ABSTRACT
Objective: To elucidate the effects of different sits fat removal on glucose and lipid metabolism in C57BL/6J mice.
Methods: Animals were randomly divided into five groups. Subcutaneous fat removal (Sub-FR group), epididymis fat removal (Epi-FR group), subcutaneous and epididymis fats removal (WAT-FR group), subcutaneous and epididymis and brown fats removal (WAT+BAT-FR group). Sham group mice were performed all operations but retained all adipose tissue. All animals were fed on chow diet after fat removal, and the plasma lipids and glucose levels were measured. Finally, liver and residual fats were collected, and the expression of genes and proteins related to lipid synthesis and metabolism were analyzed.
Results: The plasma triglyceride level in WAT+BAT-FR group was significantly decreased than that in Sham group after overnight-fasting (P<0.05) and increased rapidly after refeeding (P<0.05). Compared with sham-operated group, cholesterol and triglyceride contents in livers were significantly increased (P<0.05) and insulin resistance was decreased in WAT+BAT-FR group (P<0.05). The expression of genes related to lipid synthesis and transport in livers were up-regulated (P<0.05), and the weight of residual retroperitoneal fat was significantly increased (P<0.05) in WAT+BAT-FR group compared with Sham group. However, no significant differences, such as plasma lipids level, lipids content and genes expression in liver, or weight of residual fats were observed in the other three fat removal groups compared with Sham group.
Conclusion: Surgical removal of three sites adipose tissue but not one or two sits in healthy mice can lead to mild lipid metabolism disorder and insulin resistance.
Keywords: Lipid Metabolism Disorder; Fat Removal; Insulin Resistance; Liver Lipid Deposition; Mice
Introduction
Obesity has been affecting the global population. Between 1975 and 2018, the number of obese adults in 200 countries has increased from 105 million to 650 million [1]. Approximately 50% adults will suffer from obesity in the United States by 2030 [2]. Adipose tissue is the major energy storage site and a very important endocrine organ. Therefore, it plays a crucial role in regulating systemic metabolic homeostasis [3,4]. Increase of adipose tissue can lead to obesity, while decrease of adipose tissue can lead to lipodystrophy. Adipose dysfunction caused by obesity or lipodystrophy can lead to dyslipidemia, fatty liver, insulin resistance, cardiovascular diseases, etc. [5,6]. Lipodystrophy is mainly caused by congenital genetic defects or acquired diseases [7], while obesity is mostly related to over-nutrition, hormonal imbalance, and aging [8]. The incidence of lipodystrophy is relatively very low. However, the number of overweight and obese people has increased rapidly. For obesity, clinical treatment is mainly achieved by lifestyle changes, drugs or surgery [9-12]. Numerous studies supported that fat removal in obese subjects could improve obesity-related metabolic disorders [13-19]. Liposuction and body sculpting technology in obese people can achieve the dual goals of losing weight, preventing disease and reshaping the human body, however, many young people who are not obese are trying to achieve the goal of losing weight and body sculpting through partial liposuction or partial fat dissolving at present [20,21]. Fat removal from obese ones can improve metabolism disorders, while, whether removing adipose from healthy young adults can affect glucose and lipid metabolism or not has not yet been studied. In this study, different sits adipose tissue of C57BL/6J mice were surgically removed to investigate the effects of adipose tissue in healthy young mice fed on normal chow diet.
Materials and Methods
Animals
Thirty male C57BL/6J mice were purchased from Vital River Laboratories (Beijing, China) and housed in a 12h light/12h dark cycle at 24 ℃, with free access to water and standard laboratory chow diet. All the animal experiments followed the principles of laboratory animal care (NIH Publication No. 85Y23, revised in 1996), the experimental protocol was approved by the Animal Protection Committee of Hebei University of Traditional Chinese Medicine. When mice were 12 weeks old, they were randomly divided into five groups: sham-operated group (Sham), subcutaneous fat removal group (Sub-FR), epididymis fat removal group (Epi-FR), subcutaneous and epididymis fats removal group (WAT-FR), subcutaneous, epididymis fats and brown fat removal group (WAT+BAT-FR group), with 6 mice in each group. Fat removal surgery, according to the sits of fat removed, was performed as what we had reported [22,23]. Animals were fed on chow diet for 10 weeks after fat removal surgery and anesthetized with 1% pentobarbital sodium. Livers and resident fats were harvested for further analysis. Tissues used for subsequent Western Blot or realtime fluorescence quantitative PCR (qPCR) analysis were frozen in liquid nitrogen and stored at -80 ℃ until analyzed.
