ABSTRACT
Of particular relevance in the context of the demographic crisis is the timely diagnosis and correction of potentially preventable causes of stillbirth and perinatal mortality, which include umbilical cord abnormalities. This determined the aim of the study - to identify the role of umbilical cord abnormalities in the development of acute intrapartum fetal asphyxia and perinatal mortality in singleton term delivery in cephalic presentation. Materials and methods: To achieve the purpose, an analysis was made of the causes of 200 cases of acute intrapartum fetal asphyxia in the Ryazan region for the period 2011-2020, according to the inclusion and non-inclusion criteria for the study. The work uses methods of expert evaluation, system-structural analysis and statistical data processing using the computer software package Statistica v. 11 (StatSoft, Inc., USA). The results of the study revealed a significant contribution of umbilical cord abnormalities to the development of acute intranatal fetal asphyxia (23.5%), the birth of children in a state of severe asphyxia and perinatal mortality, as well as an extremely low prenatal diagnosis of umbilical cord abnormalities (17.7%). Conclusion: Increasing the efficiency of prenatal diagnosis of umbilical cord abnormalities will make it possible to correctly determine the obstetric tactics of delivery, which will help reduce the likelihood of developing acute intranatal fetal asphyxia, severe neonatal asphyxia, stillbirth and perinatal mortality.
Keywords: Umbilical Cord Abnormalities; Pathology of Wharton’s Jelly; Umbilical Cord Prolapse; Acute Intrapartum Fetal Asphyxia; Stillbirth; Intrapartum Fetal Death; Early Neonatal Fetal Death
Introduction
Despite the constant improvement of medical technologies and an increase in the quality of obstetric and perinatal care, acute intrapartum fetal asphyxia and stillbirth continue to remain urgent problems of modern medicine [1,2]. Therefore, the timely diagnosis and correction of potentially preventable causes of stillbirth and perinatal mortality is of particular relevance today. Such causes include umbilical cord abnormalities, which, according to various scientific data, account for about 10% of the possible or probable causes of stillbirth, and are more common after 32 weeks of pregnancy [3-7]. According to R. Bukowski (2017) and H. Mantakas (2018) et al, the contribution of umbilical cord abnormalities to stillbirth can vary widely, and range from 8% to 65% [8,9]. The human umbilical cord is a multi differentiated, constantly growing, extraembryonic organ that ensures the connection of the fetus with the placenta and its life support in the dynamics of pregnancy and childbirth [3-5]. Umbilical cord abnormalities, that can cause acute intrapartum fetal asphyxia include: entanglement around fetus neck and body parts [8-11]; umbilical cord prolapse [3-5]; true nodes, torsion, or strictures with blood clots [3,5,8]; vessels previa [12-15]; marginal or membrane attachment [3-5,16]; excessive or insufficient number of coils, pathology of Wharton’s jelly, vessels and umbilical cord length [3-5,17-22].
According to J.E. Lawn et al (2016), stillbirth is currently not declining, and continues to increase at an accelerated rate by 2030 [23]. Therefore, prenatal diagnosis of umbilical cord abnormalities is an urgent task of modern obstetrics in the 21st century. According to many researchers, the role of umbilical cord abnormalities as a cause of intrapartum fetal asphyxia is insufficiently understood [3- 5,24,25], which determined the purpose of this study. The aim of the study was to identify the role of umbilical cord abnormalities in the development of acute intrapartum fetal asphyxia and perinatal mortality in singleton term delivery in cephalic presentation.
