SUMMARY
Introduction: Considerable amounts of vitamin D are found in the brain and the vitamin D receptor is widely distributed throughout various brain regions. The effects of vitamin D on the regulation of genes involved in neuroprotection and neurotropism or neuronal regeneration have been described. There is a high prevalence of vitamin D deficiency or insufficiency among the elderly. The aim of this article is to review the relationship between vitamin D and cognition. We have attempted to answer three questions: Is there a physiological rationale that allows us to conclude that vitamin D has cognitively relevant effects? Is hypovitaminosis D associated with cognitive impairment? Does vitamin D supplementation have any effect on cognitive impairment or dementia?
Development: A descriptive review has been conducted on the research that we have considered to be the most relevant published in the last decade: cross-sectional, prospective, and retrospective studies, and randomized placebo-controlled trials have been included.
Conclusions: Preclinical studies show wide proof of concept that vitamin D may play a relevant role in cognitive functions. There is no consensus on the association of hypovitaminosis D and cognitive impairment, although most studies have found such an association. There is also no conclusive scientific evidence on beneficial effects of vitamin D supplementation on cognitive impairment, although the positive evidence available -mainly with cholecalciferol or vitamin D3 as preferred vitamin D form- is leading many authors and some clinicians to recommend it in usual practice.
Keywords: Cognition; Cholecalciferol; Dementia; Cognitive Impairment; Memory; Vitamin D
Abbreviations: VDR: Vitamin D Receptor; NGF: Nerve Growth Factor; GDNF: Glial Cell Line-Derived Neurotrophic Factor; AD: Alzheimer’s-type dementia; MOCA: Montreal Cognitive Assessment
Introduction
The functions of vitamin D in relation with calcium absorption, its integration in bone and the regulation of the calciumphosphorus metabolism, have been known since the beginning of the last century. Numerous other functions have been discovered over time both in relation to health and disease. Its extra-bone effects are being explored on cardiovascular, endocrine, and neurological level, among others, including its implications in cognitive function [1,2]. In the current COVID-19 pandemic, its relationship with the function of immune system regulation is being investigated. In this regard, the Spanish Society of Geriatrics and Gerontology has prepared a document that states its position on vitamin D supplementation in this context and points out several recommendations [3]. Vitamin D is found in nature in two physiological forms: ergocalciferol (vitamin D2), from mainly vegetal origin, and cholecalciferol (vitamin D3) from animal origin. Most vitamin D in the form of vitamin D3 is synthesized in the skin by ultraviolet rays from 7-dehydrocholesterol; only a small part is obtained from food. The major circulating form of vitamin D is 25(OH)D (calcidiol or calcifediol), which is the metabolite tested in plasma to assess vitamin D status, although the most active form is 1,25(OH)D (calcitriol or hormone D). The most recommended form of supplementation is cholecalciferol or vitamin D3 [1].
In recent years, concern about vitamin D in relation to the elderly is growing in our society; the study of its blood concentration levels has been incorporated into many routine tests and vitamin D prescription is growing significantly, given the high detected prevalence of insufficiency or clear deficiency. There are also studies connecting hypovitaminosis D to cognitive impairment, particularly of neuro-degenerative type. Because of these facts we have considered performing a review of vitamin D and its implications on cognitive performance and its impairment. Since there are updated systematic reviews on the subject, cited in the text, we carried out a descriptive review whose objectives were to answer several relevant questions: Is there any data that would allow us to conclude that vitamin D has cognitive effects? Is hypovitaminosis D associated with cognitive impairment? Does vitamin D supplementation have any effect on cognitive impairment or dementia?
Development
Materials and Methods
The review conducted on the role of vitamin D in the management of cognitive impairment is descriptive, not systematic. We have used the PubMed/MEDLINE, EMBASE and Psych INFO databases to identify the most relevant study publications, establishing as search criteria the terms: vitamin D, cholecalciferol, calcifediol and vitamin D3, and crossing them with cognitive function, cognitive decline, cognitive impairment, dementia, and Alzheimer’ s disease. The following inclusion criteria have been considered for article selection: studies published from 2010 up to August 2021 in Spanish or English; epidemiological studies (both prospective, retrospective, or cross-sectional) and interventional clinical trials with vitamin D (open-label or controlled); variable (but mentioned) follow-up time; young and elderly population; diagnosis of healthy ageing, mild cognitive impairment and/or dementia; clinical trials which specific vitamin D supplementation dosage. The exclusion criteria were articles outside the specified period of time or that not mentioning relevant parameters to be compared (sample size, follow-up, assessment tests, or dose of vitamin D supplemented).
Results
Rationale of Vitamin D in Cognitive Impairment:
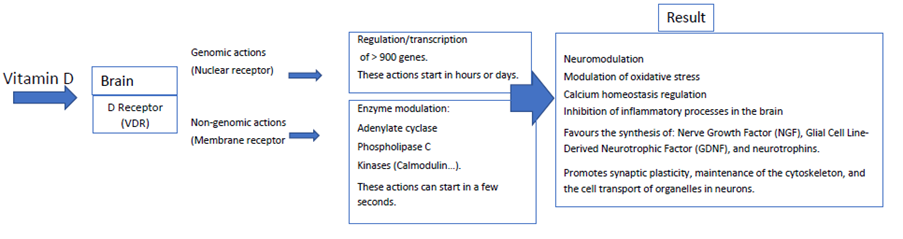
Vitamin D and Brain: Considerable amounts of vitamin D are in the brain since vitamin D can cross the blood-brain barrier, passively diffusing and binding to Vitamin D Receptor (VDR). VDR is widely spread in neurons, astrocytes, microglia of the hippocampus, orbitofrontal cortex, cingulate area, amygdala, and thalamus. These brain areas are all well-known for their relevant role in learning, memory, and other cognitive functions. It is known 1,25(OH)D-VDR coupling modulates the gene expression of neurons, astrocytes, and microglia [4]. In these cells, there are hydroxylases that can synthesize and catabolize both 25(OH)D (or calcidiol) and 1,25(OH) D (calcitriol or hormone D). Both calcidiol and calcitriol can act in a paracrine and autocrine manner by regulating different functions of these brain cells through gene modulation [5].
