ABSTRACT
Mitochondrial dysfunction causes many symptoms, such as Alzheimer’s disease, angina, attention deficit, behavioral problems, chronic fatigue syndrome (CFS), diabetes, dementia, depression, hair loss, heart disease, immune dysfunction, muscle weakness, and pain, and skin rash (Dean and English [1]). We focus on therapeutics for mitochondrial dysfunction that arises from infection by the Epstein-Barr virus (EBV), which causes CFS and insomnia.
Introduction
Epstein-Barr virus (EBV) is ubiquitous in adults (90-95%) and is a DNA, γ-herpes virus, also known as Herpes Simplex Virus 5, HSV- 5, or Human Herpesvirus 4, HHV-4). EBV is associated with chronic fatigue syndrome (CFS) (Blomberg, et al. [2-10]). CFS symptoms include persistent fatigue that is not due to ongoing exertion, not alleviated by rest, and has reduced the patient’s activity level substantially. (White, et al. [9]) notes that two post-infectious-EBV forms exist:
(1) With insomnia and
(2) With hypersomnia.
(Cooke, et al. [3]) associate latent EBV infection with psychiatric disorders (e.g., depression and manic-depressive illness). An EBV infection typically begins with a severe, acute flu-like infection: severe lethargy/fatigue, fever, and fever-induced sleep without GI upset. This acute phase is usually called infectious mononucleosis. (McFadden, et al [11]) found that EBV initially induces hyperproliferation that is quickly suppressed by activation of DNA damage response and G1/S-phase growth arrest with a rapid recovery (<72 hours) in immuno-competent patients. Recovery may take 6 months or more in immune-compromised patients. The typical virus size is 0.02-0.4 microns, allowing viral penetration of a human-cell wall (Subramanian, et al. [12]). Inside the cell, the viral genome is injected into the cell’s cytoplasm, hidden from the immune system, while the virus replicates and gains control of cellular functions after the acute infection. This latent infection causes metabolic hijacking (Lange, et al. [13-16]) of acetyl-CoA, acetylation, glycolysis, Krebs cycle, lipid synthesis, and EBV-infected immune cells [B-cells (Hulse, et al. [16-18]); T- and natural-killer-cells (Imashuku [19]). The latent EBV infection normally continues for the rest of the patient’s life. The arrested cells have a reduced level of mitochondrial respiration and a decrease in gene expression for the Krebs cycle and oxidative phosphorylation. Arrested cells are characterized by increased expression of p53 pathway gene targets, including sestrins leading to activation of AMPK, reduction in mTOR signaling, and increased autophagy. A concomitant increase in glucose imports and surface glucose transporter 1 (GLUT1) occurs, leading to elevated glycolysis, oxidative phosphorylation, and suppression of basal autophagy. (Xiao, et al. [20, 21]) found that latently infected cells have enhanced glucose and glutamine uptake with deregulated glycolysis. (Li, et al. [18]) showed that fatty acid synthesis is important for EBV lytic replication. The EBV latent membrane protein 1 (LMP-1) is expressed in many (but not all types) of EBV-1 and can induce SREBP1 and FASN, leading to increased lipid droplets (Lo, et al. [44]), sometimes leading to slightly elevated LDL.
