ABSTRACT
Since December 2019, COVID-19 (coronavirus disease 2019) originating in Wuhan, China, caused by SARS-CoV-2, responsible for severe acute respiratory syndrome, has become a major global public health problem. The presentation of these diseases ranges from asymptomatic to severe cases, which may lead to death. Such differences in pictures are due to both clinical and epidemiological factors. This study aims to evaluate epidemiological, clinical and laboratory information from patients in the community with a positive result for the coronavirus obtained at a primary laboratory in Juiz de Fora/Minas Gerais. This research was based on the analysis of data from individuals of both sexes, over 18 years old, exclusively outpatients, with a positive result for COVID-19. This study showed a predominance of male individuals as carriers of SARS-CoV-2 (65%, n=13) and a mean age of 41.9 years, ranging from 28 to 64 years. According to the medical records, all evaluated patients had some symptoms for COVID-19, the most frequent being myalgia (50%, n=10), fever and cough (45%, n=9, both). Regarding comorbidities, the most prevalent were alcoholism (25%, n=5) and systemic arterial hypertension (25%, n=5), with a portion also presenting upper respiratory tract disease 10%, n=2). The biomarkers (red cells, hemoglobin, hematocrit, white blood cells, eosinophils, rods neutrophils, segmented neutrophils, lymphocytes, platelets, fibrinogen, partial thromboplastin time, prothrombin time, lactate dehydrogenase, ferritin, D-dimer and C-reactive protein) determined 14 days after diagnosis of COVID-19 were mostly within the reference or negative values. From this research, it was concluded that the clinical, epidemiological and laboratory data are compatible with clinical findings in the literature on mild cases of COVID-19.
Keywords: COVID-19; SARS-CoV-2; Epidemiology; Laboratory Biomarkers
Introduction
COVID-19 (coronavirus disease 2019) is an infectious disease caused by SARS-CoV-2, responsible for severe acute respiratory syndrome, which may lead to death [1]. COVID-19 has several clinical manifestations, it can be asymptomatic or generate mild, moderate and severe conditions [2]. Other clinical manifestations may include fever, difficulty in breathing, cough, coryza, sore throat, loss of smell and taste and even death from multiple organ failure. The first case was reported in December 2019 in Wuhan, China and has spread around the world at an accelerated rate, including Brazil [1]. There are currently more than 490 million records associated with infected individuals and more than 6 million deaths worldwide, in addition to unreported cases. The clinical form of the disease will depends on genetics, age, sex and comorbidities. The risk factors that are most associated with severe clinical conditions of COVID-19 are advanced age, male gender and chronic diseases such as diabetes mellitus, high blood pressure, cardiovascular diseases and chronic kidney disease [2,3]. It is believed that the pathogenesis of the coronavirus is associated with a dysregulated immune response, which generates changes in serum Cytokines levels. Excess production of pro-inflammatory cytokines results in several changes, as plasma leakage and vascular alterations [3]. Available studies on the correlation between changes in laboratory markers and COVID-19 show divergent results. The most common laboratory findings are increased C-reactive protein (CRP), lactic dehydrogenase, white blood cells, neutrophils, ferritin, D-dimer, fibrinogen, prothrombin time, thrombotic tendency and decreased lymphocytes, eosinophils, erythrocytes and platelets. Significant differences in laboratory markers were found in severe COVID-19 compared to non-severe, suggesting that changes in these markers predict disease severity [4-7]. According to one of the studies, CRP and procalcitonin levels were above the normal range in all COVID-19 patients regardless of severity [5]. The analysis of risk factors and laboratory markers associated with the severity of COVID-19 is of great importance for the management of the disease, because from this knowledge, patients with these changes will be able to have access to more intense monitoring and early medical intervention in order to prevent a worse prognosis [6]. COVID-19 pandemic has been a challenge. Then, this study aims to evaluate epidemiological, clinical and laboratory information from community patients with a positive result for the coronavirus treated at a primary laboratory in Juiz de Fora/Minas Gerais. This date can be useful to analyze the clinical prognosis of asymptomatic patients or with moderate COVID-19, with a focus on management and appropriate allocation of healthcare resources in the pandemic.