Blood Sample Analysis
Blood samples were gained by retro-orbital bleeding and centrifuged to obtain plasma. Enzymatic methods were performed to estimate the levels of plasma total cholesterol (TC), triglyceride (TG) and glucose (GLU) (Bio Sino, Beijing, China). After fasted for 4 hours, animals were challenged with p. glucose (2 g/kg body weight; Abbott) or insulin ((0.75 mIU/g body weight; Humulin) for glucose and insulin tolerance tests. Blood sample were collected before (time 0) and at 15, 30, 60 and 120 (for GTT) or 90 (for ITT) minutes after the intraperitoneal injection. The levels of plasma glucose were determined by using enzymatic methods.
RNA Isolation and Quantitative Real-Time PCR
Trizol reagent (Invitrogen, USA) was used to extract the total RNA from the adipose tissues or livers. First-strand cDNA was generated by a RT kit (Invitrogen, USA) and then was performed quantitative real-time PCR by using primers listed in Table 1. Applied Biosystems and Quant Studio™ Design & Analysis Software were applied for amplifications in 35 cycles, with SYBR green fluorescence (TransGene Biotech, Beijing, China). Samples were quantitated by the comparative CT method, normalized to GAPDH.
Western Blot Analysis
Tissues were homogenized in RIPA. Protein content were measured by using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred on nitrocellulose membranes (Applygen Technologies, Beijing, China), and then were identified with the required primary antibodies. Thereafter, secondary antibodies recognized the protein-antibody complex and the enhanced chemi-luminescence detection reagents (ThermoFisher Scientific, Shanghai, China) was applied for the development of the blots. Primary antibodies used in the study includes Akt, phospho- Akt (Ser473) (Cell signaling technology, Danvers, MA).
Liver Lipid Analysis
The livers were fixed, embedded and cryosectioned at 7 μm, as previously described [24]. Lipid disposition was visualized by Oil Red O staining and HE staining. For quantitation analysis of hepatic lipid disposition, approximately 100 mg of liver sample was weighed and homogenized in 1 mL PBS. Lipids were extracted by chloroform and dissolved in 0.5 mL 3% Triton X-100. The content of triglyceride and cholesterol were determined by enzymatic methods and normalized to liver weights.
Statistical Analysis
All of the data were shown as means ± SEM. And statistics was performed using the student’s t test or One-way ANOWA with GraphPad Prism 5.0. P value < 0.05 was considered statistically significant.
Results
Mild Insulin Resistance of Multi-Site Fat-Removed Group Mice
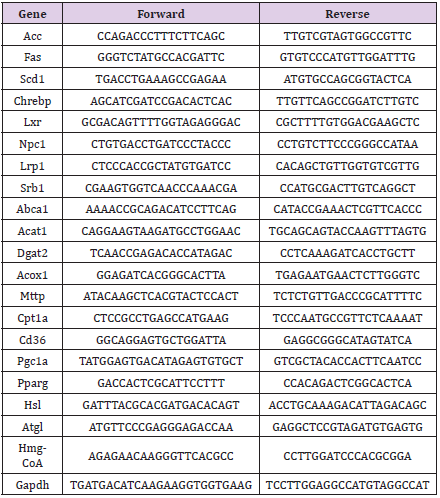
Figure 1: Blood glucose levels of five fat removal group mice.