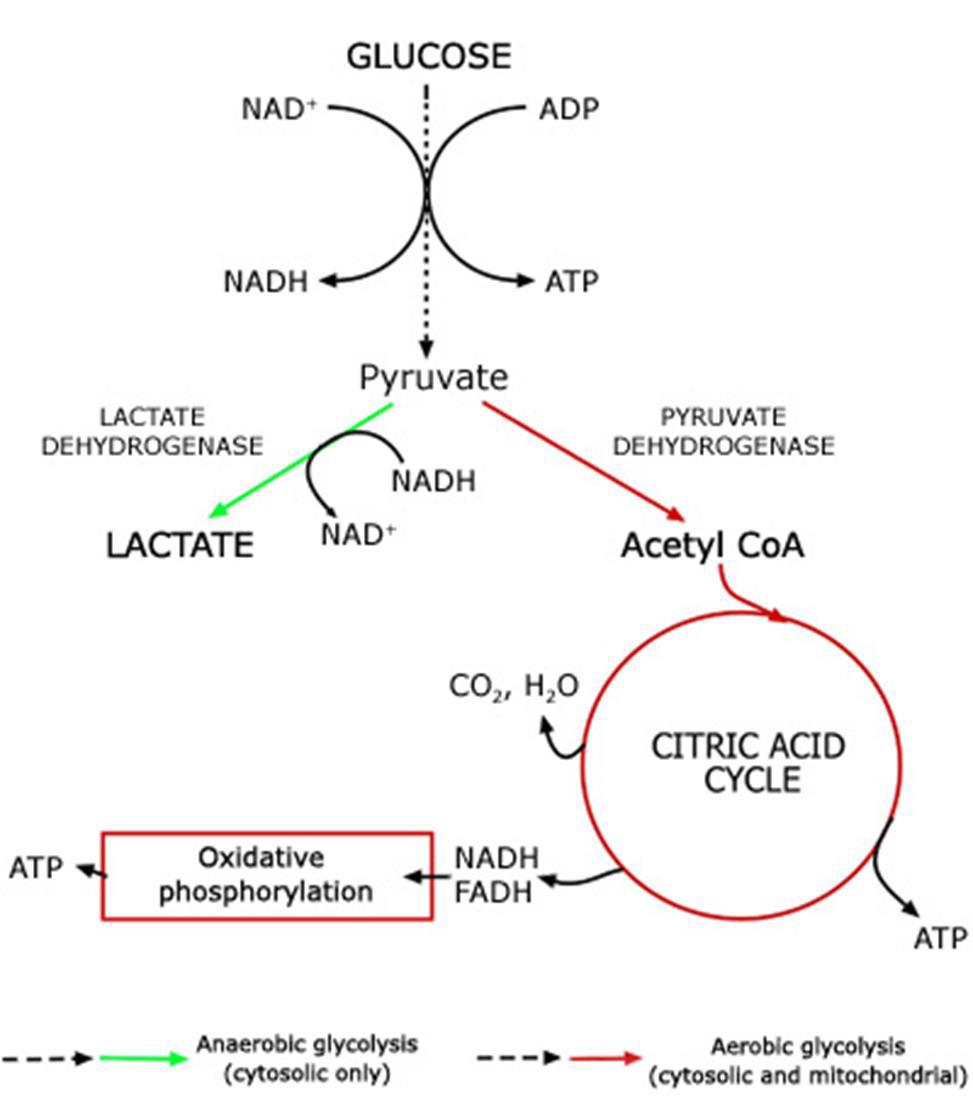
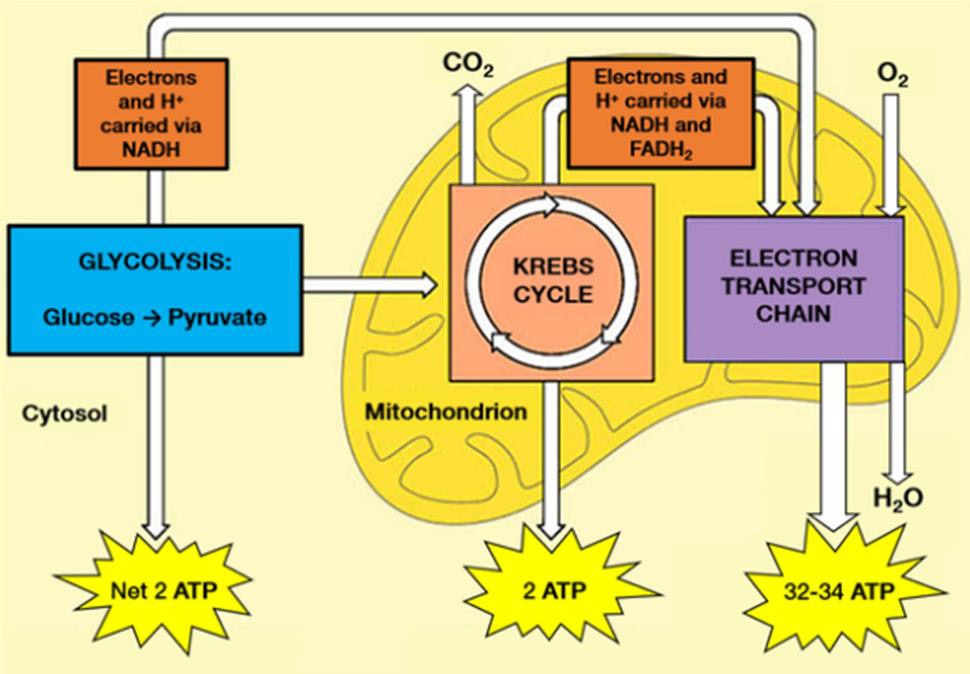
Adenosine triphosphate (ATP) is the body’s currency of energy transfer. Figure 1 shows metabolism beginning with glycolysis that processes glycose into pyruvate, ATP, and lactic acid. Lactic acid (lactate) is a waste product that is converted via the liver’s Cori cycle into glucose; this conversion is particularly efficient in highly trained athletes [McArdle, et al. [22,23]). Pyruvate enters the Krebs’ cycle, which produces more ATP (Figure 2). Excess electrons and H+ from the Krebs’ cycle are converted into much more ATP in the electron transport chain (ETC). The Krebs’ cycle and ETC occur inside mitochondria. A typical cell has hundreds of mitochondria, while ~2000 are present in liver cells and none occur in mature red blood cells. Metabolic hijacking involves mitochondrial dysfunction.