Materials and Methods
This study was based on the analysis of data from individuals of both sexes, over 18 years old, exclusively outpatients, with a positive result for COVID-19 (n=20). Epidemiological, clinical and laboratory information of patients treated at a private laboratory, located in Juiz de Fora - Minas Gerais, Brazil. Thus, this research was is based on the observation of electronic medical records of these participants at the Laboratory, containing epidemiological information and test results of the individuals. The epidemiological data used were age, gender, smoking, alcoholism, systemic arterial hypertension, diabetes, upper respiratory tract disease, autoimmune disease, pregnancy, anticoagulant therapy, vitamin therapy, Influenza vaccine, Pneumococcal vaccine and travel history. The clinical information collected was fever, cough, myalgia, diarrhea, runny nose and sensorial loss. The laboratory test used for the diagnosis of COVID-19 was RT-PCR, based on the finding of SARS-CoV-2 RNA in nasopharyngeal swab. The researched laboratory biomarkers were red cells, hemoglobin, hematocrit, white blood cells, eosinophils, rods neutrophils, segmented neutrophils, lymphocytes, platelets, fibrinogen, partial thromboplastin time, prothrombin time, lactate dehydrogenase, ferritin, D-dimer and C-reactive protein (CRP). All biomarkers were determined by blood collection after 14 days of testing for COVID-19. It is important to note that this study was carried out between March and May 2020, a period in which only wild-type SARS-CoV-2 was circulating and individuals had not received any dose of vaccine for COVID-19. This study was carried out through consultation and consent of the participants, according to a project approved by the Research Ethics Committee of UFJF, with number 4.057.992, CAAE 31527720.3.0000.5147.
Results
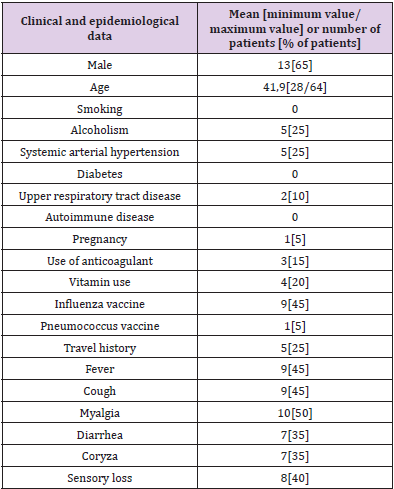
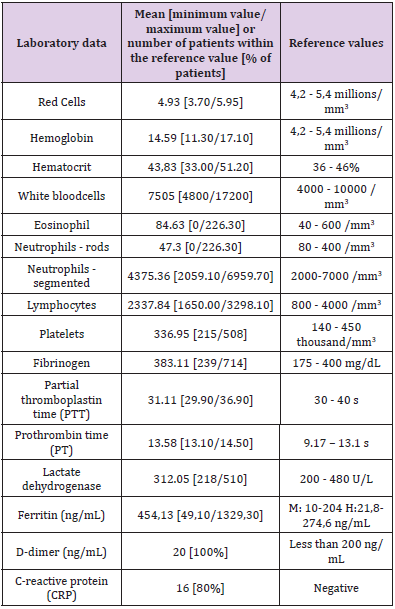
According to medical records, none of the patients evaluated in this research were asymptomatic for COVID-19. Among the most frequent symptoms were myalgia (50%, n=10) and fever and cough (45%, n=9). 35% (n=7) reported runny nose and diarrhea, and 40% (n=8) reported sensorial loss (Table 1. Clinical and epidemiological profile of patients with moderate COVID-19 and supplementary material). Assessment of comorbidities reported by patients diagnosed with COVID-19 in this study revealed 25% of patients with alcoholism and systemic arterial hypertension, 10% with upper respiratory tract disease and 5% with pregnancy (Table 1. Clinical and epidemiological profile of patients with moderate COVID-19 and supplementary material). According to Table 1, 15% (n=3) of patients reported anticoagulant therapy, 20% (n=4) revealed therapy vitamin, 45% (n=9) reported having received influenza vaccine, 5% (n=1) pneumococcal vaccine, and 25% (n=5) reported travel history. None of the patients reported smoking, diabetes or autoimmune disease. There was an increase in hemoglobin, white blood cells, segmented neutrophils and platelets in 10% of patients. 20% had an increase in red cells and 10% had a decrease, 40% had an increase in hematocrit and 10% had a decrease, 15% had a decrease in eosinophils, 50% had a decrease in neutrophils rods, 40% had an increase in fibrinogen, 95% had an increase in partial thromboplastin time, 50% had an increase in prothrombin time, 5% showed an increase in lactic dehydrogenase, 60% showed an increase in ferritin and 20% showed positive CRP (Supplementary material). Lymphocytes and D-dimer were within the reference value in all patients (Table 2. Laboratory data of patients with moderate COVID-19 and supplementary material).
