Abstract
Objective: This study aimed to compare the effectiveness of three post-extraction techniques for alveolar preservation.
Material and Methods: Twenty-three fresh adult alveoli were randomly allocated to three experimental groups: Group one, gingival flap + blood clot; Group two, polypropylene barrier + blood clot; Group three, bovine bone mineral matrix + gingival flap. Tomographic scans were performed within 1 day after tooth extraction and at 6 months post-extraction. At implant installation, a bone biopsy of the treated alveolus was obtained, prepared, and examined under hematoxylin-eosin, picrosirius, and Goldner stains.
Results: Statistically significant differences between Group two and Group three (p=0.0338) were found regarding the remodeling of the cervical region and the whole volume of the treated alveolar ridge (p=0.0217). Histologically, statistically significant differences between the polypropylene barrier technique and the gingival flap techniques were observed in terms of collagen (p<0.0001) and calcified tissue distribution (p<0.0001).
Conclusions: Our findings suggest that the bovine bone mineral matrix + gingival flap strategy provides greater dimensional stability on tomography, whereas the polypropylene barrier + blood clot provides better quality and quantity of mature bone on microscopy.
Keywords: Bone Substitutes; Bone Regeneration; Guided Tissue Regeneration; Clinical Research
Introduction
The alveolar ridge is a tooth-dependent tissue that develops in conjunction with tooth eruption and undergoes resorption and atrophy after dental extraction (Barone, et al. [1]). These morphologic changes have been widely studied (Araújo, et al. [2-10]) and the decrease in height and thickness of the alveolar ridge can hinder the three-dimensional positioning of implants, especially in the anterior region of the maxilla, where bone volume is important for aesthetic and biological reasons (Crespi, et al. [6]). Minimizing alveolar ridge atrophy is critical for ensuring adequate installation of the prosthesis on the implant (Buser, et al. [11]). However, careful extraction and immediate installation of the implant does not interrupt or inhibit the remodeling of the alveolar ridge after dental extraction (Araújo, et al. [12,13]). Therefore, it is necessary to strive to preserve this structure as much as possible (Artzi, et al. [3,7,13-17]). Several filling techniques and materials have been proposed to help preserve the alveolar structure, including blood clot (Karaca, et al. [7]), bone or bone substitutes of various origins (Aimetti, et al. [14,17-23]) with or without membranes or barriers (Artzi, et al. [14,15,17,19,22-24]). In this study, we aimed to determine the comparative effectiveness of three techniques for preserving alveolar bone structure after dental extraction by microscopic evaluation of the newly formed bone tissue, osteoid matrix, organized collagen, and remnant graft material, as well as tomographic measurements of the width, height, area, and volume of the remaining alveolar bone after socket healing.
Materials and Methods
Patients
All procedures were approved by the local ethics committee
for experiments involving humans (CAAE: 48634415.8.0000.5374
available for check at: http://plataformabrasil.saude.gov.br/login.jsf) and were conducted in accordance with the Declaration of
Helsinki principles. The paper was prepared in accordance with
the CONSORT 2010 Statement. All patients included in the study
provided written informed consent to participate. Patients were
recruited at Department of Basic Health Sciences, Faculty of
Medicine, Federal University of Mato Grosso, from September 2015
through August 2016. We included adult’s patient with at least
one upper incisor, or premolar tooth indicated for extraction after
clinical and radiographic examination. Patients who fulfilled any of
the following conditions before or during the study were excluded:
i. Absence of bone reference for tomographic measurements
ii. Diabetes, osteoporosis, or osteopenia
iii. Use of bisphosphonates
iv. Smoking
v. Radiation therapy or chemotherapy
vi. Medical contraindication for dental surgeries
vii. Periodontal disease or periapical lesions in the treated or
adjacent teeth
viii. Pregnancy and
ix. Bone wall defects
Randomization and Group Allocation
The sample randomization process was performed using Microsoft Excel for Mac 2011 software, version 14.5.8. Initially, a spreadsheet was elaborated where in row one, the cells that formed the column headings of the spreadsheet were named: randomized order (A one), order (B one), patient Identification (C one), randomized number (Done), and copy (E one). Column A, in the first stage of the preparation of the spreadsheet, was left without data insertion, since this was later used for the rank and division of the sample between the groups. Column B has a sequential numbering of integers with values in the range of one to 23, arranged in ascending order. Column C identified the dental element to be randomized. In all rows in column D, the formula =RANDBETWEEN (1; 23)+B2/30 (using row two of the worksheet as a reference, the rest the formula was copied to subsequent rows). The values returned in column D were copied and pasted into column E. Based on the values of column E, which represented the result of randomization, patients were ranked in column A through the function: = RANK(E2;$E$2:$E$24;1) to assign a serial number to each patient listed.
Experimental Design
This was a single-center study, with all procedures performed
at Federal University of Mato Grosso (Brazil) by a master surgeon.
All patients underwent extraction followed by alveolar preservation
according to group allocation:
(i) Group one (control), filling the extraction socket with blood
clot and closing with a gingival flap from the hard palate (Jung
R, et al. [25]);
(ii) Group two, filling the socket with blood clot and closing with a
polypropylene barrier (Bone HealTM; INP, Brazil);
(iii) Group three, filling the socket with acellular bovine mineral
matrix (Lumina Porous; Critéria Biomateriais, Brazil) and
closing with a gingival flap from the hard palate (Jung R, et al.
[25]).