Figure 1: Conversion of glucose into ATP, lactate and pyruvate that then enters the Krebs’ cycle.
Note: https://acutecaretesting.org/en/articles/lactate-and-lactic-acidosis/; accessed 12Sep2021
Figure 2: Glycolysis processes glucose from food into pyruvate, plus two molecules of ATP. Pyruvate then enters the Krebs’ cycle (also called the citric acid cycle or tricarboxylic acid cycle) and is converted into two more ATP molecules. Excess electrons (carried by electron donors) and excess protons (H+) from the Krebs’ cycle are metabolized into many more ATP molecules in the electron transport chain (ETC). See [A.L. Lehninger et al. [43] for details.
Treatment of Mitochondrial Dysfunction
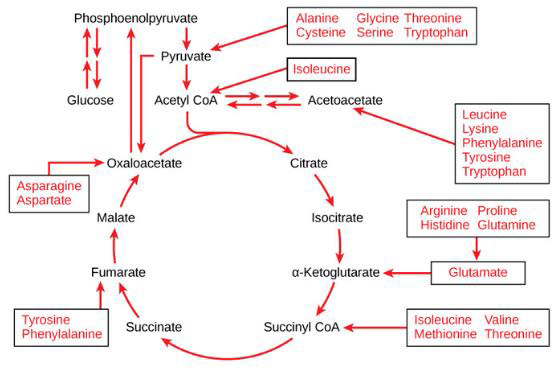
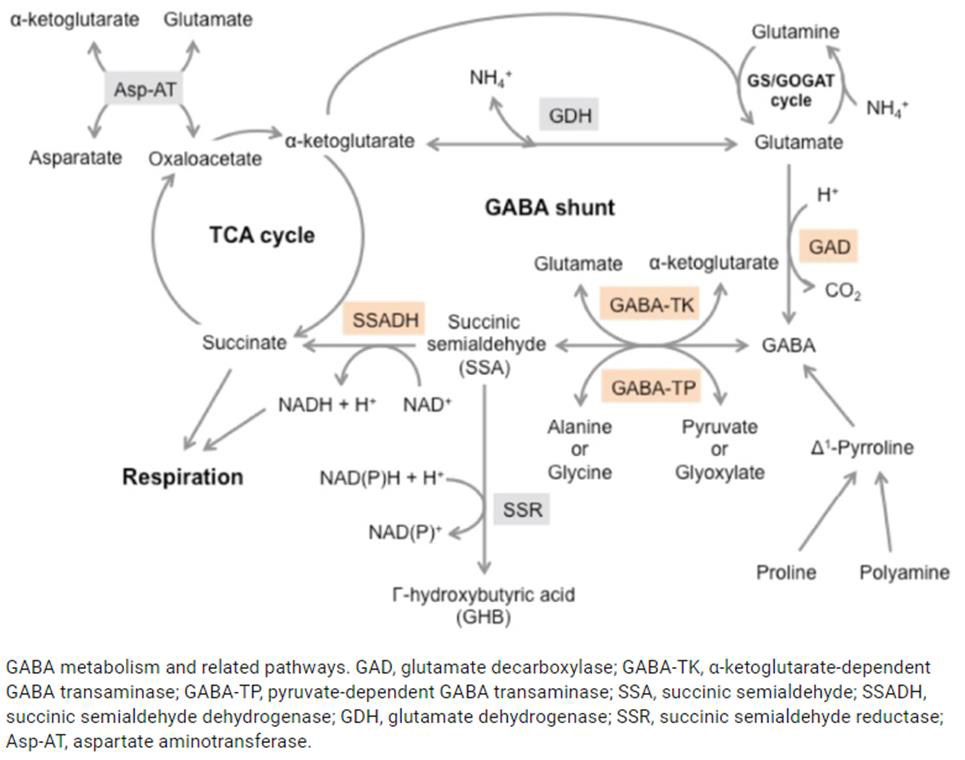
Mitochondrial dysfunction impairs ATP production, causing the diseases, as noted above. Amino and fatty acids from food can enter the Krebs’ cycle (Figure 3). Thus, supplementation with freeform amino acids can boost energy and mitigate hypoglycemia under stress conditions (e.g., trauma, exercise, starvation, and disease) (Dohm, et al. [24-26]). The GABA shunt diverts GABA (gamma-aminobutyric acid) from the production of GHB (gamma hydroxybuterate) into production of succinate (Figure 4) under stress conditions (e.g., latent EBV infection that causes insomnia due to GABA and GHB deficiencies. Mitochondrial dysfunction can also arise from cofactor deficiency, for which vitamin and mineral supplementation is appropriate (DiPasquale [27]). Many co-factors are helpful in treating insomnia [e.g., B1 (250 mg), B2, B3 (250 mg), B5 (500 mg), B6 (25 mg, as P5P), B9 (as methyl folate—680 mg), B12, vitamin C, calcium citrate (315 mg), GABA (300 