Discussion
Like most infections, the virulence of SARS-CoV-2 occurs on a continuum, the disease may display symptoms ranging from those found in normal cases of flu to breathing difficulties and pneumonia, some individuals who attain the infection are asymptomatic, others experience mild disease, whereas a small subset of individuals progress to serious or life-threatening COVID-19 [3,8]. Patients with COVID-19 experience varying degrees of severity, and 80% of them have mild infection [9]. In the present study, the 20 patients analyzed had mild cases of COVID-19 and were treated without the need for hospitalization. The RT-PCR test is used to identify coronavirus RNA in nasopharynx, oropharynx and lower respiratory tract samples as well [10]. Although it is a very effective method, infection cannot be absolutely ruled out by a negative result, and it is important to analyze the patient’s clinical conditions, as well as the epidemiological information [10]. Thus, based on the results obtained in this study, it is possible to infer that the laboratory diagnosis performed early (acute phase of infection), as well as using an analytical methodology considered gold standard such as RT-PCR for COVID-19 may have contributed to the favorable outcome of these individuals. The most common clinical symptoms of COVID-19 reported in several researches were fever, cough, headache, sore throat, anorexia, myalgia, dyspnea, and sputum production [3,9]. In addition, the patients may lose the ability to taste and smell, and some have vomiting and diarrhea [3,8]. 50% of patients in the study reported myalgia although other symptoms have also been reported, such as fever, cough, diarrhea, runny nose and sensorial less, corroborating previous research. Male sex has been shown in previous studies as one of the risk factors for the development of COVID-19 [2]. 65% of the patients analyzed in the present study were men, which confirms the finding. COVID-19 is assumed to be able to damage organs including the liver, kidneys, heart, and other organs, and pre-existing comorbidities of these organs further promote the progression of COVID-19 and lead to severe and fatal outcomes [2].
Many studies have demonstrated that the presence of comorbidities is more common among patients with severe COVID-19, including cardiovascular disease, hypertension, diabetes, COPD, malignancy, chronic obstructive pulmonary disease, cerebrovascular diseases, and chronic kidney disease [2]. The sustained low level of immunity in patients with diabetes and hypertension will lead to reduced resistance to viral infections [2]. Long-term diabetes and hypertension can damage the vascular structure and weaken the heart function, which makes these patients more likely to develop critical disease in COVID-19 [2]. A significant portion of the patients (25%) had systemic arterial hypertension and alcoholism. A smaller portion (10%) had upper respiratory tract disease. None of the analyzed patients reported diabetes and autoimmune disease. The lower prevalence of comorbidities, in this study, is a result that corroborates the current literature, since the patients analyzed had mild cases of COVID-19, while pre-existing pathologies influence the development of severe conditions. Most patients received the vaccine against the Influenza virus. These data support the hypothesis of the existence of cross-immunity between the Influenza virus and SARS-CoV-2, a fact that has been previously observed with other coronaviruses (17). It was also suggested that the immunity induced by the vaccine against Influenza could generate a maintained immunity (bystander immunity), which will “help” in the fight against infection by the new coronavirus. This fact reinforces the potential benefit of Influenza vaccination to reduce the severity of COVID-19 disease [11].
Considering the multisystemic effects of both excessive alcohol consumption and COVID-19, alcohol abuse synergistically increases the risk of cardiac injury, acute respiratory distress syndrome, pulmonary fibrosis, and liver damage, thereby worsening disease prognosis and outcome [12]. The report of alcohol consumption among the individuals in this research (45%, n=5) was not relevant for understanding the unfavorable prognosis of COVID-19. as it was associated with being controlled, of the occasional type, reaffirming that the absence of comorbidities predisposes to mild conditions. The same occurs with smoking, which was not reported by any patient analyzed. However, epidemiological meta-analyses findings suggest that active smoking is significantly linked with the risk of more severity of COVID-19 [13]. It was shown, in this pandemic period, that there are biomarkers related to SARS-CoV-2, thus demonstrating their importance, either as an indicator of the current state of the disease, or as a prognostic marker [14]. In the analysis of the blood count, an important element that must be evaluated is lymphopenia (reduction in the number of lymphocytes), in addition to the fact that in some cases neutrophilia (increase in the number of neutrophils) is also highlighted [15]. The reduction in the number of lymphocytes is a marker suggesting inhibition of the immune response during COVID-19 [16,17]. Such result was not found in our study, since the lymphocyte counts of the patients were within the expected range. With regard to the neutrophil count, an increase in this marker was observed in 10% of patients with COVID-19.
It was observed that an increase in ferritin occurred in some patients who tested positive for COVID-19. Ferritin is an important mediator of immune dysregulation, since hyperferritinemia has a pro-inflammatory effect and contributes to cytokine storming [18]. Markers such as elevated D-dimer, prothrombin time and lactate dehydrogenase and reduced hemoglobin were also found in laboratory tests of individuals with the infection [3]. In relation to the analyzed patients it is noticed that some showed an increase of prothrombin time and lactate dehydrogenase, but all of them were with the rates of d-dimer in the parameter of normality. In relation to hemoglobin it was observed an increase. Another alteration found in hemostasis is the increase in partial thromboplastin time [19]. Such alteration was also observed in the analyzed medical records. Based on the biomarkers analyzed, it was found that leukocyte and platelet counts, fibrinogen determination, lactic dehydrogenase and ferritin may be tools of choice for monitoring and prognosticating community COVID-19 cases, as they have been shown to be altered in individuals with this disease profile, even late (14 days after laboratory diagnosis of COVID-19)
Conclusion
In conclusion, our results do not indicate that adult patients with a low incidence of comorbidities affected by COVID-19 have little significant changes in laboratory biomarkers. These findings indicate a good prognosis for the disease, denying the need for hospitalization. Future studies are needed to understand the correlation between the presence of the coronavirus and its various variants of concern for the microbiota of the upper respiratory tract of its host.