Materials and Methods
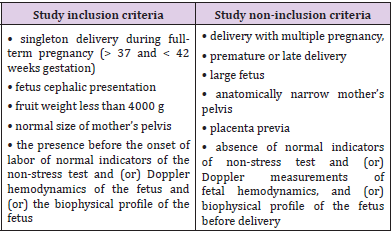
A systemic structural analysis was made of 200 cases of acute intrapartum fetal asphyxia in the Ryazan region in 2011–2020. The analysis included every first 20 cases of this pathology in each year. The study inclusion and non-inclusion criteria were clearly defined (Table 1). The main study inclusion criteria: singleton delivery during full-term pregnancy; fetus cephalic presentation; normal size of the fetus and mother’s pelvis; the presence of normal indicators of non-stress test and (or) Doppler measurements of fetal hemodynamics, and (or) biophysical profile of the fetus before delivery. The main study non-inclusion criteria: delivery with multiple pregnancy; premature or late delivery; large fetus; anatomically narrow mother’s pelvis; placenta previa; absence of normal indicators of non-stress test and (or) Doppler measurements of fetal hemodynamics, and (or) biophysical profile of the fetus before delivery. A positive non-stress test and (or) normal Doppler parameters of the fetal hemodynamics and (or) the biophysical profile of the fetus before delivery were an indirect confirmation of the development of acute intranatal fetal asphyxia.
The work used methods of expert evaluation of clinical, laboratory, instrumental and special methods of research, tactics planning and management of delivery, as well as a systemstructural analysis of the causes of acute intrapartum fetal asphyxia. The source of information was the primary medical documentation - an individual card of the pregnant woman and the puerperal, the historys of delivery and newborns, protocols for pathoanatomical and histological studies of the placenta and fetus (in case of stillbirth and early neonatal death). An expert evaluation of the protocols of delivery, the results of ultrasound examination and the biophysical profile of the fetus (if any), the protocols of pathoanatomical and histological examination of the placenta, as well as the fetus (in case of stillbirth and early neonatal death) was completed. The biophysical profile of the fetus was assessed by five indicators from 0 to 2 points each: non-stress test, physical activity, respiratory movements and muscle tone of the fetus, the amount of amniotic fluid. The criterion for the normal state of the fetus was a score of 8–10 points. The condition of the newborns was assessed in a comprehensive manner - according to the results of pH-metry of umbilical cord blood (if the study was timely) and according to the Apgar scale at 1 and 5 minutes of life. Criteria for severe fetal asphyxia - pH below 7.2, Apgar score from 3 to 0 points. Criteria for moderate fetal asphyxia - pH from 7.20 to 7.25, Apgar score from 7 to 4 points. Criteria for the normal state of the fetus - pH above 7.25, Apgar score 8-10 points. Statistical processing of the results was carried out using the Statistica v. 11 (StatSoft, Inc., USA) using parametric and nonparametric statistics methods
Results and Its Discussion
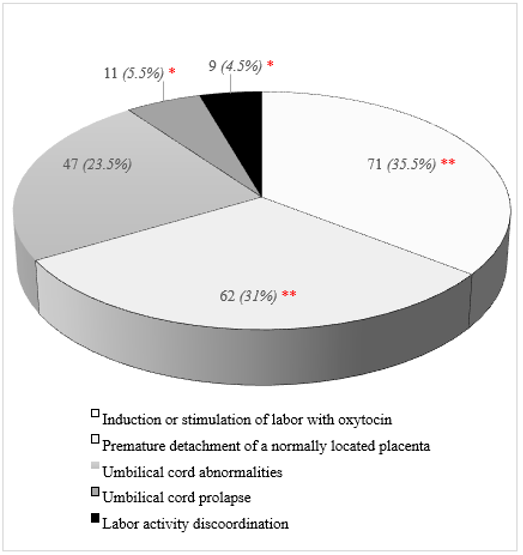
The structure of the main identified causes of acute intranatal fetal asphyxia shown in the (Figure1). The first ranking place in the structure of causes of acute intrapartum fetal asphyxia was taken by the use of oxytocin in labor – 35.5%, the second – premature detachment of the normally located placenta – 31% (рχ2 <0.05). Umbilical cord abnormalities ranked third in the structure of the causes of acute intrapartum fetal asphyxia (23.5%) and were found 1.3-1.5 times significantly less frequently than the previous causes (рχ2 <0.05). Umbilical cord prolapse and labor activity discoordination were significantly less frequent than other causes of intrapartum fetal asphyxia, and amounted to 5.5% and 4.5%, respectively (рχ2 <0.05). Methods of emergency delivery were used in all cases of acute intranatal fetal asphyxia, in the first stage of labor - abdominal delivery in 111 (55.5%) cases, in the second stage of labor - obstetric forceps in 53 (26.5%) and vacuum extraction of the fetus in 36 (18%) cases.