mg), Mg (1.2 g), Zn, lipoic acid, tryptophan (1 g), fish oil (1 g)]. These co-factor deficiencies cause melatonin deficiency (Figure 5) and deficiencies in GABA and GHB (Figure 6). The commonalities in these pathways are magnesium, B6, zinc, and copper (an inhibitor to avoid after noon). B6 supplementation markedly improves sleep, along with a GABA-agonist (e.g., 1 mg Clonazepam). A review on mitochondrial dysfunction proposes antioxidants (CoQ10, alpha lipoic acid, and acetyl-L-cysteine) that are useful in singleton and paired combinations, but never tested as a triad (Pagano, et al. [28]). Other antioxidants are glutathione (poorly absorbed orally) and N-acetylcysteine (precursor of glutathione). Anti-inflammatories include turmeric (450 mg) and vitamin D (5000 IU).
Note: https://courses.lumenlearning.com/boundless-biology/chapter/connections-of-carbohydrate-protein-and-lipidmetabolic- pathways/
Figure 3: Proteins are converted into amino acids by a variety of enzymes. Usually, amino acids are recycled into the synthesis of new proteins or are used as precursors for synthesis of other important biological molecules (e.g., hormones, nucleotides, neurotransmitters). A deficiency in Krebs’ cycle reactant can cause shunting of some amino acids into the Krebs’ cycle, as shown here.
Figure 4: The GABA shunt bypasses two steps (oxidation of α-ketoglutarate to succinate) of the tricarboxylic acid (TCA) cycle via reactions catalyzed by three enzymes: glutamate decarboxylase, GABA transaminase, and succinic semialdehyde dehydrogenase. The GABA shunt plays a major role in primary carbon and nitrogen metabolism and is an integral part of the TCA cycle under stress conditions [Takayama and Ezura [45]].