Conflict of Interest
None to declare.
Funding
Cortes Villela Clinical Laboratory, UFJF, FAPEMIG.
References
- Yang Di, Xuesong Gao, Yijin Zhang, Ping Gao, Hongjie Li, et al. (2020) A suspected case of COVID-19 turned into a confirmed case a case report. Future Virology 15(6): 335-339.
- Zhang, Jin jin (2022) Risk and Protective Factors for COVID-19 Morbidity Severity and Mortality. Clinical Reviews in Allergy & Immunology, p. 1-18.
- Zafer, Mai M, Hadir A El Mahallawy, Hossam M Ashour (2021) Severe COVID-19 and sepsis: Immune pathogenesis and laboratory markers Microorganisms 9(1): 159.
- Al Harbi, Mariam, Nawal Al Kaabi, Asma Al Nuaimi, Jehad Abdalla, et al. (2022) Clinical and laboratory characteristics of patients hospitalised with COVID-19 clinical outcomes in Abu Dhabi United Arab Emirates. BMC Infectious Diseases 22(1): 1-9.
- Damiati, Laila A, Sami Bahlas, Ahmed Aljohaney, Yasser Bawazir, Mohammad Mustafa, et al. (2022) Implications of SARS-CoV-2 infection on the clinical, hematological, and inflammatory parameters in COVID-19 patients: A retrospective cross-sectional study." Journal of infection and public health 15(2): 214-221.
- Huyut, Mehmet Tahir (2022) What is the impact and efficacy of routine immunological, biochemical and hematological biomarkers as predictors of COVID-19 mortality. International Immunopharmacology 105: 108542.
- Bonakdaran, Shokoufeh, Parvin Layegh, Solmaz Hasani, Mozhgan Afkhamizadeh, Zahra Mazloum Khorasani, et al. (2022) The Prognostic Role of Metabolic and Endocrine Parameters for the Clinical Severity of COVID-19. Disease Markers 2022: 5106342.
- AlMalki, Salim Albukhaty, Amal A Alyamani, Moayad N Khalaf, Sabu Thomas, et al. (2022) The relevant information about the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using the five-question approach (when, where, what, why, and how) and its impact on the environment. Environmental Science and Pollution Research 17: 1-25.
- Rahman Sayeeda, Maria Teresa Villagomez Montero, Kherie Rowe, Rita Kirton, Frank Kunik, et al. (2021) Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: a review of current evidence." Expert review of clinical pharmacology 14(5): 601-621.
- Pavão, Luiza (2020) Nota Té Considerações sobre o diagnóstico laboratorial da Covid-19 no Brasil.
- Prada L, J Ferreira (2020) COVID-19, diabetes e vacinas. Revista Portuguesa De Diabetes 15(4): 131-138.
- Ojo, Ademola Samuel (2020) COVID-19 and Alcoholism a Dangerous Synergy. Journal of Contemporary Studies in Epidemiology and Public Health 1(1): 20002.
- Kashyap, Vivek K, Anupam Dhasmana, Andrew Massey, Sudhir Kotnala, et al. (2020) Smoking and COVID-19 adding fuel to the flame. International journal of molecular sciences 21(18): 6581.
- Guan, Wei jie, Zheng yi Ni, Yu Hu, Wen hua Liang, Chun quan Ou, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. New England journal of medicine 382(18): 1708-1720.
- Del Carpio Orantes, Luis, Edna Rosario Contreras-Sánchez, Olga González-Segovia, Azael Ahumada Zamudio, et al. (2020) Caracterización clínica y del hemograma de pacientes con neumonía por COVID-19 en Veracruz, Mé Revista de Hematología 21(4): 205 209.
- Huang Chaolin, Yeming Wang, Xingwang Li, Lili Ren, Jianping Zhao, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China The lancet 395(10223): 497-506.
- Merad, Miriam, Jerome C Martin (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature reviews immunology 20(6): 355-362.
- Vargas Vargas, Manuel, Christian Cortés Rojo (2020) Ferritin levels and COVID-19. Revista Panamericana de Salud Pública 44: 72.
- Villa Palacio, María Isabel, Elizabeth López Henao (2020) Hematological Findings in COVID-19. Nova 18: 75-79.

 Research Article
Research Article