Volumetric computed tomography scans of the operated region were obtained at up to 24 hours and at 6 months postoperatively. After the second tomographic examination, an osseointegrated implant was installed at the treated site and a bone biopsy was collected for histomorphometric analysis.
Surgical Procedures
All surgical procedures were performed, under local anesthesia (mepivacaine hydrochloride with corbadrine), as well as under aseptic and antiseptic conditions. Postoperatively, the patients were prescribed analgesic, anti-inflammatory, and antiseptic mouthwashes. Flapless extraction was performed, in a minimally traumatic manner. Afterwards, the integrity of the alveolus was verified clinically. For teeth allocated to groups one or three, a gingival flap compatible with the recipient area and composed of epithelium and connective tissue was harvested from the hard palate using a circular scalpel; the gingival flap was accommodated onto the recipient bed and sutured to the gingival margins using nylon suture. For teeth allocated to group two, guided bone regeneration was induced by performing an incision into the interdental papillae, followed by tunneling of the vestibular and palatine flaps. The polypropylene barrier was lodged in the surgical bed according to the manufacturer’s recommendations, and the soft tissue was sutured with nylon suture. The barrier and the suture were removed after 7 days (for all patients). For teeth allocated to group three, the alveolus was filled with acellular bovine mineral matrix, hydrated in saline solution, lodged in the fresh alveolus, and sutured as described above. At 6 months postoperatively, the patients received osseointegrated implants at the treated site. A bone biopsy was obtained from the surgical bed using a trephine drill (SIN, Brazil), with an internal diameter of 2.0mm. The bone fragment was carefully removed from the trephine and placed in buffered paraformaldehyde for fixation and histological processing. The wound was sutured with nylon thread.
Tomographic Analysis
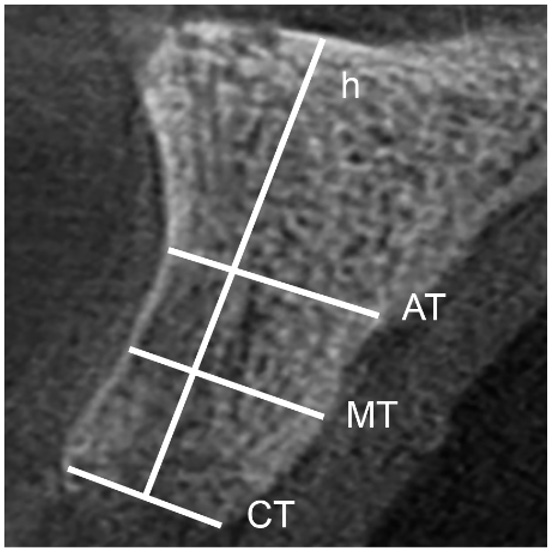
Tomographic images were analyzed using Imaging Studio version 3.3 for Windows (Anne Solutions, São Paulo, Brazil). Parasagittal sections with a thickness of 1mm were obtained at 1mm intervals. Three non-adjacent sections corresponding to the mesial, middle, and distal regions of each treated site were selected. Four types of measurements were made for each section: three measurements of thickness (cervical, middle, and apical) and one measurement of height (perpendicular to cervical thickness) (Figure 1). In the three-dimensional analysis, the roots of adjacent teeth were considered the mesial and distal references. The superior axial plane crossing the apex of the roots of the two adjacent teeth was considered the superior reference, and the cervical alveolar remnant and the vestibular and palatal cortical bones were considered references for the inferior, vestibular, and palatal references, respectively. In the first tomographic examination, the area and volume of the fresh alveolus were included in the area and total volume of the region studied, whereas only the remaining bone tissue was included in the second tomography evaluation. Tomographic measurements were performed using the “Threedimensional polygonal editing” tool available in the software. For that, the area of interest was demarcated in each axial slice (0.1mm thickness), and from the overlap of all these demarcated areas, a three-dimensional solid was constructed, which had its total area and volume mathematically calculated by the software.
Figure 1: Diagram illustrating the reference standards used for liner measurements of the alveolar ridge on computed tomography. CT, thickness in the cervical region; MT, thickness in the middle region; AT, thickness in the apical region; h, height.
Histological Analysis
The bone biopsy samples were fixed in 4% paraformaldehyde with 0.2M phosphate buffer for 24 h at 4°C. The samples were then washed in running water, decalcified with EDTA (Sangeetha [26]), and processed in a carousel tissue processor (SLEE MTP MAINZ; Carl Zeiss Meditec AG, Germany) according to the following protocol: washing in running water, dehydration in increasing ethanol concentration solutions, clarification in xylol, and embedding in paraffin overnight. For analysis, each sample was cut into 7μm sections using a HIRAX M60 microtome (Carl Zeiss), and the sections were mounted on histological slides. After deparaffinization and rehydration, some sections were stained with hematoxylin-eosin for histomorphometric analysis. Other sections underwent Goldner trichrome staining using the Von Kossa Histokit (EasyPath, Brazil), according to the manufacturer’s recommendations. The remaining sections of each specimen underwent picrosirius staining (Junqueira, et al. [27]). Tissue analyses were performed on an AxioScope A one microscope (Carl Zeiss). The software AxioVision (Carl Zeiss) was used for the quantification of tissue area. The parameters used for this evaluation were the determination of the area of the vital bone tissue, organized collagen, calcified tissue, and remnants of grafted material. These parameters were calculated as a percentage of the total area of the histological section.
Statistical Analysis
Data were analyzed using GraphPad Prism 6.01 for Windows (GraphPad Software Inc., La Jolla, CA). Two-way analysis of variance and Tukey tests were employed to evaluate the statistical significance of differences between groups.