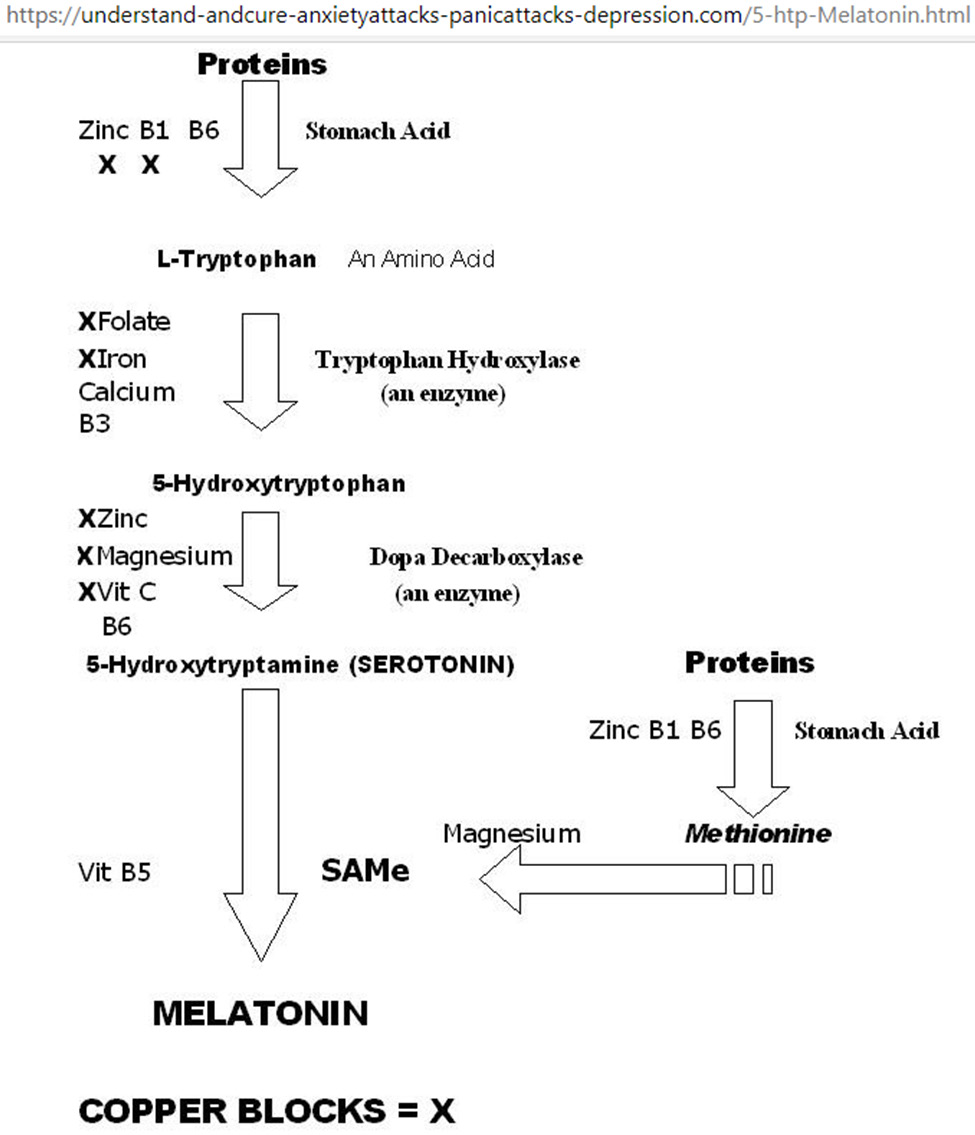
Figure 5: Metabolic network for tryptophan-5HTP-setotonin-melatonin. Starting from the top, vitamins B1/B6 and zinc are required for stomach acid production to break down proteins into amino acids; excess copper blocks B1 and zinc as co-factors for stomach acid production. Serotonin promotes happiness; serotonin deficiency results in depression, and/or anxiety, and/ or panic attacks. Melatonin promotes sleep, melatonin deficiency results in insomnia. Supplementation with Mg, Ca, Zn, and vitamins B1, B3, B5, B6, B9, and C improves sleep quality.
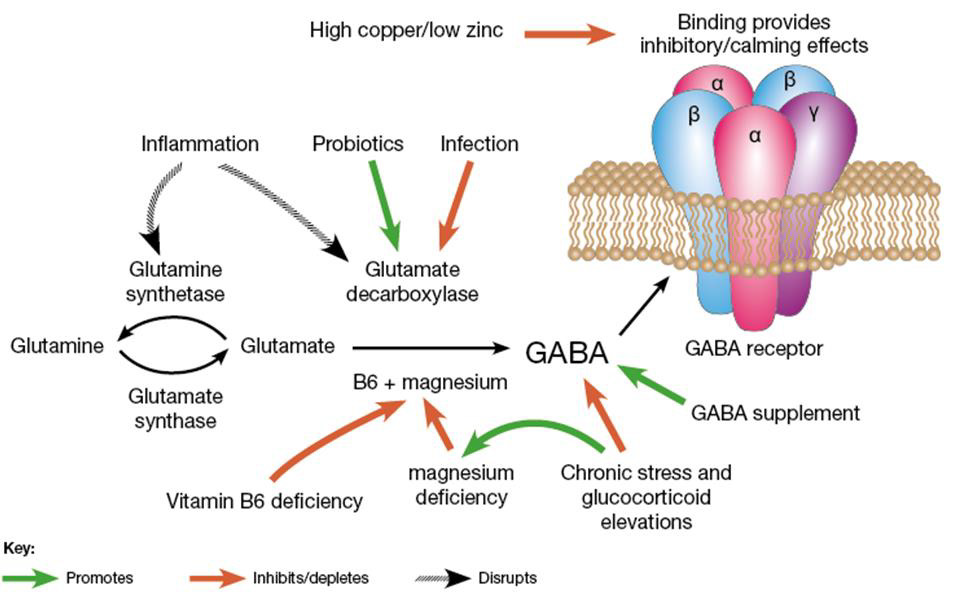
Figure 6: Metabolic network for glutamine-glutamate-GABA. Excess Cu inhibits this network as in Figure 5. B6, Mg, Zn are also co-factors in this network. [(https://drjockers.com/gaba/) accessed 15May2021].
