ABSTRACT
Abbreviations: SPR: Surface Plasmon Resonance; RSV: Resveratrol; RSV: Resveratrol
Perspective
Gold nanoparticles (GNPs) are wine-red solutions with antioxidant capabilities whereas gold is a yellow solid that is inert in nature [1]. As a result, GNPs qualities differ from bulk gold. GNPs come in a variety of sizes and forms, ranging from 1nm to 8 μm, and include nanospheres, nanorods, nanoshells, nanocubes, nanocages, and branched [2,3]. Gold nanoparticles have a unique combination of chemical, physical, optical, and electrical capabilities, and might be used as a novel platform in a variety of sectors, including medicine. GNPs have a lot of potential as a medication delivery method since they can efficiently carry drugs into diverse cell types [4]. GNPs have unique physicochemical characteristics such as Surface Plasmon Resonance (SPR) and the ability to bind amine and thiol groups, permitting surface modification and use in biological applications [5]. The categorization of synthesis procedures of gold nanoparticles may be done in two ways. The reduction of chloroauric acid by trisodium citrate at 100°C is one of the first and most popular chemical procedures [6].
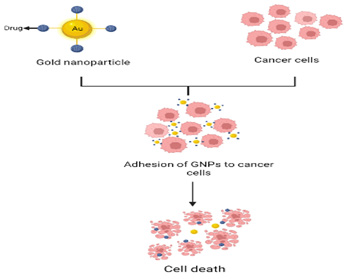
Depending on the starting concentration of sodium citrate, this reaction allowed the production of aqueous solutions of moderately monodisperse spherical nanoparticles with sizes ranging from 5-100 nm [7]. While other methods produce predominantly spherical AuNPs, gold nanoparticles may be made into a variety of geometries, including rods, cages, tubes, and other configurations. The most suitable method for synthesizing different structures of AuNPs is seed-mediated growth, which involves reducing gold salts with a strong reducing agent to produce seed particles, which are then added to a metal salt solution in the presence of a weak reducing agent and a structure directing agent. The mechanism of anticancer action of GNPs has been depicted in Figure 1. Cancer is the most prevalent cause of death in the world. Chemotherapeutics are typically used to treat cancer by intravenous dosing; however, they can cause toxicity in normal cells, resulting in severe side effects in patients. As a result, a novel and improved treatment technique to target tumour foci is required, as well as increased cytotoxicity on cancer cells and fewer side effects [8]. Because of their particular characterizations, inorganic nanoparticles such as Gold Nanoparticles (GNPs) have recently been researched and exploited as a prospective contender for diverse biotechnological applications [9]. GNP-conjugated drug delivery has a greater perfusion rate in targeting tumour foci, resulting in lower anticancer, medication dose and lesser toxicity to normal tissues, as well as fewer side effects [10].
Gold Nanoparticles as Anticancer Properties
Thilagam and Gnanamani developed gold nanoparticles of globular [albumin (AP)] and fibrillar [keratin (KP)] which consist of four forms- plain gold nanoparticles AuNps, keratin functionalized gold nanoparticles known as KP-AuNps, albumin functionalized gold nanoparticles (AP-AuNps) and combinations of KP-AP-AuNps. These particles were characterized by UV- visible spectroscopy and FTIR. The secondary structure of protein was determined by Circular dichorism spectroscopy. Anticancer activity was evaluated using MTT assay to determine the cell viability. Results showed that AuNPs showed 90% cell viability at 5μM. pH stability were also seen by changing pH from 2-10. At both acidic and alkali pHs, AuNps displayed complete aggregation, but only at pH 7.0 they demonstrate resonance. On the other hand, plasma excitation of KP-AuNps was at 4.0 pH and in AP-AuNps, no plasmon resonance was obtained. As a result, they believed that protein functionalization significantly improved the functional characteristics of gold nanoparticles, which will find uses in human health care [11]. Clarance et al. have synthesized gold nanoparticles by using endophytic fungi known as Fusarium solani by green synthesis for treating cancer.
The synthesized gold nanoparticles were evaluated against HeLa and MCF-7 cancer cell line. UV, FTIR, SEM, and XRD were used to examine the NPs’ production. The average diameter of the synthesized nanoparticles was between 40 and 45 nm, according to SEM examination. Anticancer results showed that, synthesized compounds showed inhibitory concentration of 0.8μg/mL against MCF-7 cell line. These nanoparticles showed dose dependent cytotoxic effect means by changing dose the cytotoxic effect also changes. Cell cycle analysis exhibited that the synthesized nanoparticles causes cell cycle arrest at G0 and G1 phase with 55.13 %, 52.11 % and 51.10% apoptotic inhibition against MCF- 7cell lines. Overall, the findings suggest a biomedical application for a less toxic chemotherapeutic drug [12]. Nguyen and co-workers synthesized gold NPs with dispersion of 10% as anticancer. Anticancer activity was assessed on two breast cancer cell lines - MDA-MB231, MCF-7 and MCF10. The average diameter of Au@PDA NPs was 44.4 nM.