Vitamin D exerts its effect through genomic and non-genomic pathways. Genomics is performed through the VDR receptor which is a member of the nuclear transcription factor family, thus influencing gene transcription and regulating the expression of more than 900 genes [6]. Due to positioning in DNA, it is also involved in the expression of numerous protein complexes throughout the body, including the brain. Its effects through nongenomic pathways are like the ones of other steroid hormones. It has fast actions mainly through VDR and also through an associated protein (the disulfide isomerase of A-Pdia3 family). In this way it influences the modulation of adenylate cyclase, phospholipase C, protein kinases (among others, calmodulin) and participates in the phosphorylation of cellular proteins. It also promotes the intracellular release of calcium, and it is known that high levels of calcium produce neurotoxicity associated to cognitive impairment; several calcium-binding proteins are regulated by vitamin D: calbindin, parvalbumin and calretinin [7]. In this sense, Eyles, et al. [8] have mapped out the presence of metabolites of vitamin D in the brain, as well as its enzymes and VDR, indicating that vitamin D acts as a neurosteroid in processes related to learning and memory. These genomic and non-genomic actions are the basis for their effects on neurocognition (see Figure 1).
Vitamin D and Neurotransmission: In in vitro and preclinical research models, vitamin D has been shown to be involved in the gene expression of neurotransmitters such as acetylcholine, dopamine, serotonin, and gamma butyric acid, and may reduce the hyperphosphorylation of the tau protein, attenuating the neuronal death process [9]. Vitamin D has neuroprotective effects by decreasing the neurotoxic glutamatergic hyperactivity and the positive regulation of the expression of genes involved in the formation of new synapses in the hippocampus [10], as well as genes involved in neuronal myelination [11].
Vitamin D promotes the synthesis of Nerve Growth Factor (NGF), which acts on neurons in the fronto-basal area of the cholinergic system protecting it from glutamate toxicity, and also on Glial Cell Line-Derived Neurotrophic Factor (GDNF), which performs maintenance and repair functions of dopaminergic and noradrenergic neurons. It likewise promotes the synthesis of several neurotrophins which are involved in growth and differentiation of neurons, and in attenuation of programmed neuronal death [10]. Vitamin D is involved in synaptic plasticity, maintenance of the cytoskeleton and cell transport of cell organelles through regulation of numerous proteins involved including tubulin, drebrin, GAP43 and Dynactin [12]. It has anti-inflammatory effects on the brain, and reduces the activation of cytokines by activated microglia, decreasing the synthesis of nitric oxide, an enzyme that is also boosted in degenerative diseases, and regulates the expression of gamma glutamyl transpeptidase enzyme, so it has an important role on antioxidant mechanisms in the brain [9].
Vitamin D and Cognitive Impairment: Vitamin D deficiency is associated with psychiatric and neurological diseases in which cognitive disorders are involved, such as schizophrenia, autism spectrum disorders, attention deficit hyperactivity disorder, Parkinson’s disease and dementia. In patients with Alzheimer’stype dementia (AD) there is often a vitamin D insufficiency or even deficiency. However, it is not clear whether hypovitaminosis D is a factor involved in their aetiology or a consequence of dementia lifestyle involving lower sun exposure. There are several mechanisms on which its effects on neurocognition are based, mainly induction of neuroprotection, oxidative stress modulation, calcium regulation and inflammation reduction [12]. Furthermore, some polymorphisms of the VDR gene have been found to increase the risk of AD, specifically the haplotype of the TaqI, ApaI, Tru9I, BsmI and FokI alleles. Several studies consider that these haplotypes can be risk factors for AD [13]. Some research indicates that vitamin D might ease the clearance of amyloid plaques in the brain. This can be performed by at least two mechanisms: to increase the transportation of β-amyloid through brain-blood barrier by regulating the expression of transporters (P-glycoprotein or P-gp, and low-density protein receptor related to protein 1 or LRP-1) via VDR; and to stimulate macrophages to allow phagocytosis of the β-amyloid [14]. Based on these positive findings, Łukaszyk, et al. [15] and Patel, et al. [16] raised the consideration of vitamin D as a marker of cognitive dysfunction and possible treatment option. Nevertheless, vitamin D supplementation studies in patients with cognitive impairment in dementias have yielded contradictory results, as we will see below. It has been found that low 25(OH)D levels may increase the risk of cardiovascular disease. Therefore, vitamin D would decrease risk of cognitive impairment and dementia through beneficial vascular-type actions in hypertension, diabetes, atherosclerosis, and subsequent strokes [10]. In conclusion, there is sufficient neurophysiological rationale that supports a potential positive effect of vitamin D on cognitive functions.
Prevalence of Low Vitamin D Levels in Individuals with Cognitive Impairment: is there a Relationship Between Hypovitaminosis D and Poorer Cognitive Function?: 25(OH) D serum levels are considered to show objective deficiency when they are < 20 nanograms per millilitre (ng/ml) or < 50 nanomoles/ litre (< 50 nmol/l) [conversion factor x 2.5]; 20 - 30 ng/ml (50 nmol/l – 75 nmol/l) vitamin D deficiency; and optimum level above 30 ng/ml (75 nmol/l). In our country, most studies find vitamin D deficiency in a percentage of older adults greater than 50% [17].