High-GABA food (e.g., tomatoes, spinach, oats, potatoes, sweet potatoes, corn) improves sleep. High-glutamate food (e.g., tomatoes, eggs, cheese, salmon, beef, spinach, nut butter, Parmesan cheese) is converted to GABA via B6 and magnesium for sustained sleep. Whole wheat bread (2 slices at supper) facilitates tryptophan transport across the blood-brain barrier to improve sleep. Latent EBV infection activates immune response of interferon-gamma (IFN- γ) that in turn triggers indoleamine-2,3-dioxygenase (IDO), which metabolizes tryptophan to kynurenine (rather than to 5HTP→serotonin→melatonin (Mehraj, et al. [29,30]). EBV’s diversion of tryptophan to kynurenine decreases serotonin, and thus increases depression (Anderson, et al. [31]), making 5HTP a potential alternative to anti-depressive medications. (Schwarcz [32]) found that metabolites of the kynurenine pathway influence glutamatergic activity, including ionotropic and metabotropic receptors, vesicular glutamate transport, and generation/ scavenging reactive free radicals.
(Kermani [5]) notes that chronic (latent) EBV infection is associated with
a) Over-burdened organs, including the liver, pancreas, spleen, and gut
b) Cardiac dysrhythmias,
c) Elevation of IgG, IgM-antibodies and anti-nuclear body,
d) Reduction in natural killer and lymphocyte helper cells,
e) Deficiencies in trace elements, vitamins, antioxidant capacity,
f) Excess of heavy metals,
g) Hashimoto’s thyroiditis, which is usually associated with heavy metal toxicity, e.g., amalgam fillings,
h) Dental infections,
i) Additional lymphotropic pathogens, such as cytomegaloviruses,
chlamydia, toxoplasma, and borrelia.
Treatment recommendations by (Kermani [5]) include alkaline infusion, vitamins B6/B9/B12/C, magnesium, selenium, zinc, and algae.
Numerous antivirals are used to treat EBV (Lieberman [6]), including:
• Glycyrrhizin in licorice root (Lin, et al. [33])
• Lauric acid/monolaurin,
• Butyrate in the form of tributyrin (Szentirmai [34])
• Quercetin
• N-acetyl cysteine,
• Co-enzyme Q-10,
• Exercise,
• Purified protein A from calf thymus (Riordan, et al. [35]),
• Proteflazid [Chopyak, et al. 2008],
• Valtrex (Sunde [36]).
Acknowledgment
Enlightening discussions with Rachel A. Wilkenson MD motivated this work, which was unfunded.
Conclusion
Ongoing research seeks novel diagnostics and therapeutics for EBV/CFS and EBV-induced cancers, such as EBV nuclear antigen 1 and EBV-oncoproteins, LMP1 and LMP2A (Andrei, et al. [37-48]).
References
- W Dean, J English (2013) Krebs’ Cycle intermediates. Nutrition Rev.
- J Blomberg, Rizwan M, Böhlin-Wiener A, Elfaitouri A, Julin P, et al. (2019) Antibodies to human herpesviruses in myalgic encephalomyelitis/ chronic fatigue syndrome. Front Immunol 10: 1946.
- RG Cooke, Warsh JJ, Hasey GM (1989) Epstein-Barr virus as a cause of autoimmune disease and other medical morbidity in patients with affective disorders. Med Hypoth 29(3): 177-185.
- K Kawai, A Kawai (1992) Studies on the relationship between chronic fatigue syndrome and Epstein-Barr Virus in Japan. Internal Med 31(3): 313-318.