Results
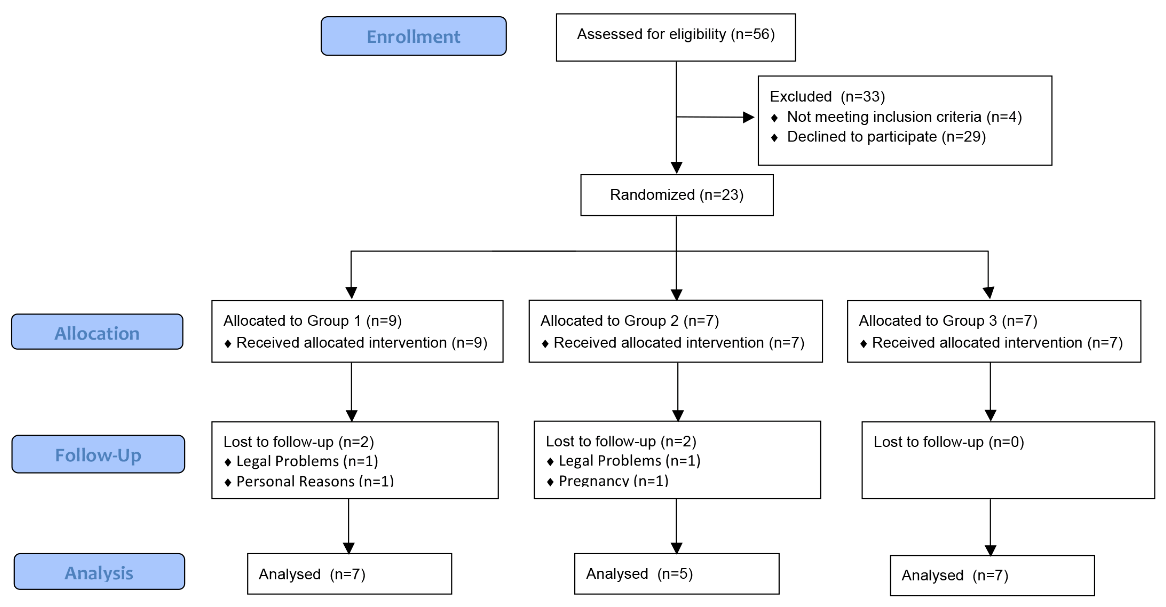
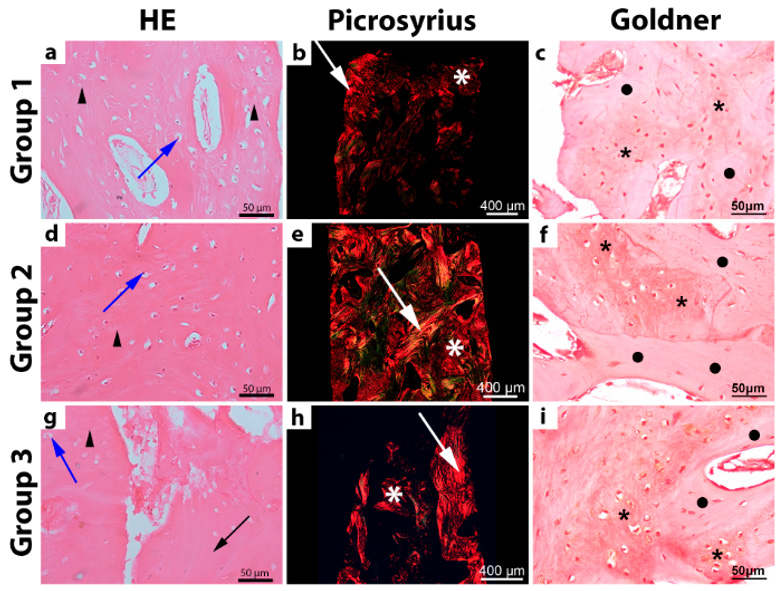
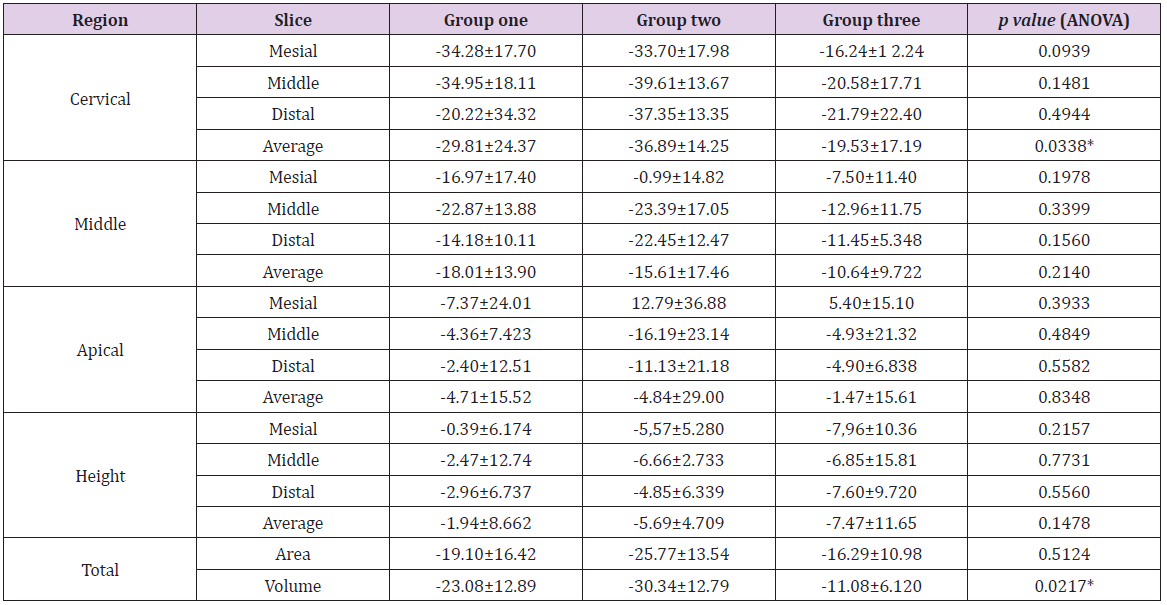
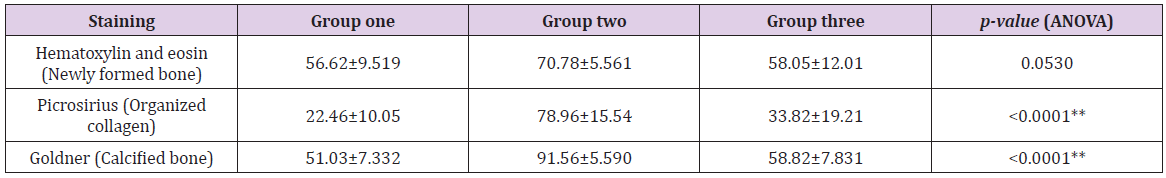
Of 56 teeth considered for this study, 23 were selected (male, 11; female, 12; age, 41.91±11.69 years) as shown in Table 1. The teeth were randomly allocated to three experimental groups according to the alveolar preservation technique employed. Four teeth were lost to follow-up (Figure 2). After the randomization process, teeth ranked from one to nine were allocated to group one, whereas those ranked from 10 to 16 were allocated to group two, and those ranked from 17 to 23 were allocated to group three. The sample size used in this study was based on other previously published articles used as reference in this study (Artzi, et al. [7,10,14,28]). The percent change in alveolar ridge dimensions on computed tomography (immediately post-extraction vs. 6 months post-extraction) did not differ among groups when considering individual slices. However, when averaging across the mesial, middle, and distal slices, groups two and three differed significantly in terms of the percent change in cervical thickness and bone volume (Table 2). On histomorphometric analysis, group two differed significantly from groups one and three in terms of the percent areas of organized collagen and calcified bone (Table 3). In all groups, hematoxylin-eosin staining revealed newly formed bone tissue with an organization representative of bone maturity, with osteocytes, and without inflammatory cells (Figure 3).