A. Blood glucose levels of five groups mice fasting for 4 hours before fat removal;
B. Blood glucose levels of five groups mice fasting for 4 hours after fat removal for 1week;
C. GTT for 4 weeks of five groups mice after fat removal.
D. AUC data of Figure C;
E. Insulin tolerance test (ITT) for 8 weeks of five groups mice after fat removal;
F. AUC data of Figure E. Mean±SEM. n = 6. # P<0.05.
Adipose tissue often plays a crucial role in insulin sensitivity. Therefore, plasma glucose level was determined. No significant decrease in plasma glucose level was found after 4 h fasting between the fat-removed groups and the sham-operated group mice before and after fat removed (Figures 1A & 1B). At 4 weeks after fat surgery, glucose clearance was significantly delayed in the WAT+BAT-FR group compared with the sham-operated group (Figures 2C & 2D). Insulin tolerance, at 8 weeks after surgery, further showed that multi-site fat removal aggravated insulin resistance (Figures 2E & 2F). While no significant differences in glucose clearance and insulin tolerance were observed in the three other fat-removed groups and sham-operated group (Figures 2C- 2F). Our data suggested that multiple sites fat removal in healthy young mice could aggravate insulin resistance.
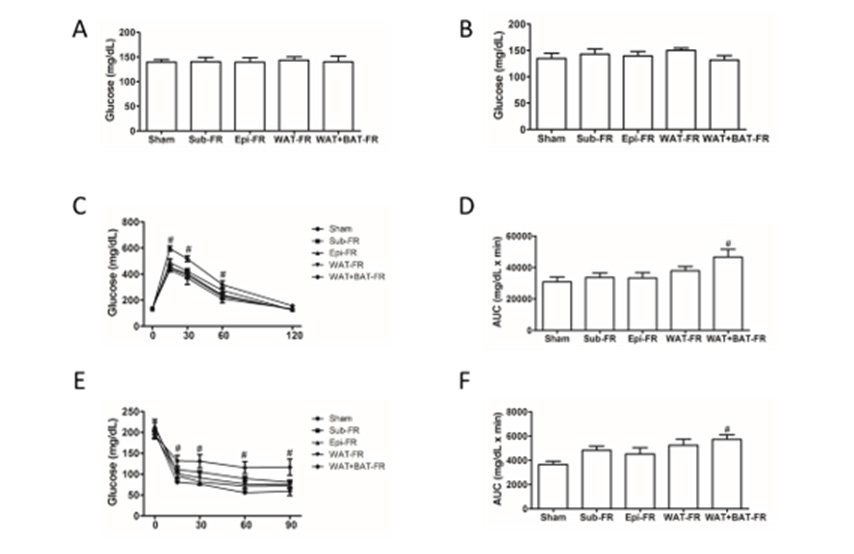
Figure 2: Blood lipid levels of five fat removal group mice.
• A, B: Plasma TG and TC levels of mice fasting for 4 hours before fat removal;
• C, D: Plasma TG and TC levels of mice fasting for 4 hours after fat removal.
• E, F: Plasma TG and TC levels of mice fasting for 14 hours after fat removal.
• G, H: Plasma TG and TC levels of the mice refed for 6 hours after fasting for 14 hours after fat removal. Mean±SEM. n = 6. # P<0.05.
Hypotriglyceridemia of Multi-Site Fat-Removed Group Mice After Overnight Fasting
Next, we determined plasma lipids level of the experimental mice. Though there was no significant difference in plasma TG level between the fat-removed groups and sham-operated group before and after fat removal, plasma TG level in WAT+BAT-FR group decreased significantly after overnight fasting (Figures 3A &3C) and increased after refeeding for 6h (Figure 3G), whereas no significant difference was found between the other three fatremoved groups and sham-operated group (Figures 3A,3C,3E,3G). However, there was no significant difference in plasma TC levels between the four fat-removed groups and sham-operated group (Figures 3B,3D,3F,3H). Our data indicated that multiple sites fat removal in healthy young mice could lead to hypotriglyceridemia after overnight fasting and increased after refeeding.