The hydrodynamic size was 54.1 nM. The average size of Au@ PDA NPs was in between 40-6-nM with zeta potential of -21.67mV. TEM images explained that these nanoparticles formed PDA shell with thickness of 4nm. These plasma nanoparticles were negatively charged with polygonal shaped instead of spherical. They also found that synthesized NPs caused cell apoptosis with 18% against MCF- 7 and MDA-MB231 breast cancer cells. Overall, they anticipated that this research would help to pique interest in the rapid green fabrication of PDA-coated NPs for future biomedical applications in nanomedicine [13]. Venditti and co-workers have synthesized hydrophilic gold nanoparticles with citrate and L-cysteine for anticancer. They improved the bioavailability by adding Resveratrol (RSV) vehicle. Two conjugate system were investigates named as AuNPs@RSV1 and AuNPs@RSV2. The chemical and molecular stability of AuNPs following interaction with RSV in both conjugated systems is confirmed by SR-XPS analysis.
Dynamic light scattering system was assessed to confirm the hydrophilic behavior and nano-dimension of both NPs with value of <2RH>1 = 45 ± 12 nm and <2RH>2 = 170 ± 30 nm). NEXAFS results explained that, in C k spectrum of both NPs, peaks were found in between 284-292 with angle dependency π* transition only in sample 2 which showed that aggregation of RSV on gold nanoparticles was due to physisorption for sample AuNPs@RSV2. Bioavailability of AuNPs and AuNPs@RSV1 were assessed on breast cancer cell line. Results showed that, these NPs reduces the ER alpha +ve breast cell number by activating the PARP-1 cleavage [14].
Application of Gold Nanoparticles in Anticancer Therapy
Photothermal Therapy: Gold nanoparticles are of great relevance for photothermal therapy because of their unique features, such as electromagnetic radiation absorption and scattering [15,16]. The use of electromagnetic radiation to generate heat for the thermal death of cancer cells is part of this therapy technique [17-19]. Radiofrequency therapy: Radiofrequency therapy is also one of the least destructive cancer treatments (RFA). It involves the use of a moderate frequency alternating current to destroy cancer tissue [20]. A syringe probe is inserted into the tumour bulk during the RFA procedure to elevate its temperature when radiofrequency diathermy is used to kill it [21,22]. RFA is particularly effective in the treatment of small primary metastatic cancers in the lungs, liver, and kidneys [23,24]. Furthermore, the use of RFA allows for the mild but effective eradication of benign bone lesions. In contrast to surgical techniques, it causes less bone loss and has less recurrence.
Gold Nanoparticles as Drug Carriers: To improve tumour accumulation in a nanoparticle-based drug delivery system, passive targeting (using the EPR effect), motivated to pursue (by conjugating tiny aiming molecules), or a mix of such tactics can be used [25]. Physical encapsulation or chemical (covalent or noncovalent) bonding can be used to attach the therapeutic to the nanocarrier. A prodrug method could be used in the later approach, with the therapeutic molecule be transformed into an inactive state or connected to a bridge which can only be digested in the tumour microenvironment [26]. This approach exploits pathophysiological abnormalities in normal cells and cancer cells, including acidic pH or upregulation of certain cellular components.
Gold Nanoparticles as Therapeutics – Modulation of Angiogenesis
Another possible technique for cancer treatment is to use gold nanoparticles as therapeutic agents or even of themselves. AuNPs, as previously indicated, may have cytotoxic capabilities towards specific cancer cell lines. Surface modification of nanogold nanoparticles with targeted receptors may also enhance its selectivity of activity while reducing non-specific interactions with healthy cells and tissues. Nevertheless, because the mechanisms of cytotoxicity of nanogold particles are not well understood and defined, further study into their usage as anticancer treatments is required [27,28].
Conclusion
GNPs have gotten a lot of attention in biomedical applications because of their unusual physical and chemical features. The use of GNPs in conjunction with an anticancer therapy plan gives a unique opportunity. This perspective comprises application of GNPs in anticancer therapy such as photothermal therapy, radiofrequency therapy, GNPs as drug carriers and therapeutics – modulation of angiogenesis. GNP conjugates have multivalence and functional plasticity, allowing them to have stronger binding affinity, a longer circulation half-life, improved biocompatibility, improved internalization within cancer cells, and considerably better drug targeting selectivity to tumours.
References
- Saudagar R, TM Kanchan (2016) A review on gold nanoparticles. Asian J Pharm Res 6(1): 45-48.
- Nejati K (2021) Biomedical applications of functionalized gold nanoparticles: a review. Journal of Cluster Science, p. 1-16.
- LING KW (2015) Gold Nanoparticles Internalization Affects Viability And Liver Functions Of Primary Rat Hepatocytes.