Figure 2: CONSORT diagram, prepared in accordance with the CONSORT 2020 statement. Teeth were stratified according to the alveolar preservation technique: group one, gingival flap + blood clot; group two, polypropylene barrier + blood clot; group three, bovine mineral matrix + gingival flap.
Figure 3: Microscopic appearance of alveolar bone biopsies at 6 months after extraction. Samples were stratifying according to the alveolar preservation technique: group one, gingival flap + blood clot; group two, polypropylene barrier + blood clot; group three, bovine bone mineral matrix + gingival flap. Representative brightfield photomicrographs, under hematoxylin and eosin staining are shown for groups one (A) two (D), and three (G). Newly formed bone tissue (triangles), remnant graft material (black arrow), and osteocytes (blue arrow) are labeled. Representative photomicrographs obtained, under picrosirius sating polarized, light reveal the distribution of collagen fibers in areas of bone (white arrows) and osteoid matrix (white asterisk) in teeth from groups one (B), TWO (E), And three (H). Representative brightfield photomicrographs, under Golder trichome staining, are shown for newly formed bone belonging to group one (C), two (E), and three (I). Areas of calcified bone tissue (black asterisk) and osteoid matrix (circles) are labeled.
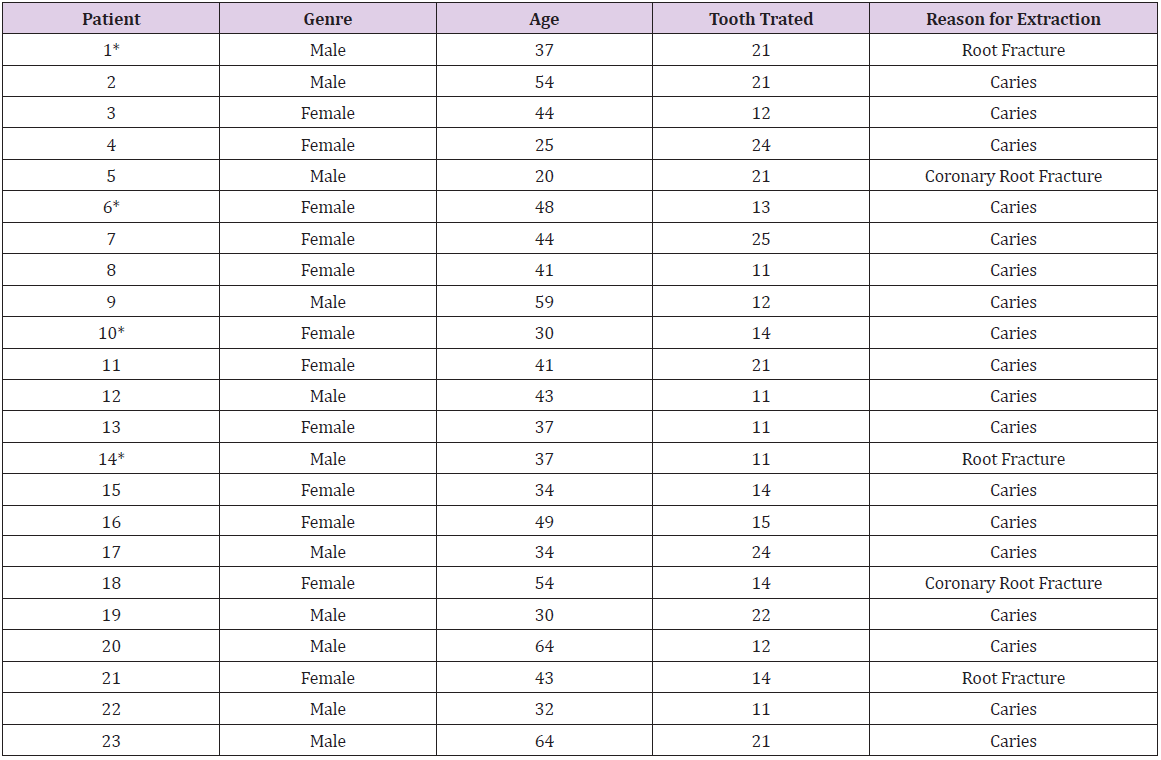
Table 1: Baseline demographic and clinical characteristics for each patient.