- H Kermani (2009) Chronic Epstein-Barr infection. Verlag fur Naturheilkunde.
- S Lieberman (2004) Antiviral Intervention for Chronic Fatigue Syndrome. (encognitive.com). Townsend Lett Doc & Patients p: 74-76.
- SH Mozhgani, Farid Rajabi, Mohsen Qurbani, Yousef Erfani, Somayeh Yaslianifard, et al. (2021) Human Herpesvirus 6 infection and risk of chronic fatigue syndrome: A systematic review and meta-analysis. Intervirology 65(1): 49-57.
- ES Pankanin (2018) Chronic fatigue syndrome - criteria for diagnosis, etiology, treatment. Journal of Education, Health and Sport (umk.pl) 8: 430-435.
- PD White (2007) What causes prolonged fatigue after infectious mononucleosis and does it tell us anything about chronic fatigue syndrome. J Infect Diseases 196(1): 4-5.
- Marshall V Williams, Brandon Cox, William P Lafuse, Maria Eugenia Ariza (2019) Epstein-Barr virus dUTPase induces neuroinflammatory Mediators: Implications for myalgic encephalomyelitis/chronic fatigue syndrome. Clin Therapeutics 41(5): 848-863.
- McFadden, Amy Y Hafez, Rigel Kishton, Joshua E Messinger, Pavel A Nikitin, et al. (2016) Metabolic stress is a barrier to Epstein-Barr virus-mediated B-cell immortalization. Proc National Acad Sci 113(6): E782-E790.
- RP Subramanian, Robert J Geraghty (2007) Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins DHL and B. Proc National Acad Sci 104(8): 2903-2908.
- PT Lange, Michael Lagunoff, Vera L Tarakanova (2019) Chewing the Fat: the conserved ability of DNA viruses to hijack cellular lipid metabolism. Viruses 11(2): 119.
- Katharina A Mayer, Johannes Stöckl, Gerhard J Zlabinger, Guido A Gualdoni (2019) Hijacking the supplies: Metabolism as a novel facet 0f virus-host interaction Front Immun 10: 1533.
- Shivani K Thaker, James Ch'ng, Heather R Christofk (2019) Viral hijacking of cellular metabolism. BMC Biology 17(1): 59.
- Liang Wei Wang, Zhonghao Wang, Ina Ersing, Luis Nobre, Rui Guo, et al. (2019) Epstein Barr virus subverts mevalonate and fatty acid pathways to promote infected B cell proliferation and survival. PloS Pathog 15(9): e1008030.
- M Hulse, Sarah M Johnson, Sarah Boyle, Lisa Beatrice Caruso, Italo Tempera (2021) Epstein-Barr virus-encoded latent membrane protein 1 and B-cell growth transformation induces lipogenesis through fatty acid synthase. J Virology 95(4): e01857-20.
- Chao-Yuan Tsai, Shuhei Sakakibara, Teruhito Yasui, Takeharu Minamitani, Daisuke Okuzaki, et al. (2018) Bystander inhibition of humoral immune responses by Epstein-Barr virus LMP1. Intl Immunol 30(12): 579-590.
- S Imashuku (2002) Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol 44(3): 259-272.
- L Xiao, ZY Hu, X Dong, Z Tan, W Li, et al. (2014) Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene 33(37): 4568-4578.
- AK Lo, Christopher W Dawson, Lawrence S Young, Chuen Wai Ko, Pok Man Hau, et al. (2015) Activation of the FGFR1signalling pathway by the Epstein-Barr virus-encoded LMP1promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J Pathol 237(2): 238-248.
- WD McArdle, FI Katch, VL Katch (1996) Exercise Physiology-Energy, Nutrition and Human Performance (4th)., Dona Balado ed. Williams & Wilkins, Pennsylvania, publ. 199: 577-581.
- WL Kenney, JH Wilmore, DL Costill (1999) Physiology of Sport and Exercise (2nd)., Human Kinetics Publishing Champaign IL.
- GL Dohm, GJ Kasperek, EB Tapscott, HA Barakat (1985) Protein metabolism during endurance exercise. Fed Proc 44(2): 348-352.
- DL Shaw, MA Chesney, IF Tullis, HP Agersborg (1962) Management of fatigue: A physiological approach. Am J Med Sci 243:758.