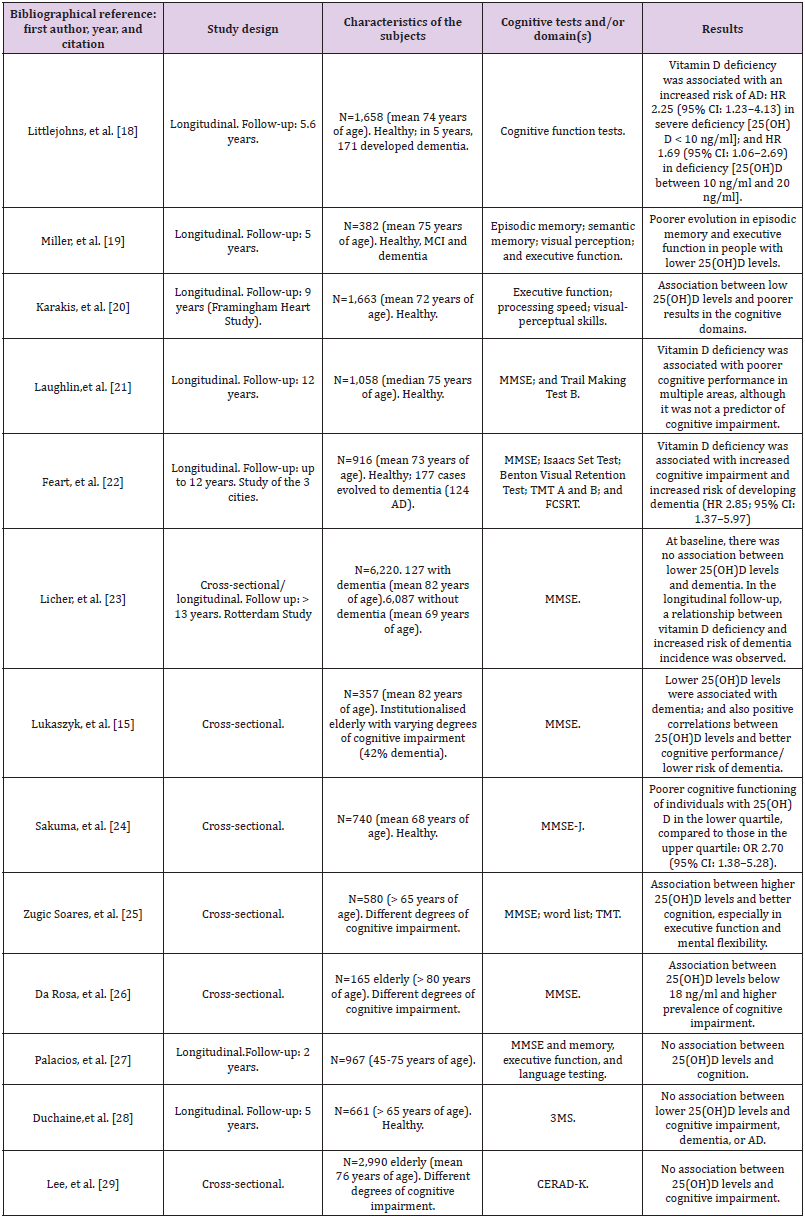
Observational Association Studies and Mendelian Randomization Studies: In this section we raise several questions, among others: is there a relationship between the level of vitamin D and global cognitive functions, especially in areas such as memory, executive function, or language? Is hypovitaminosis D associated with cognitive impairment or dementia? Is there an association indicating a risk, or is it one of the direct causes of cognitive impairment? Can hypovitaminosis D be an effect -more than a cause- of the lifestyle of people with cognitive impairment or dementia? There are studies that have shown associations between low vitamin D levels and cognitive impairment; however, there are others that did not find such an association. Characteristics of these studies can be found in Table 1 [18-29].
Table 1: Epidemiological studies of association between 25(OH)D levels and cognitive impairment.
Note: 25(OH)D (25-hydroxy-vitamin D), 3MS (Modified Mini-Mental State examination), AD (Alzheimer’s type Dementia), CERAD-K (Korean version of the consortium to establish a registry for Alzheimer disease), FCSRT (Free and Cued Selective Reminding Test), HR (Hazard Ratio), MCI (Mild Cognitive Impairment), MMSE (Mini-Mental State Examination), MMSE-J (Japanese version of the Mini-Mental State Examination), TMT (Trail Making Test).
In the North American longitudinal population study (US Cardiovascular Health Study), 1,658 older adults without dementia were followed for more than 5 years. Hazard Ratio (HR) of risk of occurrence of dementia of any type was statistically significantly higher in individuals with severe vitamin D deficiency (<10 ng/ml, HR 2.25; 95% CI - 95% confidence interval- 1.23 - 4.13) or more moderate vitamin D deficiency (between 10 and 20 ng/ml, HR 1.53; 95% CI 1.06 – 2.21) [18]. In 2016, based on data from individuals who participated in the Framingham Heart Study, the relationship between vitamin D and cognitive impairment was studied based on 9-year follow-up data. Epidemiological information was analyzed from two cohorts of individuals: on the one hand, those with a diagnosis of dementia diagnosed throughout the follow-up (n = 1,663, mean age 72 years, mean baseline levels of 25(OH)D 25 ng/ ml or insufficiency); and those who, although without development of dementia, were performed with systematic cognitive tests, as well as brain imaging techniques by magnetic resonance (n = 1,139, mean age 60 years, mean baseline levels of 25(OH)D 20 ng/ ml or deficiency). In the first cohort, it was observed that 16.1% developed dementia; in this group, no association was found between vitamin D deficiency and increased risk of progression to dementia. However, in the cohort of individuals undergoing cognitive and imaging tests, an association was observed between low 25(OH)D levels and lower hippocampal volumes, as well as poorer performance in some neuropsychological function tests [20].
In the Rotterdam prospective study, covering more than 13 years of follow-up, it was observed that, in patients with initial dementia, no statistically significant difference in vitamin D levels was found, compared to the rest of the patients. However, when the incidence of dementia was analyzed in the 6,087 patients without a previous diagnosis (795 participants developed it, 641 with AD), an association was observed between lower baseline levels of 25(OH)D and higher risk of dementia incidence [23].These results from white North American and European individuals have also been replicated in other populations: in the Asian population, a cross-sectional epidemiological study [24] showed about a threefold risk of cognitive impairment in people with low vitamin D concentrations [OR -odds ratio- 2.70 (95% CI 1.38 – 5.28; p = 0.011)]. Similarly, in another cross-sectional study in 2,990 Koreans of 73 years mean age, an association was found between cognitive performance, measured by MMSE -Mini-Mental State Examination-, TMT -Trail Making Test- and digit scales, and the different levels of 25(OH)D, although losing the statistically significant association in an adjusted logistic regression model where sociological items were introduced [29]. Epidemiological studies carried out with Mendelian Randomization (MR) methodology have shown conflicting results and conclusions.