Note: *Lost of follow up.
Table 2: Morphometric percent change in alveolar ridge dimensions on computed tomography (immediately post-extraction vs. 6 months post-extraction), in a linear two-dimensional analyses for each studied region (mesial, middle, distal and height) and averaged over the same regions; and and three-dimensional full analyses for area and volume.
Note: Positive and negative values, respectively, indicate an increase and decrease in bone tissue. Teeth were stratified according to the alveolar preservation technique: group one, gingival flap + blood clot; group two, polypropylene barrier + blood clot; group three, bovine bone mineral matrix + gingival flap. *Statistically significant difference; Tukey test indicates differences between groups two vs. three.
Table 3: Percentage area of new bone, organized collagen, and calcified bone at 6 months after extraction.
Note: Teeth were stratified according to the alveolar preservation technique: group one, gingival flap + blood clot; group two, polypropylene barrier + blood clot; group three, bovine bone mineral matrix + gingival flap. **Statistically significant difference; Tukey test indicates differences between groups one vs. two, and two vs. three.
Samples from group two displayed some areas of lamellar bone formation. Samples from group three revealed remnant graft material in contact with newly formed bone tissue which presented an average of 9.61% of the total slice area, 6 months after the first surgery. Connective tissue and blood vessels were observed in all groups. Picrosirius staining revealed a predominance of immature collagen fibers alternating with small areas of mature collagen fibers and permeated by areas of connective tissue in group one, whereas vast regions of organized collagen (sometimes with radial and concentric organization) interlaced with scattered areas of connective tissue were observed in group two; in group three, picrosirius staining revealed organized and dense collagen interspersed with disorganized collagen which, in some regions of concentric collagen bundles, reflected immature bone (Figure 3). In all three groups, Goldner trichrome staining revealed regions of newly formed bone tissue, calcified or as osteoid matrix, with calcification foci throughout the section (Figure 3).
Discussion
This study had a similar design and sample size as employed by previous studies focused on two-stage dental implant surgery (Aimetti, et al. [17,18,20,28]). Moreover, we employed a similar evaluation strategy, including microscopic analysis with hematoxylin-eosin staining for evaluating bone vitality and cellular components (Artzi, et al, [1,6,10,14,20,29,30]), picrosirius staining for evaluating collagen fibers (Vivan, et al. [31]), and Goldner trichrome staining for distinguishing the osteoid matrix from mature bone (Vivan, et al. [31]). However, while previous studies measured only alveolar ridge height and thickness (Araújo, et al. [5,23,29,32,33]), this study also measured alveolar ridge area and volume. Such three-dimensional measurements provide a more perspective on bone remodeling after dental extraction. Reports on socket healing duration are contradictory and vary widely, from 3 months (Aimetti, et al. [18,29,30]) to 4 months (Araujo-pires, et al. [6,34-36]), 6 months (Lekovic, et al. [37,38]), 7 months (Barone, et al. [1,36]), 8 months (Mardas [39]), and even 15 years (Carmagnola, et al. [36]). The discrepancies are likely directly related to the technique and filling material used for preserving the alveoli.
As dimensional stability is higher for palatal bone than for vestibular bone (Botticelli, et al. [4]), palatal bone can be used as a stable reference for evaluating dimensional changes in the alveoli after dental extraction. Using the alveolar apex as the apical reference (Jung, et al. [33]) is controversial. Therefore, it was used an adjacent bone structure for apical reference. The presence of biomaterial in the alveolus is believed to delay socket healing (Heberer, et al. [30]), which is supported by the present results obtained for group three. Specifically, the rate of new bone formation and the percent area of organized collagen were lower in group three than in group two, as new bone formation is preceded by resorption of the grafted biomaterial. However, from a clinical perspective, 58.05% vital bone bioavailability (i.e., average percentage area of new bone in group three) is perfectly adequate for implant installation and osseointegration. On microscopic analysis, we found that the rate of new bone formation depends on the graft material. In group three patients, the rate of new bone formation was higher than that reported for freeze-dried bone allografts (Froum, et al. [20]), Bio- Oss CollagenTM (Heberer, et al. [30]), xenogenous corticocancellous bone grafts (Barone, et al. [1]), calcium sulphate (Crespi, et al. [29]), magnesium-enriched hydroxyapatite (Crespi, et al. [29]), pig bone (Crespi, et al. [6]), and autogenous bone marrow (Pelegrine, et al. [40]), but similar to the rate reported for bioactive glass (Froum, et al. [20]).
