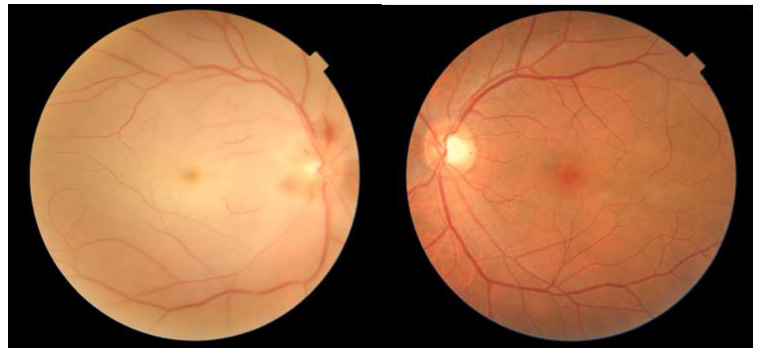
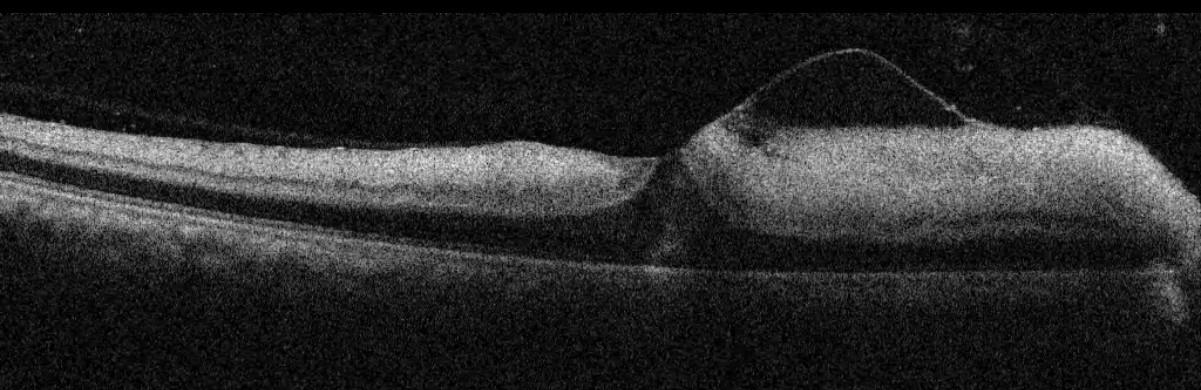
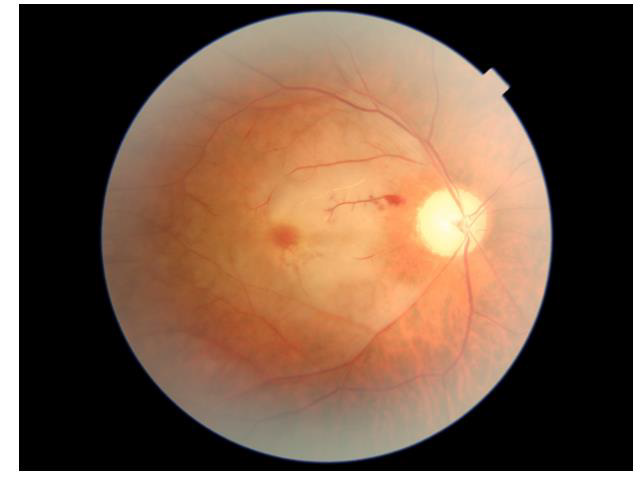
A healthy 51 years old Hispanic male with no past medical history presented with painless loss of vision in his right eye. He was vaccinated with Ad26.COV2.S COVID-19 (Janssen/Johnson & Johnson) four days prior to loss of vision. On the day of examination, his best corrected visual acuity was hand motion in the right eye and 20/20 in the left eye. The ophthalmologic examination of the left eye was unremarkable, while the right eye showed a relative afferent pupillary defect. Dilated fundus ophthalmoscopy revealed the presence of severe arterial narrowing, retinal whitening in the macular region, and loss of the physiological macular reflex. In addition, his optic nerve head exam showed elevation with pallor and superior flame hemorrhages. Ocular coherence tomography (OCT) of the right macula showed thickening with hyperreflectivity of the inner retinal layers (Figure 1). Color fundus photography of the right eye revealed whitening of the macula sparing the fovea in the typical cherry red spot appearance (Figure 2). A diagnosis of CRAO was hence made. Blood tests including CBC, CMP, PT, INR and PTT were within normal limits. Inflammatory markers were also examined including Rheumatoid Factor, ANA, Westergren Sedimentation Rate and C-Reactive protein were within normal limits and not suggestive of an arteritic CRAO (Table 1). At the 3 week follow-up visit, vision in the right eye did not improve. OCT scans showed improvement in hyperreflectivity but the beginning of inner retinal atrophy (Figures 3 & 4).