The first relevant study of this type was published in 2016 and was conducted by choosing 4 Single Nucleotide Polymorphisms (SNP) previously related to vitamin D deficiency from the genome of 33,996 patients, weighting the actual influence of each of the 4 SNPs on cognitive impairment from data from 2,347 individuals; thus, the corresponding risk estimates of developing AD could be obtained. The decrease of a standard deviation in 25(OH)D levels increased the risk of AD diagnosis (OR 1.25; 95% CI 1.03 – 1.51; p = 0.021) and, consequently, the authors concluded that vitamin D deficiency may represent a risk factor for AD [30]. Two years later another MR study was published with similar positive conclusions. In this case, researchers were able to identify 7 SNPs related to vitamin D deficiency and mutations in the 7 alleles were associated with an increased risk of diagnosis of AD [31]. However, other MR studies have been published not confirming these association findings. In 2017, a study was published without any finding of correlation between low 25(OH)D levels and risk of onset of dementia or cognitive impairment using data from Swedish men from the Uppsala Longitudinal Study of Adult men (12-year followup of a cohort of 1,182 elderly individuals) [32]. Also in 2017, the possible relationship between hypovitaminosis D and cognitive impairment was assessed based on data from 172,349 individuals from 17 cohorts with records of 2 polymorphisms (DHDR7- rs12785878 and CYP2R1-rs12794714). The authors found no associations between global or memory-related cognitive function and either of the two polymorphisms [33].
In addition to the above, systematic literature reviews and meta-analyses started to be published from the early 2010s, revealing a possible relationship between low vitamin D levels and poor cognitive functioning. Some concluded that there was an inverse association between cognitive performance and vitamin D levels [34-37], but others were considered negative [38-40]. If we try to answer the questions raised at the beginning of this section by summarizing the published evidence, most authors conclude that hypovitaminosis D is associated with cognitive impairment or dementia, although there are also relevant studies that did not find this association. Regarding the question of whether low 25(OH) D level a risk factor is or not, we have seen that several studies showed that people with vitamin D deficiency or insufficiency have a higher risk of cognitive impairment, in comparison with those with normal values. However, no study has clearly ascertained the vitamin D deficiency causality for cognitive impairment or dementia, although genetic studies results may point in that direction. Current research still cannot rule out the hypothesis that hypovitaminosis D could be a consequence of lifestyle of people with cognitive impairment or dementia including poorer sun exposure and nutrition, as well as even lower sociability. This latter approach would have to be demonstrated through the execution of appropriately designed prospective clinical trials. These studies should compare interventions with vitamin D supplementation versus placebo in patients with different stages of cognitive impairment. We will analyze this point in detail in the following section, according to the available evidence.
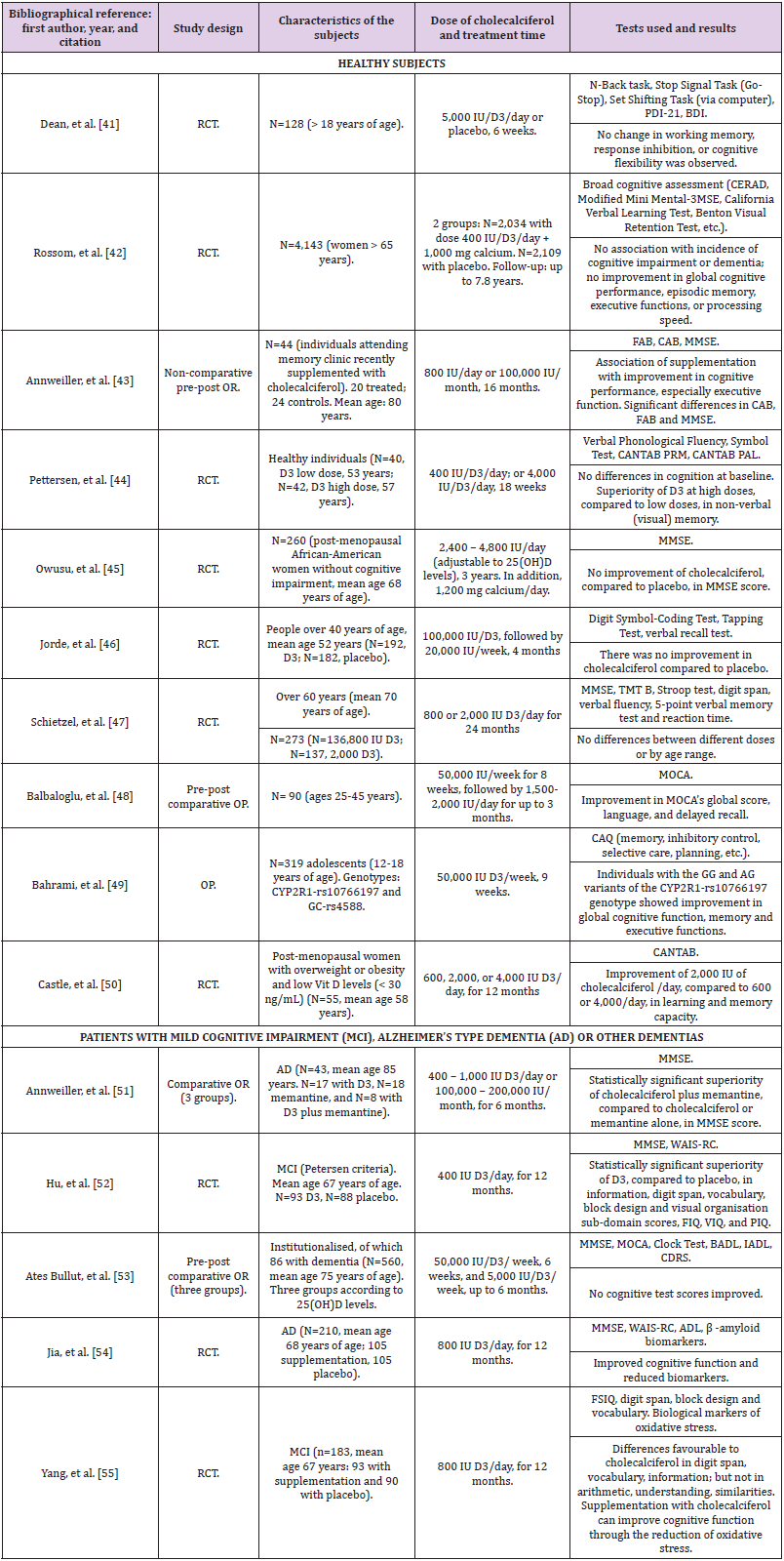
Effect of Vitamin D (Cholecalciferol) Supplementation on Cognitive Function: What Do Recent Clinical Trials Indicate Us?: Most vitamin D supplementation studies were conducted using vitamin D3/cholecalciferol (see Table 2); however, several questions could be raised in relation to vitamin D supplementation and cognitive functions: when cognitive impairment or dementia, especially AD, is already present, can supplementation reverse the damage or slow progression of disease? Does supplementation produce beneficial effects in healthy individuals without cognitive impairment? Is this cognitive improvement, if occurring, dependent or not on normal, deficient or insufficient vitamin D plasma levels? Which are the convenient or necessary doses? To investigate the results of vitamin D supplementation on cognitive function, studies have been carried out with healthy elderly people or with patients suffering from cognitive impairment or dementia, mainly AD. Since the effects in both groups could be different and this difference would be of utmost importance, we analyzed separately the studies conducted with each of these two groups (Table 2) [41-55].