- JT Hicks (1964) Treatment of fatigue in general practice: A double blind study. Clin Med 71: 85-90.
- M Di Pasquale (2007) Amino Acids and Proteins for the athlete: The Anabolic Edge. CRC Press Boca Raton Florida.
- G Pagano, Federico V Pallardó, Alex Lyakhovich, Luca Tiano, Maria Rosa Fittipaldi et al. (2020) Aging-related disorders and mitochondrial dysfunction. Int J Molec Sci 21: 7060.
- V Mehraj, JP Routy (2015) Tryptophan catabolism in chronic viral infections: handling uninvited guests. Int J Trypt Res 8: 41-48.
- Rosa Bellmann Weiler, Katharina Schroecksnadel, Claudia Holzer, Clara Larcher, Dietmar Fuchs, et al. (2008) IFN-gamma mediated pathways in patients with fatigue and chronic Epstein Barr virus infection. J Affective Disorders 108(2): 171-176.
- G Anderson, M Berk, M Maes (2014) Biological phenotypes underpin the physio‐somatic symptoms of somatization depression and chronic fatigue syndrome. Acta Psych Scandin 129(2): 83-97.
- R Schwarcz (2016) Kynurenines and glutamate: multiple links and therapeutic implications. Adv Pharamacol 76: 13-37.
- JC Lin, Jaw Ming Cherng, Man Shan Hung, Lidia A Baltina, Lia Baltina, et al. (2008) Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus Infection: Structure-activity relationships. Antiviral Res 79(1): 6-11.
- Éva Szentirmai, Nicklaus S Millican, Ashley R Massie, Levente Kapás (2019) Butyrate, a metabolite of intestinal bacteria enhances sleep. Sci Rpts 9: 7035.
- NH Riordan, JA Jackson, HD Riordan (1998) Pilot study of the effects of thymus protein on elevated Epstein-Barr virus titers. Townsend Lett Doc Patients 78-79.
- Pia Titterud Sunde, Ingar Olsen, Morten Enersen, Bjørn Grinde (2008) Patient with severe periodontitis and subgingival Epstein-Barr virus treated with antiviral therapy. J Clin Virology 42(2): 176-178.
- Graciela Andrei, Erika Trompet, Robert Snoeck (2019) Novel therapeutics for Epstein-Barr virus. Molecules 24(5): 997.
- TE Messick, Garry R Smith, Samantha S Soldan, Mark E McDonnell, Julianna S Deakyne, et al. (2019) Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein Barr virus infection and tumor growth. Sci Transl Med 11(482): 5612.
- HF Chau, Yue Wu, Wan-Yiu Fok, Waygen Thor, William Chi-Shing Cho, et al. (2021) Lanthanide-based peptide-directed visible/near-infrared imaging and inhibition of LMP1. JACS Au 1(7): 1034-1043.
- A Sakudo, Hirohiko Kuratsune, Takanori Kobayashi, Seiki Tajima, Yasuyoshi Watanabe, et al. (2006) Spectroscopic diagnosis of chronic fatigue syndrome by visible and near-infrared spectrometry in serum samples. Biochem Biophys Res Comm 345(4): 1513-1516.
- S Soldan, Emma M Anderson, Drew M Frase, Yue Zhang, Lisa B Caruso, et al. (2021) EBNA1 inhibitors have potent and selective antitumor activity in xenograft models of Epstein Barr virus-associated gastric cancer. Gastric Cancer 24(5): 1076-1088.
- J Zhu, Saidu K, Cen D, Tang W, Gu M, et al. (2020) Generation of novel affibody molecules targeting the EBV LMP2A N-terminal domain with inhibiting effects on the proliferation of nasopharyngeal carcinoma cells. Cell Death Disease 11(4): 213.
- DL Nelson, A Lehninger, MM. Cox, (2008) Principles of Biochemistry. WH Freeman & Co. New York, NY.
- AK Lo, Raymond Wai Ming Lung, Christopher W Dawson, Lawrence S Young, Chuen Wai Ko, et al. (2018) Activation of sterol regulatory element binding protein 1 (SREBP1) mediated lipogenesis by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) promotes cell proliferation and progression of nasopharyngeal carcinoma. J Pathol 246(2): 180-190.
- M Takayama, H Ezura (2015) How and why does tomato accumulate a large amount of GABA in the fruit. Frontiers in Plant Science 6: 612.

 Mini Review
Mini Review