Previous reports indicate that the extraction socket heals without graft material (i.e., simple closure by flap advancement), but the rate of new bone formation ranges from 17% to 53.1% (Froum, et al. [20]), which is comparable to the value we found for group one (56.62%; gingival flap + blood clot) but much lower than the value for group two (70.78%; polypropylene barrier + blood clot). The discrepancy might be related to differences in techniques including flap elevation and interposition of an impermeable barrier between the bone plate and the periosteum, which may interfere with bone healing. Histological analysis of the connective tissue in areas not filled by bone tissue revealed results similar to those obtained using bioactive glass (Froum, et al. [20]), better than those obtained with Bio-OssTM (Carmagnola, et al. [36]), and worse than those obtained with blood clot filling (Froum, et al. [20]). However, such observations should be interpreted in consideration of the potential effect of biopsy manipulation and processing. Furthermore, the presence of remnant graft material in biopsies is related to the speed of material resorption and subsequent replacement by bone tissue. Thus, a large volume of remnant graft material indicates lower availability of vital bone tissue.
The amount of remnant graft material in group three was comparable to that reported for Bio-Oss CollagenTM (Heberer, et al. [30]) and bioactive glass (Froum, et al. [20]), but lower than that reported for pig bone (Barone, et al. [1]), Bio-OssTM (Carmagnola, et al. [36]), and hydroxyapatite (Crespi, et al. [6]), suggesting that bovine bone mineral matrix has a relatively high resorption rate, which contraindicates its use when aiming to maintain the alveolar ridge for longer but supports its use when aiming to maximize the percentage of vital bone tissue at 6 months postoperatively. Furthermore, in group three, the remnant graft material was in contact with new bone in the absence of inflammation, confirming that this graft material is highly biocompatible (Froum, et al, [20,39]). Guided bone regeneration displayed the best microscopic results, proving that, although the inclusion of biomaterial in the alveolus affects healing, it can be biocompatible. With regard to the percentage of mature bone, bovine bone mineral matrix was similar to Bio-Oss CollagenTM (Schulz, et al. [38]). However, use of a polypropylene barrier (group two) was associated with higher bone calcification and thus bone maturity. Taken together, our microscopic observations suggest that alveolar treatment with graft to preserve its structure affect bone healing. Since the mature bone, osteoid matrix, and grafted material are difficult to assess precisely on tomographic images, caution is recommended when analyzing exclusively tomographic examination results of alveoli treated for structure preservation; several aspects should be considered in such cases, including tomographic findings, nature of the filling material, and time elapsed since extraction.
The mean change in linear dimensions of the dental alveoli was reported to range between 1.7% and 77.5%, depending on the grafting material (Jung, et al. [33]). In our study, the mean dimensional changes ranged between 5.4% tissue gain and 39.61% tissue reduction. A mean cervical remodeling of 18.1% was reported for Bio-OssTM used together with a gingival flap (Jung, et al. [33]), which is similar to the value obtained in our study for group three (19.53% reduction) but smaller than the value noted for group two (36.89% reduction). The discrepancy likely stems from the choice of grafting material, since group three also showed lower rates of remodeling than those noted for group one and for beta-tricalcium phosphate (Jung, et al. [33]). Our finding that morphometric remodeling was the highest in group two suggests that installing an impermeable barrier may decrease the blood supply to the bony crest. Therefore, such a strategy would be more suitable for regions with thicker vestibular and palatal/lingual cortical bone. While the use of bovine bone mineral matrix (group three) provided superior results over those of Bio-Oss CollagenTM (Araújo, et al. [34]), some discrepancy might be attributable to the choice of anatomical reference used in the measurements. Importantly, our results confirm that alveolar height reduction also occurs at grafted sites (Araújo, et al. [34]). Previous reports estimate a 2.7% reduction in the availability of bone tissue (Araújo, et al. [34]), but methodological differences likely account for most of the discrepancies (e.g., use of two-dimensional vs. threedimensional data, one side vs. entire alveolar ridge). In fact, group three exhibited the least pronounced decrease in alveolar volume, which was significantly smaller than the decrease noted in group two. Further studies based on three-dimensional parameters are required to confirm these findings.
Our observations in alveoli preserved using gingival flaps suggest that primary closure of the alveolus after tooth extraction can also help maintain alveolar ridge height and that the flapless surgery to maintain bone tissue vascularization helps preserve the alveolar process. On the contrary, minor losses of the grafted material in the early postoperative period should be considered. Tomographic and clinical observations agree on the changes in the grafted bone upon healing (Barone, et al. [1]), as measurements conducted at the center of the ridge crest (clinical) are equivalent to those conducted in the cervical region of the middle slice (tomography). Dimensional alterations of the alveolar ridge cannot be completely prevented by alveolar preservation (Horváth, et al. [41]), which is in agreement with the results of the present study; however, guided bone regeneration without biomaterial filling appears to have no advantage. The central portion of the alveolus is clinically interesting because increased bone resorption occurs at this site (Barone, et al. [1,5]), thus carrying increased risk of alveolar ridge micro-fractures during tooth extraction maneuvers. Therefore, we emphasize the importance of performing the extraction carefully to minimize trauma to the tissue and maintain the remaining bone structure in the medium or long term. The optimal clinical protocol for alveolar preservation after dental extraction has yet to be established. We recommend the surgeon’s decision be based on the following factors: thorough knowledge of the material to be grafted, its origin, and its potential interaction with the host tissue; cortical bone thickness of the alveolus after tooth extraction; the need to elevate the mucoperiosteal flap; the opportunity to minimize tissue trauma during tooth extraction; and the timing and technique of alveolar closure.