Table 2: Clinical trials of supplementation on the effect of vitamin D3 (cholecalciferol) on cognitive function.
Note: AD (Alzheimer’s type Dementia), ADAS-COG (Alzheimer Disease Assessment Scale-COGnitive), ADL (Activity of Daily Living scale), BADL (Basic Activities of Daily Living), BDI (Beck Depression Inventory), CAB (Cognitive Assessment Battery), CANTAB (Cambridge Neurological Test Automated Battery), CAQ (Cognitive Ability Questionnaire), CDRS (Clinical Dementia Rating Scale), FAB (Frontal Assessment Battery), FSIQ (Full Scale Intelligence Quotient), IADL (Instrumental Activities of Daily Living), MCI (Mild Cognitive Impairment), IU (International units of vitamin D), MMSE (Mini-Mental State Examination), mRNA (messenger RiboNucleic Acid), MOCA (Montreal Cognitive Assessment), OP (Open Prospective), OR (Open Retrospective), PAL (Paired Associates Learning Task) / PRM (Pattern Recognition Memory task), PDI-21 (Peters Delusion Inventory - 21 Items), PIQ (Performance Intelligence Quotient), RCT (Randomized, double-blind, placebo-controlled Clinical Trial), TMT (Trail Making Test), VIQ (Verbal Intelligence Quotient), WAIS-RC (Wechsler Adult Intelligence Scale-Revised).
Studies in Elderly People Without Cognitive Impairment: In 2011, a RCT (Randomized, double-blind, placebo-controlled Clinical Trial) was conducted for six weeks with 128 young adults receiving vitamin D with average normal 25(OH)D levels without finding changes in the cognitive functions assessed [41]. If the treatment and assessment time could be considered insufficient in this study, the same cannot be said of another study published in 2012, when another RCT was carried out in 4,143 women over the age of 65 followed for about 8 years, probably the longest follow-up time of a study of these characteristics. There were no differences between the group with calcium supplementation and 400 IU of vitamin D3 and the one with placebo on the incidence of MCI or dementia, nor in other cognitive tests evaluating memory, language, or executive functions [42]. Pettersen, et al. [44] assessed the effect of high doses versus low doses of vitamin D3 in a single-blinded RCT by supplementing 82 healthy individuals with low (400 IU/day) or high (4,000 IU/day) doses for 16 weeks. Significant improvement was observed with high doses in non-verbal visual memory, an effect that was more pronounced in those who had lower 25(OH) D levels. No differences were detected in other cognitive domains [36]. Balbaloglu, et al. [48] also tested if there was an effect with high doses (starting dose: 50,000 IU per week). They selected 90 healthy women between 25 and 45 years of age with deficient levels of vitamin D and supplemented them for up to 3 months, finding improvements with respect to the baseline score in the MOCA (Montreal Cognitive Assessment) score, especially in the items of language and delayed recall [48].Unlike previous studies, another RCT compared supplementation at different doses (800 IU/day and 2000 IU/day) in individuals over 60 years old; they supplemented 273 subjects but found no differences after 24 months [47].
We found only one study that analyzed genetic variability and vitamin D supplementation in subjects without cognitive impairment; they studied 319 healthy adolescents who were supplemented with high doses of vitamin D3 (50,000 IU/week) for 9 weeks and observed that those who had the CYP2R1- rs10766197 genotype GG and AG alleles performed better than the ones with AA alleles in various cognitive functions assessed with the cognitive skills questionnaire: global cognitive function, memory, and executive functions. The authors concluded that some individual genotypes could particularly benefit from high doses of cholecalciferol [49].
