ABSTRACT
The drug delivery application of Porous metal–organic frameworks (MOFs) have been investigated due to their unique structures which are built of inorganic nodes and organic ligands. In present study, zsm-5-5-FU was successfully prepared by applied for delivery of 5-fluorouracil (5-FU). Using variety of analytical methods including FTIR, FESEM, EDS, and the prepared nanostructure was characterized. Results revealed the placement of the drug in zeolite is well done and also the in vitro loading and releasing studies, for 5-FU was evaluated. In addition, Based on the in vitro cytotoxicity results, zsm-5-5Fu was able to increase cytotoxicity compared to that of 5-Fu on HT-29 cancerous cells indicating the highlighted role of this drug delivery system.
Keywords: ZSM-5; Zeolite; Drug Delivery; 5-FU; Cytotoxicity; ZSM-5-5-FU
Introduction
Cancer as the most prevalent diseases worldwide is one of the main public health concerns. (Figure 1) In spite of intensive efforts for treatment of cancer, the necessity of developing effective agents isn’t ignorable [1]. Designing an ideal drug delivery system for targeting cancer cell is considered as a hot topic in life science research. MOFs with crucial features including high drug loading capacity, high surface area, as well as tunable pore size is used for drug delivery intensively[2]. MOFs plays an important role as an carriers in drug delivery because they are non-toxic as well as the uptake of drugs and getting across the cell membrane has been facilitated via controlling the size of MOFs [3]. 5-florouracil (5-FU) is anticancer drugs which is able to induces cytotoxic and increase DNA damage [4]. Although, 5 FU frequently applied, developed drug resistance and severe side effects affected its clinical application [5]. Encapsulate of 5-FU using various DDS could be an effective idea [6]. In present work, the drug loading capacity of zsm-5 for 5-FU as an anticancer drug was evaluated. Upon exposure by zsm- 5-5Fu the in vitro cytotoxicity against cancer cells were assessed. Finally but contrary to the original goal of this project, which was to use a muff, because of the simpler and faster synthesis, we carried out this project with a zeolite. (Figure 2)
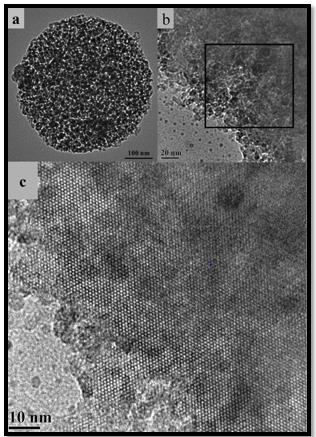
Figure 2: TEM images (a, b) of mesoporous ZSM-5 microsphere of different magnifications and the HR-TEM image (c) from the area marked by a black square in (b).
Nano Composite
Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material. [7] The idea behind Nanocomposite is to use building blocks with dimensions in nanometre range to design and create new materials with unprecedented flexibility and improvement in their physical properties. In the broadest sense this definition can include porous media, colloids, gels and copolymers, but is more usually taken to mean the solid combination of a bulk matrix and nano-dimensional phase(s) differing in properties due to dissimilarities in structure and chemistry. [8] The mechanical, electrical, thermal, optical, electrochemical, catalytic properties of the nanocomposite will differ markedly from that of the component materials. Size limits for these effects have been proposed.
1. <5 nm for catalytic activity
2. <20 nm for making a hard magnetic material soft
3. <50 nm for refractive index changes
4. <100 nm for achieving superparamagnetism, mechanical strengthening or restricting matrix dislocation movement [9].
Nanocomposites are found in nature, for example in the structure of the abalone shell and bone. [10] The use of nanoparticle-rich materials long predates the understanding of the physical and chemical nature of these materials (Figure 3). Some researchers investigated the origin of the depth of color and the resistance to acids and bio-corrosion of Maya blue paint, attributing it to a nanoparticle mechanism. From the mid-1950s nanoscale organo-clays have been used to control flow of polymer solutions (e.g. as paint viscosifiers) or the constitution of gels (e.g. as a thickening substance in cosmetics, keeping the preparations in homogeneous form). By the 1970s polymer/clay composites were the topic of textbooks, although the term “nanocomposites” was not in common use. [11] In mechanical terms, nanocomposites differ from conventional composite materials due to the exceptionally high surface to volume ratio of the reinforcing phase and/or its exceptionally high aspect ratio. The reinforcing material can be made up of particles (e.g. minerals), sheets (e.g. exfoliated clay stacks) or fibers (e.g. carbon nanotubes or electrospun fibers). [12] The area of the interface between the matrix and reinforcement phase(s) is typically an order of magnitude greater than for conventional composite materials. [13] The matrix material properties are significantly affected in the vicinity of the reinforcement. Some scientists be aware that with polymer nanocomposites, properties related to local chemistry, degree of thermoset cure, polymer chain mobility, polymer chain conformation, degree of polymer chain ordering or crystallinity can all vary significantly and continuously from the interface with the reinforcement into the bulk of the matrix (Figure 4). This massive quantity of reinforcement surface area means that a relatively small amount of nanoscale reinforcement can have an observable effect on the macroscale properties of the composite [14].