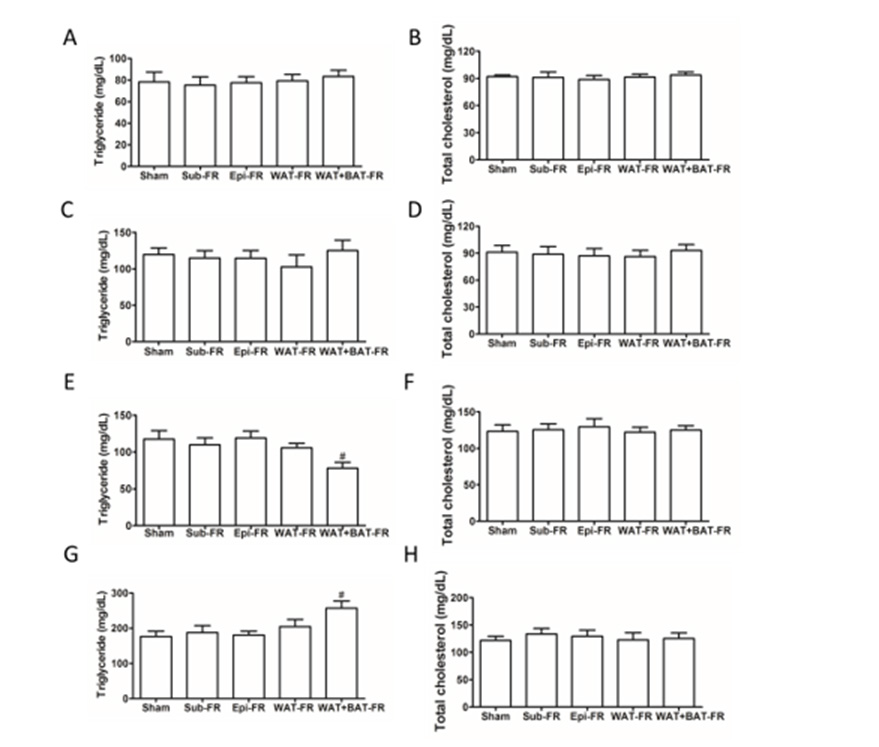
Figure 3: Characterization of residual fats of fat removal mice.
A. The weight of fat removed by surgery.
B. Body weight of five fat removal group mice.
C. Residual mesenteric fat weight in the five group mice.
D. Residual retroperitoneal fat weight in the five group mice.
E. mRNA levels of residual retroperitoneal fat related genes in the WAT+BAT-FR group and sham-operated group mice.
F. mRNA levels of residual mesenteric fat related genes in the WAT+BAT-FR group and sham-operated group mice.
Mean±SEM. n = 6. # P<0.05.
Compensatory Increased in Residual Retroperitoneal Fat of Multi-Site Fat-Removed Group Mice
The body weight of the four fat-removed group mice were not significantly different from the sham-operated group mice though the removed fat weight was difference (Figures 3A & 3B). The weight of retroperitoneal fat but not mesenteric fat was significantly increased in WAT+BAT-FR group after fat removed for 10 weeks (Figure 3D). No significant difference was found in retroperitoneal fat or mesenteric fat in the other fat-removed groups compared with sham-operated group (Figure 3B). Therefore, mRNA expression levels of genes related to lipolysis (Atgl, Hsl), lipid synthesis (Fasn, Acc, Scd1, Dgat2), adipogenesis (Pparg, Pgc1a), fatty acid oxidation (Cpt1a, Acox1) and lipid transport (Cd36, Mttp) of the adipose tissues in sham-operated group and WAT+BAT-FR group were detected by qPCR. We found that lipid synthesis, transport and related gene expression were up-regulated in adipose-deprived mice (Figures 3E & 3F). The expression of Pcg1a in retroperitoneal fat in the WAT+BAT-FR group was increased, suggesting that cell proliferation might be increased (Figure 3E). These results suggest that the residual retroperitoneal fat was compensatory increased in multi-site fat-removed group mice.