- Wilson R (2008) The use of gold nanoparticles in diagnostics and detection. Chemical Society Reviews 37(9): 2028-2045.
- Li Y, HJ Schluesener, S Xu (2010) Gold nanoparticle-based biosensors. Gold Bulletin 43(1): 29-41.
- De Souza CD, BR Nogueira, MEC Rostelato (2019) Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. Journal of Alloys and Compounds 798(25): 714-740.
- Agnihotri S, S Mukherji, S Mukherji (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Advances 4(8): 3974-3983.
- Kwiatkowski S, Jolanta Kotlińska, Olga Michel, Krzysztof Kotowski, Julita Kulbacka, et al. (2018) Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomedicine & Pharmacotherapy 106: 1098-1107.
- Hammami I, NM Alabdallah (2021) Gold nanoparticles: Synthesis properties and applications. Journal of King Saud University-Science 33(7): 101560.
- Ajnai G, Tzuchun Kan, Chun Chia Cheng, Teh Hua Tsai, Jungshan Chang, et al. (2014) Trends of gold nanoparticle-based drug delivery system in cancer therapy. Journal of Experimental & Clinical Medicine 6(6): 172-178.
- Thilagam R, A Gnanamani, (2020) Preparation, characterization and stability assessment of keratin and albumin functionalized gold nanoparticles for biomedical applications. Applied Nanoscience 10(6): 1879-1892.
- Clarance P, Ben Luvankar, Jerin Sales, Ameer Khusro, Paul Agastian, et al. (2020) Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi Journal of Biological Sciences 27(2): 706-712.
- Nguyen LN, Pradeep Bhartiya, Jae Sung Kwon, Liem Quang Nguyen, Neha K, et al. (2020) In situ plasma-assisted synthesis of polydopamine-functionalized gold nanoparticles for biomedical applications. Green Chemistry 22(19): 6588-6599.
- Venditti I, Giovanna Iucci, Ilaria Fratoddi, Manuela Cipolletti, Emiliano Montalesi, et al. (2020) Direct conjugation of resveratrol on hydrophilic gold nanoparticles: structural and cytotoxic studies for biomedical applications. Nanomaterials 10(10): 1898.
- Khlebtsov, B, Vladimir Zharov, Andrei Melnikov, Valery Tuchin, Nikolai Khlebtsov, et al. (2006) Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters. Nanotechnology 17(20): 5167.
- Spyratou, Mersini Makropoulou, Efstathios P Efstathopoulos, Alexandros G Georgakilas, Lembit Sihver (2017) Recent advances in cancer therapy based on dual mode gold nanoparticles. Cancers 9(12): 173.
- Lavanya AB (2003) Effects of electromagnetic radiation on biological systems: a short review of case studies. in 8th international conference on electromagnetic interference and compatibility. IEEE.
- IP Soares P, Isabel M M Ferreira, Rui A G B N Igreja, Carlos M M Novo, Joao P M R Borges (2012) Application of hyperthermia for cancer treatment: recent patents review. Recent patents on anti-cancer drug discovery 7(1): 64-73.
- Johnson CC, AW Guy (1972) Nonionizing electromagnetic wave effects in biological materials and systems. Proceedings of the IEEE 60(6): 692-718.
- Sutherland LM, John A R Williams, Robert T A Padbury, David C Gotley, Bryant Stokes, et al. (2006) Radiofrequency ablation of liver tumors: a systematic review. Archives of Surgery 141(2): 181-190.
- McGahan JP, GD Dodd III (2001) Radiofrequency ablation of the liver: current status. American Journal of Roentgenology 176(1): 3-16.
- Sztandera K, M Gorzkiewicz, B Klajnert Maculewicz (2018) Gold nanoparticles in cancer treatment. Molecular pharmaceutics 16(1): 1-23.
- Vroomen L, EN Petre, F Cornelis, SB Solomon, G Srimathveeravalli (2017) Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: What are the differences? Diagnostic and interventional imaging 98(9): 609-617.
- Gillams A, EN Petre, FH Cornelis, SB Solomon, G Srimathveeravalli (2008) Tumour ablation: current role in the liver, kidney, lung and bone. Cancer Imaging (Spec Iss A), p. S1-S5.
- Wang X, Z Guo (2013) Targeting and delivery of platinum-based anticancer drugs. Chemical Society Reviews 42(1): 202-224.
- He Q, Gary Bryantc, Ravi Shuklaab, Vipul Bansal (2020) Tumor microenvironment responsive drug delivery systems. Asian Journal of Pharmaceutical Sciences 15(4): 416-448.
- Carnovale C (2016) Size, shape and surface chemistry of nano-gold dictate its cellular interactions, uptake and toxicity. Progress in Materials Science 83: 152-190.
- Bergers G, LE Benjamin (2003) Tumorigenesis and the angiogenic switch. Nature reviews cancer 3(6): 401-410.

 Perspective
Perspective