Conclusion
Within the context of a clinical randomized, not blinded study, we conclude that the strategy bovine bone mineral matrix + gingival flap is superior to gingival flap + blood clot or polypropylene barrier + blood clot, providing higher dimensional stability of the alveolar ridge, though guided bone regeneration provided better quality and quantity of the newly formed bone tissue. Further randomized clinical studies and comparative studies using different techniques in different clinical situations are needed to establish a consensus.
Authors Contribution Statements
• Lima, F.F.B.; Sotto-Maior, B.S.; Francischone, C.E. Contributed to
conception and design.
• Lima, F.F.B.; Damazo, A.S. Contributes to acquisition, analysis
and interpretation of the data.
• Lima, F.F.B. Drafted the Manuscript.
• Lima, F.F.B; Damazo, A.S.; Sotto-Maior, B.S.; Francischone, C.E.
Gave final approval and agrees to be accountable for all aspects
of work ensuring integrity and accuracy.
• Sotto-Maior, B.S.; Francischone, C.E. critically revised the
manuscript.
Acknowledgment
This study did not receive any funding from the public or private sector. The authors report no competing interests.
References
- Barone A, Aldini N N, Fini M, Giardino R, Calvo Guirado JL, et al. (2008) Xenograft versus extraction alone for ridge preservation after tooth removal: a clinical and histomorphometric study. Journal of Periodontology 79(8): 1370-1377.
- Araújo M G, Lindhe J (2005) Dimensional ridge alterations following tooth extraction. An experimental study in the dog. Journal of Clinical Periodontology 32(2): 212-218.
- Barone A, Ricci M, Tonelli P, Santini S, Covani U, et al. (2013) Tissue changes of extraction sockets in humans: A comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clinical Oral Implants Research 24(11): 1231-1237.
- Botticelli D, Berglundh T, Lindhe J (2004) Hard-tissue alterations following immediate implant placement in extraction sites. Journal of Clinical Periodontology 31(10): 820-828.
- Chappuis V, Engel O, Reyes M, Shahim K, Nolte L, et al. (2013) Ridge Alterations Post-extraction in the Esthetic Zone: A 3D Analysis with CBCT. Journal of dental research 92(12 Suppl): 195S-201S.
- Crespi R, Capparè P, Gherlone E (2011) Comparison of Magnesium-Enriched Hydroxyapatite and Porcine Bone in Human Extraction Socket Healing: A Histologic and Histomorphometric Evaluation. The International journal of oral & maxillofacial implants 26(5): 1057-1062.
- Karaca Ç, Er N, Gülşahı a, Köseoğlu O T (2015) Alveolar ridge preservation with a free gingival graft in the anterior maxilla: volumetric evaluation in a randomized clinical trial. International Journal of Oral and Maxillofacial Surgery 44(6): 774-780.
- Pietrokovski J, Massler M (1967) Alveolar ridge resorption following tooth extraction. Journal of Prosthetic Dentistry 17(1): 21-27.
- Tan W L, Wong T L T, Wong M C M, Lang N P (2012) A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clinical Oral Implants Research, 23(SUPPL 5): 1-21.
- Trombelli L, Farina R, Marzola A, Bozzi L, Liljenberg B, et al. (2008) Modeling and remodeling of human extraction sockets. Journal of Clinical Periodontology 35(7): 630-639.
- Buser D, Martin W, Belser U C (2004) Optimizing Esthetics for Implant Restorations in the Anterior Maxilla: Anatomic and Surgical Considerations. The International journal of oral & Maxillofacial Implants 19: 43-61.
- Araújo M G, Sukekava F, Wennström JL, Lindhe J (2006) Tissue modeling following implant placement in fresh extraction sockets. Clinical Oral Implants Research 17(6): 615-624.
- Masaki C, Nakamoto T, Mukaibo T, Kondo Y, Hosokawa R, et al. (2015) Strategies for alveolar ridge reconstruction and preservation for implant therapy. Journal of Prosthodontic Research 59(4): 220-228.
- Artzi Z, Tal H, Dayan D (2000) Porous Bovine Bone Mineral in Healing of Human Extraction Sockets: Journal of Periodontology 71(6): 1015-1023.
- Camargo P M, Lekovic V, Carnio J, Kenney E B (2004) Alveolar bone preservation following tooth extraction: A perspective of clinical trials utilizing osseous grafting and guided bone regeneration. Oral and Maxillofacial Surgery Clinics of North America 16(1): 9-18.
- Roman A, Cioban C, Stratul S I, Schwarz F, Muste A, et al. (2014) Ridge preservation using a new 3D collagen matrix: a preclinical study. Clinical Oral Investigations, pp. 1527-1536.
- Wood R, Mealey B L (2012) Histologic Comparison of Healing After Tooth Extraction with Ridge Preservation Using Mineralized Versus Demineralized Freeze-Dried Bone Allograft. Journal of Periodontology 83(3): 329-336.