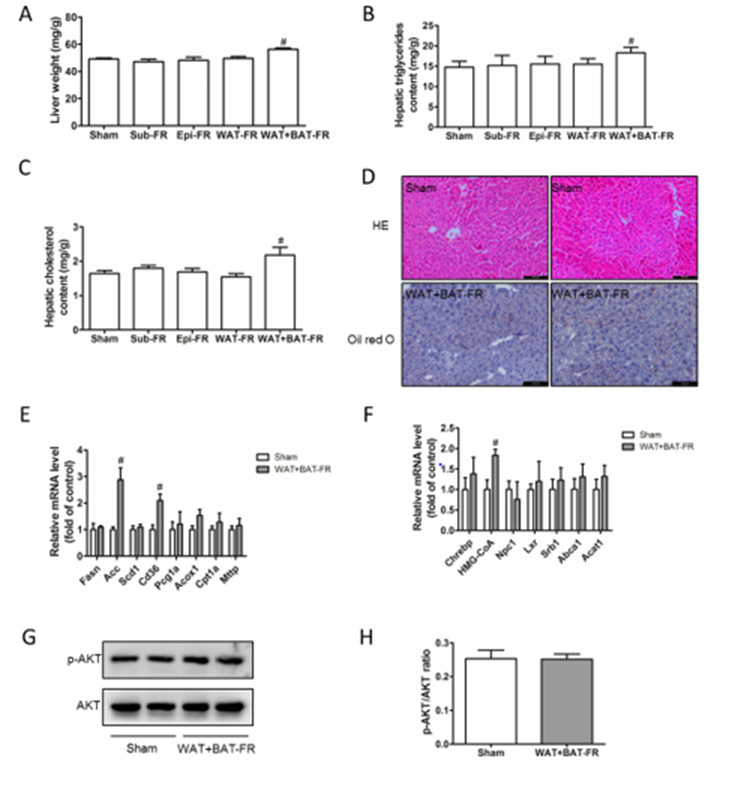
Mild Liver Lipid Deposition of Multi-Site Fat-Removed Group Mice
Figure 4: Lipid deposition in livers of fat removal mice.
A. Liver weight of the five groups mice;
B. Liver triglyceride content of the five groups mice;
C. Liver cholesterol content of the five groups mice;
D. HE staining and Oil red O staining of liver tissue of Sham group and WAT+BAT-FR mice, Bar= 200μm;
E. Expression of genes related to triglyceride (TG) synthesis and metabolism in the liver of Sham group and WAT+BAT-FR mice;
F. Expression of genes related to cholesterol (TC) synthesis and metabolism in liver of Sham group and WAT+BAT-FR mice;
G. p-Akt and AKT expression in liver tissues of Sham group and WAT+BAT-FR mice detected by Western-blot.
H. Gray value quantification of Figure 4G; Mean±SEM. n = 6. # P<0.05.
Adipose tissue is a storage site for triglycerides and cholesterol. Loss of adipose tissue, often accompanied by ectopic deposition of lipids in the live. In our fat removal model, liver weight was significantly increased in the WAT+BAT-FR group compared with sham-operated group (Figure 4A) Consistently, liver cholesterol and triglyceride content were significantly increased compared with the control group (Figures 4B & 4C). However, no ignificant differences were observed between the other fat-removed group and shamoperated group in liver weight or liver cholesterol and triglyceride content compared with sham-operated group. Therefore, the effect of adipose tissue loss on liver lipid deposition was further analyzed by pathological staining or mRNA expression detection in WAT+BAT-FR group and sham-operated group. Both HE and oil red O staining of the liver showed that the WAT+BAT-FR group did not exhibit severe liver lipid deposition compared with sham-operated group (Figure 4D). In order to clarify the cause of the increase of liver cholesterol and triglyceride contents in the WAT+BAT-FR group, we detected the mRNA expression levels of triglyceride and cholesterol-metabolisation-related genes in liver, and found that the expression of genes related to triglyceride synthesis (Acc) and lipid transport (Cd36) in the WAT+BAT-FR group was significantly up-regulated compared with sham-operated group (Figure 4E). The expression of HMG-CoA, a key enzyme in cholesterol synthesis, was also significantly up-regulated in the WAT+BAT-FR group compared with sham-operated group (Figure 4F). Liver is an insulin-sensitive target organ, and Akt is a key downstream target protein of insulin signal passway. Therefore, we examined Akt phosphorylation levels to determine the effect of mild lipid deposition on insulin sensitivity in liver tissues. Western-blot results showed that the level of p-Akt in the liver of the WAT+BAT-FR group was not significantly different from that of the sham-operated group (Figures 4G & 4H).