Studies in Patients with Cognitive Impairment: Annweiller C et al. carried out an open retrospective study in patients with AD; they studied 43 patients supplemented with vitamin D at variable doses. At the six-month evaluation, they found that those who had been treated with memantine plus vitamin D had significantly improved in MMSE, averaging 4 points, while those who had taken memantine only or vitamin D only remained stable. They concluded that the objective cognitive improvement in this series of patients suggested some type of possible synergy or enhancement of effects of the memantine-vitamin D combination [51]. In 2018, an RCT was published with patients suffering from MCI and vitamin D deficiency. For 12 months, 93 patients received a supplement of 400 IU/day of vitamin D3 and 88 received placebo. The authors found statistically significant improvements attributed to cholecalciferol, compared to placebo, in 5 cognitive subdomains (information, digit span, vocabulary, block design, and visual organization) [52]. In the same year, another RCT of 105 patients with AD was published testing 800 IU/day intervention with cholecalciferol for 12 months. The treated group improved in several cognitive functions (vocabulary, block design, digit span, figure organization and other functions), and decreased in terms of some biomarkers related to the β-amyloid protein (β-amyloid 42, amyloid precursor protein, BACE-1 -β-secretase type 1- and others), compared to placebo [54]. This positive assessment on biochemical and cognitive functions may confirm the previous research that had found a relationship between β-amyloid protein and vitamin D. It is an admitted fact that in AD the amyloid production increases with a decrease in its clearance; in a pilot study with 14 healthy subjects over 50 years of age with vitamin D deficiency who were supplemented, an increase in plasma amyloid was observed, suggesting an increase in cerebral amyloid clearance by increasing its peripheral transportation. This occurred with greater intensity in individuals over the age of 60 [56].
Recently, a RCT was performed in 183 patients with MCI (mean age 67 years; mean 25(OH)D less than 20 ng/ml) who were administered 800 IU daily of vitamin D3 (n=93) or placebo (n=90) for 12 months. Statistically significant differences were found favorable to supplementation with cholecalciferol in terms of the FSIQ (Full Scale Intelligence Quotient) score and several other cognitive tests; among them, digit span, block design and vocabulary. This work also has the interest that the researchers observed that supplementation with cholecalciferol significantly reduced biological markers of oxidative stress regulated by telomere length [55]. Conversely, a systematic review and metaanalysis was published, including 2,345 participants from 9 clinical trials on vitamin D supplementation for the prevention of AD. The authors concluded that, in their opinion, the current evidence does not support a beneficial effect of vitamin D supplementation to prevent AD [57]. In short, vitamin D supplementation has generally led to increases in 25(OH)D levels to an optimal level. Both in healthy subjects and cognitive impairment or dementia patients, there are studies that have detected positive cognitive changes due to vitamin D cholecalciferol supplementation, while others have not detected them. This difference in results cannot be attributed to the doses used, the time used for supplementation, or to the quality of the work. We cannot, therefore, answer with certainty the questions raised about the effect of vitamin D supplementation on cognition, nor the effect of different doses or the duration of supplementation.
Nevertheless, and given the reasonably favorable findings, some authors consider vitamin D as a potential therapeutic target [16] or even a “new paradigm for therapy in the prevention and treatment of dementia and AD.” [2]. However, scientific evidence available to date regarding the possible efficacy of cholecalciferol in healthy or cognitively impaired individuals is insufficient to affirm that there is a robust and consistent effect.
Conclusion
Though, traditionally, science and statistics have been used and abused by politicians for the attainment of hegemony and personal advantages, an evidence-based approach is a necessity for every person, whether as a specialist or as an average person; otherwise the outcome can be messy and unfavorable. So, no direct or indirect relationship can be assumed to exist between politics and immunization; two non-combinable objects. Though conspiracy theory can be a hypothesis that needs acceptable proof and investigation, the current pandemic is a real threat that demands instant and proper response. Disregard to lack of information or anxiety, as the main causes of hesitation for refusal or deferring vaccination, politicization of immunization drives mostly from subjective judgment, which can be pushed by leader’s, cult’s, kin’s, or media’s standpoints, as plausible sources of data for hoi polloi, in comparison with scientific texts or bulletins, as authentic resources for professionals. On the other hand, neither pro-vaccine groups nor anti-vaccine crowds have unconditional aficionados. Both of them consist of different people with unlike viewpoints. Presently, vaccination is the best approach for decreasing natural vulnerability and augmenting bodily defense. Though it is not a perfect approach with unbreakable protection, at the moment immunization is the most reliable method for management of the present pandemic. Survival is everybody’s right and its increase is a blessed objective and duty in public health and medical practice.
Limitations of the Review
This conducted review is not a systematic review. We have not scored the studies, either with statistics, or qualitatively or with any systematic review usual methodology. All the studies shown in the tables are from the last decade when interest in this topic has relevantly increased, and particularly until mid-2021. The great heterogeneity of studies and vitamin D supplementation doses and treatment duration makes it difficult to draw consistent conclusions. Well-designed clinical trials, predominantly RCTs -more appropriate for establishing causality- with sufficient sample size to counteract usual attrition, adequate follow-up periods -preferably several years-, and targeting specific populations in relation to cognitive impairment are needed.
Conclusion
Although the available scientific evidence is inconclusive, there are sufficient clinical data to consider that supplementation with cholecalciferol in elderly patients with cognitive impairment and vitamin D insufficiency or deficiency may have beneficial effects on cognitive function. Hence, vitamin D status should be monitored in these patients and hypovitaminosis D corrected with cholecalciferol dosage regimens that achieve serum 25(OH)D levels preferably above 30 ng/ml. In any case, if such monitoring is not easily accessible for economic or administrative reasons, the high prevalence of vitamin D deficiency in these patients may warrant supplementation with cholecalciferol, even without the availability of 25(OH)D serum levels.
Conflicts of Interests
González, Javier is an employee of ITF Research Pharma, S.L.U., Alcobendas, Madrid, Spain.
References
- Marazuela M (2005) Déficit de vitamina D en el adulto: clínica, diagnóstico y tratamiento. [Adult vitamin D deficiency: clinical assessment, diagnosis and treatment.] Endocrinol Nutr 52(5): 215-223.
- Sultan S, Taimuri U, Basnan SA, Waad Khalid Ai-Orabi, Afaf Awadallah, et al. (2020) Low Vitamin D and Its Association with Cognitive Impairment and Dementia. J Aging Res: ID 6097820.