Zeolite
Zeolites are a group of crystalline materials made up of evenly sized pores and tunnel systems. When purifying VOCs and hydrocarbons, we use a synthetic hydrophobic zeolite. When the contaminated air passes through the material, the hydrocarbons are adsorbed. The material can adsorb a certain amount of hydrocarbons before needing to be regenerated. [15,16] A smaller flow of hot air is then directed through the material so that the hydrocarbons release from the zeolite in a higher concentration. This enables more cost-effective incineration. One of its strengths is that it is non-combustible–meaning it can withstand very high temperatures. [17] This means that we are also able to purify volatile hydrocarbons such as fumes emitted from vulcanization, plastic smoke and styrene, all of which require very high temperatures during regeneration. The resistance to high temperatures and the structure of the material also allows the zeolite to be completely regenerated – meaning that the VOCs completely release from the zeolite when heated. This means that the system maintains its high purification rate year after year and that the material does not have to be replaced, which gives it a long lifespan and a minimal need for maintenance. [18] Our systems have an availability of over 99% and a lifespan exceeding 25 years. Combining the benefits of zeolite with our 30 years of experience in working with air purification gives our customers a supremely sustainable and customized system with low operating costs and high availability.
Reversible Hydration and Dehydration
During drying it comes to the removal of free and bound water from the crystal grid, which is then counterbalanced back in contact with materials such as stored grain and feed, pet litter, in flue gas to prevent condensation and the like. [19] Clinoptilolite stabilize moisture at a low dose of volume and avoid the adverse effects of water. [20]
Results and Discussion
Characterization
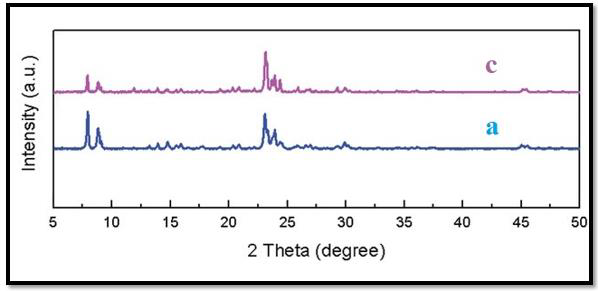
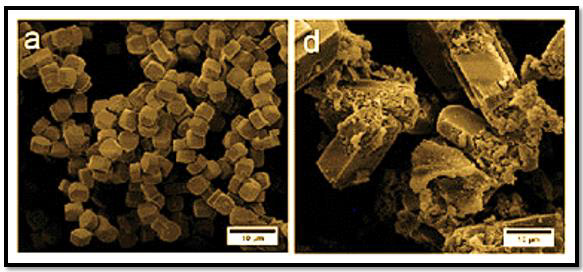
The chemical structure of the zsm-5-5Fu was characterized with different analytical methods such as XRD, SEM & TEM.
Drug Loadings and Release
The MOF- with the proper size and the accessible porosity could be used for loading and release of 5-Fu. The loading capacity of zsm-5 under physiological condition (pH 7.4) was investigated. the results showed high drug loading capacity (DLC) (90%) and drug loading efficiency (DLE) 70% by UV–Vis spectroscopy. The results of release profiles of zsm-5-5Fu revealed sustained for 72 h despite with an initial rapid release.
Cytotoxicity Assay
In order to determine the in vitro cytotoxicity of the zsm-5, 5-FU drug, and zsm-5-5Fu HT-29 cell lines, MTT assay was conducted [21]. The obtained results of the cell viability assay showed that zsm-5-5Fu and 5-FU drug inhibited cell growth in a time and dosedependent manner while the zsm-5- showed less growth inhibition after 48 h compared to drug loaded zsm-5 and free drug 5-FU. Based on this results, one may conclude that MOFs with low toxicity could be used effectively for biological applications in the future [3].