Discussion
As of August of 2021, the CDC reports that 8.16 percent (around 14 million) of the fully vaccinated population in the United States have received the Ad26.COV2.S COVID-19 vaccine (Janssen/ Johnson & Johnson). Following the administration of this vaccine, several cases reported complications including PE, DVT, and CVST with Thrombocytopenia. To summarize these complications, there were (15) cases of CVST of which 12 were from post vaccination case reports, 1 from an African cohort, and 2 from additional case reports. The range of onset for these CVST cases was 6 to 21 days post vaccination, and the platelet count range was 9 to 127k. In addition there were 10 DVT cases, for which the time to onset was 13-36 days post vaccination and the platelet count range was 10 to 127k. Furthermore, there were 10 PE cases with a range of onset 3 to 57 days post vaccination. (See, et al. [1,2,4,5]) Review of the literature revealed no cases of arterial thrombotic complications with any of the COVID-19 Vaccines. However, those related to COVID-19 infection included 59 unique cases such as: Ischemic stroke, Mesenteric ischemia, acute limb ischemia, Intraaortic thrombus and Large-vessel stroke. Out of the 59 cases there are 25 cases of Ischemic stroke, 6 cases of Large-vessel stroke, 23 cases of acute limb ischemia, 4 cases of Mesenteric and small bowel ischemia, and 1 case of Intra-aortic thrombus. As for CRAO cases after COVID-19 infection, literature search revealed 3 cases, all of which had other risk factors to develop this condition such as hypertension, hyperlipidemia, obesity, and sickle cell trait. The reported cases of CRAO developed 12 days to 21 days after diagnosis of COVID 19 was establishied (Montesel, et al. [7-9]). In addition to cases mentioned above, thrombotic complications have been reported in 8% of COVID-19 infection cohort (Lodigiani [10]). The relation between thrombotic diseases and COVID-19 infection has been explained by inflammatory homeostasis and endothelial cell dysfunction connected to direct viral infection of endothelial cells (Varga [11]).
As for the relation between COVID-19 vaccines and thrombotic events, they have been associated with elevated IgG isotypes that recognise PF4 and active platelets through the Fcgamma receptors (Greinacher, et al. [6,12]). This relation has only been described with adenoviral vector vaccines while none of the mRNA vaccines have had reported cases of arterial thrombotic events. The case reports summarized above indicate that COVID-19 infection can result in a hypercoagulable state and blockage of medium to large vessels including central retinal, mesenteric, and intracranial arteries. Since the Ad26.COV2.S COVID-19 vaccine is a viral vector vaccine, it appears to have a different safety profile compared to mRNA vaccine and that is possibly behind the difference in safety profile and the increased occurrence of venous thrombotic complications after this vaccine. The complication we report here, however, is arteritic in nature and occured in a small sized artery. Such occlusion is typically related to embolic events or arteritic inflammatory processes leading to occlusion of the artery. CRAOs are rarely associated with hypercoagulable states but its reports in COVID-19 infected patients along with other arteritic occlusions make it a plausible explanation for this case where possibly the viral vector vaccine induced antibody-related platelet activation.
References
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, et al. (2021) Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 384: 2187-2201.
- See I, Su JR, Lale A, Woo EJ, Guh AY, et al. (2021) US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 325(24): 2448-2456.
- Oster ME, Shay DK, Su JR, Gee J, Creech CB, et al. (2022) Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 327(4):331-340.
- Lale A, Krajewski A, Friedman LS (2017) Undertriage of Firearm-Related Injuries in a Major Metropolitan Area. JAMA Surg 52(5): 467-474.
- Muir K, Kallam A, Koepsell SA, Gundabolu K (2021) Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med 384: 1964-1965.
- Abou‐Ismail MY, Moser KA, Smock KJ, Lim MY (2021) Vaccine‐induced thrombotic thrombocytopenia following Ad26.COV2.S vaccine in a man presenting as acute venous thromboembolism. Am J Hematol.
- Montesel A, Bucolo C, Mouvet V, Moret E, Eandi CM (2020) Case Report: Central Retinal Artery Occlusion in a COVID-19 Patient. Front Pharmacol 11: 588384.
- Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P (2020) Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases 21: e00867.
- Dumitrascu QM, Volod O, Bose S, Wang Y, Biousse V, et al. Acute ophthalmic artery occlusion in a COVID-19 patient on apixaban. Journal of Stroke and Cerebrovascular diseases 29(8): 104982.
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, et al. (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191: 9-14.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, et al. (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234): 1417-1418.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, et al. (2021) Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med 384: 2092-2101.

 Case Report
Case Report