- Tarazona-Santabalbina FJ, Cuadra L, Cancio JM, Roca Carbonell F, Pérez-Castejón Garrote JM, et al. (2021) Vitamin D supplementation for the prevention and treatment of COVID-19: a position statement from the Spanish Society of Geriatrics and Gerontology. Revista Española de Geriatría y Gerontología 56 (2021): 177-182.
- Littlejohns TJ, Kos K, Henley WE, Kuźma E, Llewellyn DJ (2016) Vitamin D and Dementia. J Prev Alzheimer's Dis 3(1): 43‐52.
- De Abreu, DF, Eyles D, Feron F (2009) Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 34(Suppl 1): S265-S277.
- Gáll Z, Székely O (2021) Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients 13(11): 3672.
- Alexianu ME, Robbins E, Carswell S, SH Appel (1998) 1Alpha, 25 dihydroxyvitamin D3-dependent up-regulation of calcium-binding proteins in motoneuron cells. J Neurosci Res 51(1): 58-66.
- Eyles DW, Burne TH, McGrath JJ (2013) Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Frontiers in neuroendocrinology 34(1): 47-64.
- Groves NJ, McGrath JJ, Burne TH (2014) Vitamin D as a neurosteroid affecting the developing and adult brain. Annual review of nutrition 34: 117-141.
- Moretti R, Morelli ME, Caruso P (2018) Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact. Int J Mol Sci 19(8): 2245.
- Jean-Francois Chabas, Delphine Stephan, Tanguy Marqueste, Stephane Garcia, Marie-Noelle Lavaut, et al. (2013) Cholecalciferol (vitamin D₃) improves myelination and recovery after nerve injury. PLoS One 8(5): e65034.
- Bivona G, Gambino CM, Iacolino G, Ciaccio M (2019) Vitamin D and the nervous system. Neurological research 41(9): 827-835.
- Gezen-Ak D, Dursun E, Bilgic B, Hanagasi H, Ertan T, et al. (2012) Vitamin D receptor gene haplotype is associated with late-onset Alzheimer's disease. The Tohoku journal of experimental medicine 228(3): 189-196.
- Dursun E, Gezen-Ak D, Yilmazer S (2011) A novel perspective for Alzheimer's disease: vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J Alzheimers Dis 23(2): 207-219.
- Łukaszyk E (2018) Bien Barkowska, K. Bien, B. Cognitive Functioning of Geriatric Patients: Is Hypovitaminosis D the Next Marker of Cognitive Dysfunction and Dementia. Nutrients 10(8): 1104.
- Patel P, Shah J (2017) Role of Vitamin D in Amyloid clearance via LRP-1 upregulation in Alzheimer's disease: A potential therapeutic target? J Chem Neuroanat 85: 36-42.
- Navarro Valverde C, Quesada Gomez JM (2014) Deficiencia de vitamina D en Españ Realidad o mito? [Vitamin D deficiency in Spain. Reality or myth?. Rev Osteoporos Metab Miner 6 (Suppl 1): S5-10.
- Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, et al. (2014) Vitamin D and the risk of dementia and Alzheimer disease. Neurology 83(10): 920-928.
- Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, et al. (2015) C. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol 72(11): 1295-1303.
- Karakis I, Pase MP, Beiser A, Booth SL, Jacques PF, et al. (2016) Association of Serum Vitamin D with the Risk of Incident Dementia and Subclinical Indices of Brain Aging: The Framingham Heart Study. J Alzheimers Dis 51(2): 451-461.
- Gail A Laughlin, Donna Kritz-Silverstein, Jaclyn Bergstrom, Emilie T Reas, Simerjot K Jassal, et al. (2017) Vitamin D Insufficiency and Cognitive Function Trajectories in Older Adults: The Bernardo Ranch Study. J Alzheimers Dis. 58(3): 871-883.
- Catherine Feart, Catherine Helmer, Bénédicte Merle, François R Herrmann, Cédric Annweiler, et al. (2017) Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement 13(11): 1207-1216.
- Licher S, De Bruijn RFAG, Wolters FJ, Zillikens MC, Ikram MA, et al. (2017) Vitamin D and the Risk of Dementia: The Rotterdam Study. J Alzheimers Dis 60(3): 989-997.
- Sakuma M, Kitamura K, Endo N, Ikeuchi T, Yokoseki A, et al. (2019) Low serum 25-hydroxyvitamin D increases cognitive impairment in elderly people. J Bone Miner Metab 37(2): 368-375.
- Zugic Soares J, Pettersen R, Saltyte Benth J, Knapskog AB, Selbæk G, et al. (2019) Higher Vitamin D Levels Are Associated with Better Attentional Functions: Data from the NorCog Register. J Nutr Health Aging 23(8): 725-731.
- Da Rosa MI, Beck WO, Colonetti T, Budni J, Falchetti ACB, et al. (2019) Association of vitamin D and vitamin B12 with cognitive impairment in elderly 80 years or older: a cross-sectional study. J Hum Nutr Diet 32(4): 518-524.
- Palacios N, Scott T, Sahasrabudhe N, Gao X, Tucker KL (2020) Serum vitamin D and cognition in a cohort of Boston-area Puerto Ricans. Nutr Neurosci 23(9): 688-695.
- Duchaine CS, Talbot D, Nafti M, Giguère Y, Dodin S, et al. (2020) Vitamin D status, cognitive decline and incident dementia: the Canadian Study of Health and Aging. Can J Public Health 111(3): 312-321.
- Lee DH, Chon J, Kim Y, Seo YK, Park EJ, et al. (2020) Association between vitamin D deficiency and cognitive function in the elderly Korean population: A Korean frailty and aging cohort study. Medicine (Baltimore) 99(8): e19293.
- Mokry LE, Ross S, Morris JA, Manousaki D, Forgetta V, et al. (2016) Genetically decreased vitamin D and risk of Alzheimer disease. Neurology 87(24): 2567-2574.