Conclusion
In this study, zsm-5 was applied for delivery of 5-Fu. The obtained nanostructure poses spherical morphology with an average diameter of 39-52 nm. Results showed the high loading capacity (90%) and sustained drug release behavior. Moreover, upon exposure by zsm-5-5Fu, higher cytotoxicity than those for zsm-5 and 5-Fu drug against PC3 cells was determined indicating zsm-5-5Fu may could be a promising anticancer drug delivery system in the future.
References
- O Zarei, F Azimian, M Hamzeh Mivehroud, J Shahbazi Mojarrad, S Hemmati, et al. (2020) Medicinal Chemistry Research 29: 1438-1448.
- S Rojas, T Devic, P Horcajada (2017) Journal of Materials Chemistry B 5: 2560-2573.
- BH Song, X Ding, ZF Zhang, GF An (2019) Journal of the Iranian Chemical Society 16: 333-340.
- E Tawfik, M Ahamed, A Almalik, M Alfaqeeh, A Alshamsan, et al. (2017) Saudi Pharmaceutical Journal 25: 206-213.
- NM Mhaidat, M Bouklihacene, RF Thorne (2014) Oncology letters 8: 699-704.
- W Cai, J Wang, C Chu, W Chen, C Wu, et al. (2019) Advanced Science 6: 1801526.
- Song Y, Chen Y, Xu M, Wei W, Zhang Y, et al. (2020) A Cobalt‐Free Multi‐Phase Nanocomposite as Near‐Ideal Cathode of Intermediate‐Temperature Solid Oxide Fuel Cells Developed by Smart Self‐Assembly. Advanced Materials 32(8): 1906979.
- Rane AV, Kanny K, Abitha VK, Thomas S (2018) Methods for synthesis of nanoparticles and fabrication of nanocomposites. In: SM Bhagyaraj, OS Oluwafemi, S Thomas (Eds.)., Synthesis of inorganic nanomaterials. Woodhead Publishing, pp. 121-139.
- Lopez N, Janssens TVW, Clausen BS, Xu Y, Mavrikakis M, et al. (2004) On the origin of the catalytic activity of gold nanoparticles for low-temperature CO oxidation. Journal of Catalysis 223(1): 232-235.
- Hubbe Martin A, Orlando J Rojas, Lucian Amerigo Lucia, Mohini Sain (2008) Cellulosic nanocomposites: A review. BioResources 3(3): 929-980.
- Kornmann Xavier, Henrik Lindberg, Lars A Berglund (2001) Synthesis of epoxy–clay nanocomposites: Influence of the nature of the clay on structure. Polymer 42(4): 1303-1310.
- Fornes TD, DR Paul. Modeling properties of nylon 6/clay nanocomposites using composite theories. Polymer 44(17): 4993-5013.
- Wagner HD, O Lourie, Yishay Feldman, Reshef Tenne (1998) Stress-induced fragmentation of multiwall carbon nanotubes in a polymer matrix. Applied physics letters 72(2): 188-190.
- Gu Dongdong, GuangbinMeng, ChuangLi, WilhelmMeiners, ReinhartPoprawe, et al. (2012) Selective laser melting of TiC/Ti bulk nanocomposites: Influence of nanoscale reinforcement. Scripta Materialia 67(2): 185-188.
- Fechete Ioana, Ye Wang, Jacques C Vedrine (2012) The past, present and future of heterogeneous catalysis. Catalysis Today 189(1): 2-27.
- Dąbrowski A (2001) Adsorption—from theory to practice. Advances in colloid and interface science 93(1-3): 135-224.
- Shahabuddin M, Tanvir Alam, Bhavya B Krishna, Thallada Bhaskar, Greg Perkins, et al. (2020) A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresource Technology 312: 123596.
- Khan Faisal I, Aloke Kr Ghoshal (2000) Removal of volatile organic compounds from polluted air. Journal of loss prevention in the process industries 13(6): 527-545.
- Rathje William L, Cullen Murphy (2001) Rubbish!: The archaeology of garbage. University of Arizona Press.
- Gorse, Christopher, David Johnston, Martin Pritchard (2012) A dictionary of construction, surveying, and civil Engineering. Oxford University Press.
- Malamis S, E Katsou (2013) A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: examination of process parameters, kinetics and isotherms. Journal of hazardous materials 252: 428-461.

 Mini Review
Mini Review