Discussion
In this study, subcutaneous fat in the groin, epididymis fat in the viscera and brown fat in shoulder blade of C57BL/6J mice were selectively removed to observe the role of adipose tissue in healthy young mice. Our results suggest that only subcutaneous fat removal, or epididymis fat removal or subcutaneous fat and epididymis fat removal did not show significant impacts on insulin sensitivity, plasma lipid levels, weight of residual fat or liver lipids content. Whereas, multi-site fat removal in subcutaneous fat, epididymis fat and brown fat resulted in mild insulin resistance, hypotriglyceridemia after overnight fasting, expansion of residual retroperitoneal fat and increased liver lipids content without obvious lipid deposition. Our study proved that multiple sites fat removal in healthy mice can lead to increased liver weight and lipids content, and qPCR further proved that multiple sites loss of adipose tissue can promote fatty acid uptake and cholesterol synthesis in livers (Figure 4).
The main function of adipose tissue is to store triglycerides. In our fat-removed model, mice with both white and brown fat removal exhibited hypotriglyceridemia after overnight fasting, a similar phenotype of lipodystrophy [20]. However, moderate amount of fat removal did not affect metabolic homeostasis significantly. White fat plays an important role in regulating metabolism. Therefore, there are many studies on the roles of adipose tissue in insulin sensitivity. In obese mice induced by high fat diet, removal of visceral fat can increase insulin sensitivity and reduce fatty liver [11] while removing subcutaneous fat can reduce insulin sensitivity and aggravate fatty liver [21]. The increase of pathological subcutaneous fat can also result in similar pro-inflammatory effects with visceral adipose tissue. Some obese patients achieve the purpose of losing weight and alleviateing metabolic disorders by fat removal surgery [25], but now many subjects with standard weight try to reduce weight using fat removal method, so as to achieve the goal of slimming and shaping.
However, the role of adipose tissue loss in healthy subjects is unclear. In this study, mice with multiple sites fat removal showed mild insulin resistance, decreased plasma triglyceride levels and increased liver lipid levels, suggesting that the large loss of adipose tissue in healthy mice can lead to mild metabolic disorders. One or two sites fat removal did not show significant impact on metabolic homeostasis. However, whether it affects other functions are not defined in this study and need further exploration. In summary, surgical removal of subcutaneous, epididymis and brown fat in C57BL/6J mice showed mild insulin resistance, hypotriglyceridemia after overnight fasting and liver lipids content. Besides, ubcutaneous fat, or epididymis fat or subcutaneous fat and epididymis fat removal did not play a significant impact on metabolic homeostasis.
Acknowledgments
This research was funded by Hebei Traditional Chinese Medicine Administration 2022080 to L.L., 2022368 to Y.W. and 2017009 to J.Z.; Medical Science Research Project of Hebei Provincial Health Commission 20221478 to L.L.; Dr Fund of Hebei University of Chinese Medicine (BSZ2021007) to L.L. and (BSZ2021022) to Y.W.; Science and Technology Project of Hebei Education Department BJ2019005 to M.G.; Natural Science Foundation of Hebei Province H2020423061 and Key program of Scientific and technological Research projects of Colleges and Universities in Hebei Province ZD2017056 to J.Z.
Author Contributions
Conceptualization, L.L., Y.L. and J.Z.; Data curation, L.L., Y.W. and N.L.; Formal analysis, L.L. and Y.W.; Funding acquisition, L.L.; Y.W; Investigation, J.Z.; Methodology, L.L.; Supervision, Y.L., M.G., D.X. and J.Z.; Writing – original draft, L.L.; Writing – review & editing, D.X., Y.L. and J.Z.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klaus S (2004) Adipose tissue as a regulator of energy balance. Current drug targets 5: 241-250.
- Stern JH, Rutkowski JM, Scherer PE (2016) Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metabolism 23: 770-784.
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature medicine 7: 947-953.
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, et al. (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. Journal of Clinical Investigation 112: 91-100.