- Aimetti M, Romano F, Griga FB, Godio L (2009) Clinical and histologic healing of human extraction sockets filled with calcium sulfate. The International journal of oral & maxillofacial implants 24(5): 902-909.
- Crespi R, Capparè P, Gherlone E (2009a) Dental implants placed in extraction sites grafted with different bone substitutes: radiographic evaluation at 24 months. The Journal of periodontology 80(10): 1616-1621.
- Froum S, Cho S, Rosenberg E, Rohrer M, Tarnow D, et al. (2002) Histological Comparison of Healing Extraction Sockets Implanted with Bioactive Glass or Demineralized Freeze-Dried Bone Allograft: A Pilot Study. Journal of periodontology 73(1): 94-102.
- Joshi CP, D Lima C B, Samat U C, Karde PA, Patil A G, et al. (2017) Comparative alveolar ridge preservation using allogenous tooth graft versus free-dried bone allograft: A randomized, controlled, prospective, clinical pilot study. Contemporary Clin Dent 8(2): 211-217.
- Kesmas S, Swasdison S, Yodsanga S, Sessirisombat S, Jansisyanont P, et al. (2010) Esthetic alveolar ridge preservation with calcium phosphate and collagen membrane: Preliminary report. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 110(5): e24-e36.
- Mardas N, D Aiuto F, Mezzomo L, Arzoumanidi M, Donos N, et al. (2011) Radiographic alveolar bone changes following ridge preservation with two different biomaterials. Clinical Oral Implants Research 22(4): 416-423.
- Avila Ortiz G, Elangovan S, Kramer K W O, Blanchette D, Dawson D V, et al. (2014) Effect of Alveolar Ridge Preservation after Tooth Extraction: A Systematic Review and Meta-analysis. Journal of dental research 93(10): 950-958.
- Jung R, Siegenthaler D, Hämmerle C H F (2004) Postextraction Tissue Management: A Soft Tissue Punch Technique. The International Journal of Periodontics & Restorative Dentistry 24(6): 545-553.
- Sangeetha R (2013) Comparison of routine decalcification methods with microwave decalcification of bone and teeth. Journal of oral and Maxillofacial Pathology 17(3): 386-391.
- Junqueira L C U, Bignolas G, Brentani R R (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. The Histochemical Journal 11(4): 447-455.
- Clozza E, Pea M, Cavalli F, Moimas L, Di Lenarda R, et al. (2014) Healing of Fresh Extraction Sockets Filled with Bioactive Glass Particles: Histological Findings in Humans. Clinical Implant Dentistry and Related Research 16(1): 145-153.
- Crespi R, Capparè P, Gherlone E (2009b) Magnesium-enriched hydroxyapatite compared to calcium sulfate in the healing of human extraction sockets: radiographic and histomorphometric evaluation at 3 months. The Journal of periodontology 80(2): 210-218.
- Heberer S, Al chawaf B, Jablonski C, Nelson J J, Lage H, et al. (2011) Healing of ungrafted and grafted extraction sockets after 12 weeks: a prospective clinical study. The International journal of oral & maxillofacial implants 26(2): 385-392.
- Vivan R R, Mecca C E, Biguetti C C, Rennó A C M, Okamoto R, et al. (2016) Experimental maxillary sinus augmentation using a highly bioactive glass ceramic. Journal of Materials Science: Materials in Medicine 27(2): 41.
- Araújo M G, da Silva J C C, de Mendonça AF, LindheJ (2015) Ridge alterations following grafting of fresh extraction sockets in man: A randomized clinical trial. Clinical Oral Implants Research 26(4): 407-412.
- Jung R E, Philipp A, Annen B M, Signorelli L, Thoma D S, et al. (2013) Radiographic evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. Journal of Clinical Periodontology 40(1): 90-98.
- Araujo pires AC, Mendes V C, Ferreira-junior O, Sérgio P, Carvalho P, et al. (2015) Investigation of a Novel PLGA / CaP Scaffold in the Healing of Tooth Extraction Sockets to Alveolar Bone Preservation in Humans. Clinical Implant Dentistry and Related Research 18(3): 559-570.
- Canullo L, Pellegrini G, Canciani E, Heinemann F, Galliera E, et al. (2016) Alveolar socket preservation technique: Effect of biomaterial on bone regenerative pattern. Annals of Anatomy 206: 73-79.
- Carmagnola D, Adriaens P, Berglundh T (2003) Healing of human extraction sockets filled with Bio-Oss. Clinical Oral Implants Research 14(2): 137-143.
- Lekovic V, Camargo P M, Klokkevold P R, Weinlaender M, Kenney E B, et al. (1998) Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. Journal of periodontology 69(9): 1044-1049.
- Schulz M C, Kallweit M B, Kallweit S, Koch R, Lauer G, et al. (2016) Autogenous bone and a bovine bone substitute for ridge preservation - preliminary clinical and histologic findings. Australian Dental Journal 61(1): 62-70.
- Mardas N, Chadha V, Donos N (2010) Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: A randomized, controlled clinical trial. Clinical Oral Implants Research 21(7): 688-698.
- Pelegrine A A, Da Costa C E S, Correa M E P, Marques Jr J F C (2010) Clinical and histomorphometric evaluation of extraction sockets treated with an autologous bone marrow graft. Clinical Oral Implants Research 21(5): 535-542.
- Horváth A, Mardas N, Mezzomo LA, Needleman I G, Donos N, et al. (2013) Alveolar ridge preservation. A systematic review. Clinical Oral Investigations 17(2): 341-363.

 Research Article
Research Article