- Larsson SC, Traylor M, Markus HS, Michaëlsson K (2018) Serum Parathyroid Hormone, 25-Hydroxyvitamin D, and Risk of Alzheimer's Disease: A Mendelian Randomization Study. Nutrients 10(9): 1243.
- Olsson E, Byberg L, Karlström B, Cederholm T, Melhus H, et al. (2017) Vitamin D is not associated with incident dementia or cognitive impairment: an 18-y follow-up study in community-living old men. Am J Clin Nutr 105(4): 936-943.
- Maddock J, Zhou A, Cavadino A, Kuźma E, Bao Y, et al. (2017) Vitamin D and cognitive function: A Mendelian randomisation study. Sci Rep 7(1): 13230.
- Dickens AP, Lang IA, Langa KM, Kos K, Llewellyn DJ (2011) Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs 25(8): 629-639.
- Annweiler C (2016) Vitamin D in dementia prevention. Ann N Y Acad Sci 1367(1): 57-63.
- Shen L, Ji HF (2015) Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta-analysis. Nutr J 14: 76.
- Kalra A, Teixeira AL, Diniz BS (2020) Association of Vitamin D Levels with Incident All-Cause Dementia in Longitudinal Observational Studies: A Systematic Review and Meta-analysis. J Prev Alzheimer's Dis 7(1): 14-20.
- Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, et al. (2014) Plasma nutrient status of patients with Alzheimer's disease: Systematic review and meta-analysis. Alzheimers Dement 10(4): 485-502.
- Sommer I, Griebler U, Kien C, Auer S, Klerings I, et al. (2017) Vitamin D deficiency as a risk factor for dementia: a systematic review and meta-analysis. BMC Geriatr 17(1): 16.
- Yang K, Chen J, Li X, Zhou Y (2019) Vitamin D concentration and risk of Alzheimer disease: A meta-analysis of prospective cohort studies. Medicine (Baltimore) 98(35): e16804.
- Dean AJ, Bellgrove MA, Hall T, Phan WM, Eyles DW, et al. (2011) Effects of vitamin D supplementation on cognitive and emotional functioning in young adults--a randomised controlled trial. PLoS One 6(11): e25966.
- Rossom RC, Espeland MA, Manson JE, Dysken MW, Johnson KC, et al. (2012) Calcium and vitamin D supplementation and cognitive impairment in the women's health initiative. J Am Geriatr Soc 60(12): 2197-2205.
- Annweiller C, Fantino B, Gautier J, Beaudenon M, Thiery S, et al. (2012) Cognitive effects of vitamin D supplementation in older outpatients visiting a memory clinic: a pre-post study. J Am Geriatr Soc 60(4): 793-795.
- Pettersen JA (2017) Does high dose vitamin D supplementation enhance cognition? A randomized trial in healthy adults. Exp Gerontol 90: 90-97.
- Jeanette E Owusu, Shahidul Islam, Subhashini S Katumuluwa, Alexandra R Stolberg, Gianina L Usera, et al. (2019) Cognition and Vitamin D in Older African American Women- Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J Am Geriatr Soc 67(1): 81-86.
- Jorde R, Kubiak J, Svartberg J, Fuskevåg OM, Figenschau Y, et al. (2019) Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. J Neurol Sci 396: 165-171.
- Schietzel S, Fischer K, Brugger P, Orav EJ, Renerts K, et al. (2019) Effect of 2000 IU compared with 800 IU vitamin D on cognitive performance among adults age 60 years and older: a randomized controlled trial. Am J Clin Nutr 110(1): 246-253.
- Balbaloglu O, Tanık N (2019) The effect of vitamin D on cognitive functions in young female patients: a prospective controlled study using the Montreal Cognitive Assessment. Arq Neuropsiquiatr 77(1): 19-24.
- Afsane Bahrami, Sayyed Saeid Khayyatzadeh, Najmeh Jaberi, Maryam Tayefi, Farzaneh Mohammadi, et al. (2019) Common Polymorphisms in Genes Related to Vitamin D Metabolism Affect the Response of Cognitive Abilities to Vitamin D Supplementation. J Mol Neurosci 69(1): 150-156.
- Monica Castle, Nancy Fiedler, Lilliana Claudia Pop, Stephen J Schneider, Yvette Schlussel, et al. (2020) Three Doses of Vitamin D and Cognitive Outcomes in Older Women: A Double-Blind Randomized Controlled Trial. J Gerontol A Biol Sci Med Sci 75(5): 835-842.
- Annweiller C, Herrmann FR, Fantino B, Brugg B, Beauchet O (2012) Effectiveness of the combination of memantine plus vitamin D on cognition in patients with Alzheimer disease: a pre-post pilot study. Cogn Behav Neurol 25(3): 121-127.
- Hu J, Jia J, Zhang Y, Miao R, Huo X, et al. (2018) Effects of vitamin D3 supplementation on cognition and blood lipids: a 12-month randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry 89(12): 1341-1347.
- Ates Bulut E, Soysal P, Yavuz I, Kocyigit SE, Isik AT (2019) Effect of Vitamin D on Cognitive Functions in Older Adults: 24-Week Follow-Up Study. Am J Alzheimer's Dis Other Demen 34(2): 112-117.
- Jia J, Hu J, Huo X, Miao R, Zhang Y, et al. (2019) Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer's disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry 90(12): 1347-1352.
- Yang T, Wang H, Xiong Y, Chen C, Duan K, et al. (2020) Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J Alzheimers Dis 78(4): 1509-1518.
- Miller BJ, Whisner CM, Johnston CS (2016) Vitamin D Supplementation Appears to Increase Plasma Aβ40 in Vitamin D Insufficient Older Adults: A Pilot Randomized Controlled Trial. J Alzheimers Dis 52(3): 843-847.
- Du Y, Liang F, Zhang L, Liu J, Dou H (2020) Vitamin D Supplement for Prevention of Alzheimer's Disease: A Systematic Review and Meta-Analysis. Am J Ther 28(6): e638-e648.

 Review Article
Review Article