- Guzman N, Vijayan V (2021) HIV-associated Lipodystrophy.
- Friedman JM (2019) Leptin and the endocrine control of energy balance. Nat Metab 1: 754-764.
- Lin Y, Chen L, Chen Y, Chan S, Liao J, et al. (2021) Quercetin attenuates cisplatin-induced fat loss. European journal of nutrition 60: 1781-1793.
- Yuan Y, Xu P, Jiang Q, Cai X, Wang T, et al. (2020) Exercise-induced alpha-ketoglutaric acid stimulates muscle hypertrophy and fat loss through OXGR1-dependent adrenal activation. EMBO Journal 39: e103304.
- Viana RB, Naves J, Coswig VS, de Lira C, Steele J, et al. (2019) Is interval training the magic bullet for fat loss? A systematic review and meta-analysis comparing moderate-intensity continuous training with high-intensity interval training (HIIT). Br J Sports Med 53: 655-664.
- Scotto DPA, Guerra E, Orlandi C, Bazzucchi I, Sacchetti M (2017) Effect of combined resistance and endurance exercise training on regional fat loss. J Sports Med Phys Fitness 57: 794-801.
- Foster MT, Shi H, Seeley RJ, Woods SC (2011) Removal of intra-abdominal visceral adipose tissue improves glucose tolerance in rats: role of hepatic triglyceride storage. Physilogy & Behavior 104: 845-854.
- Foster MT, Shi H, Softic S, Kohli R, Seeley RJ, et al. (2011) Transplantation of non-visceral fat to the visceral cavity improves glucose tolerance in mice: investigation of hepatic lipids and insulin sensitivity. Diabetologi 54: 2890-2899.
- Foster MT, Shi H, Seeley RJ, Woods SC (2010) Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physilogy & Behavior 101: 282-288.
- Shi H, Strader AD, Woods SC, Seeley RJ (2007) The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol Endocrinol Metab 293: E1012-E1020.
- Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, et al. (2010) Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 139: 448-455.
- Herrera MF, Pantoja JP, Velazquez-Fernandez D, Cabiedes J, Aguilar-Salinas C, et al. (2010) Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes care 33: 1413-1418.
- Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P (2002) A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord 26: 193-199.
- McMahan DL, Dehbozorgi H, Lam QN, Le C, Richardson J, et al. (2021) Acute Renal Failure following Novel Subcutaneous Fat Reduction with Injected Ice Slurry. Plast Reconstr Surg Glob Open 9: e3719.
- Garibyan L, Moradi TS, Javorsky E, Farinelli WA, Wang Y, et al. (2020) Subcutaneous Fat Reduction with Injected Ice Slurry. Plastic and Reconstructive Surgery 145: 725e-733e.
- Wang M, Gao M, Liao J, Han Y, Wang Y, et al. (2015) Dysfunction of lipid metabolism in lipodystrophic Seipin-deficient mice. Biochem Biophys Res Commun 461: 206-210.
- Foster MT, Softic S, Caldwell J, Kohli R, de Kloet AD, et al. (2013) Subcutaneous Adipose Tissue Transplantation in Diet-Induced Obese Mice Attenuates Metabolic Dysregulation While Removal Exacerbates It. Physiol Rep 1.
- Liu L, Liang C, Wang X, Ding X, Lu Y, et al. (2019) Surgical fat removal exacerbates metabolic disorders but not atherogenesis in LDLR-/- mice fed on high-fat diet. Sci Rep 9(1): 17848.
- Liu L, Liang C, Wang X, Pei K, Ma X, et al. (2021) Preliminary adipose removal did not prevent diet-induced metabolic disorders in mice. Chin Med J (Engl) 134(6): 716-724.
- Liao J, Liu X, Gao M, Wang M, Wang Y, et al. (2018) Dyslipidemia, steatohepatitis and atherogenesis in lipodystrophic apoE deficient mice with Seipin deletion. Gene 648: 82–88.
- Moallemi M, Siamian H, Zargaran A (2018) A Historical Report of a 9th Century AD Surgical Fat Removal. Dermatologic surgery 44: 886.

 Research Article
Research Article