Figure 1: The structure of the acute intrapartum fetal asphyxia. Statistically significant differences with the proportion of umbilical cord abnormalities according to the χ2 fit criterion (р<0.05): ** – statistically significant higher, * – statistically significantly lower.
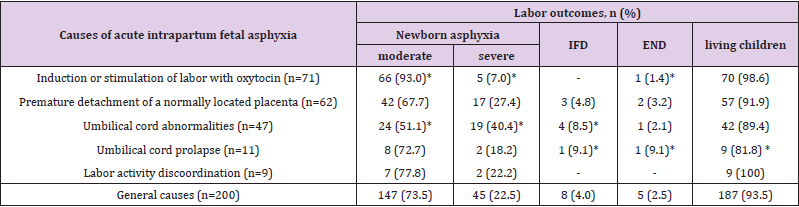
Delivery outcomes are presented in Table 2. The largest proportion of newborns in a state of severe asphyxia registered with abnormalities of the umbilical cord – 40.4%. With premature detachment of a normally located placenta, it was 1.5 times less (27.4%, рχ2<0.05), and with labor activity discoordination – it was 1.8 times less (22.2%, рχ2 <0.05). The highest specific weight of perinatal losses was registered with the loss of the umbilical cord loops (18.2%), with abnormalities of the umbilical cord, it was 10.6%, and with premature detachment of a normally located placenta - 8%, which is significantly higher than in the general structure of causes (pχ2<0.05). There were no cases of perinatal death due to incoordination of labor. Some researchers consider umbilical cord prolapse in the general structure of cord abnormalities [3,4]. In our study, out of 11 cases of umbilical cord prolapse, 3 cases (27.3%) had a long umbilical cord (more than 70 cm), 5 cases (45.5%) - polyhydramnios, and 2 cases (18.2%) - a combination of these pregnancy complications.
Table 2: Delivery outcomes in acute intrapartum fetal asphyxia.
Note: n (%) – the absolute number of cases and their proportion for each of the reasons; IFD – intrapartum fetal death; END – early neonatal death; * – statistically significant differences with general causes according to the criterion of matching χ2 (рχ2 <0,05).
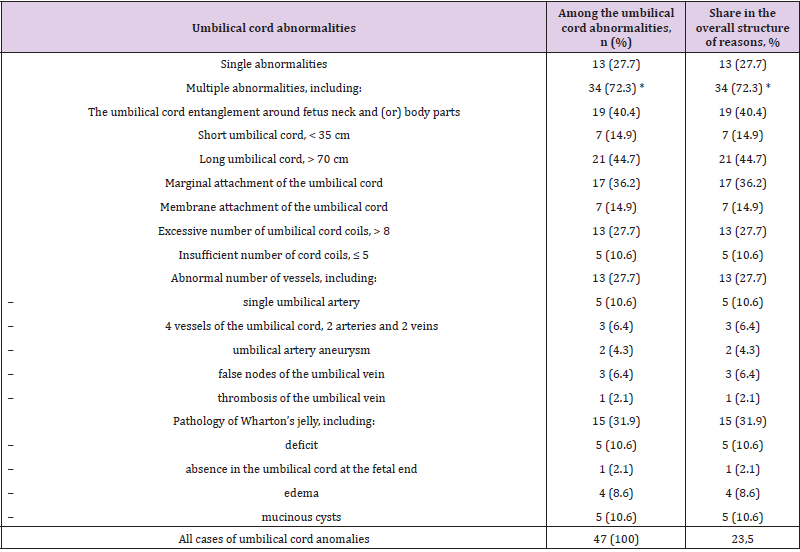
Thus, in 7 cases (63.6%) there were no other abnormalities of the umbilical cord during it prolapse. In 6 cases (54.6%), prolapse of the umbilical cord occurred either during amniotomy (2 cases) or shortly after amniotomy (4 cases), which did not allow us to exclude the iatrogenic cause of this pathology. Possible iatrogenic causes of cord prolapse may be incorrect amniotomy and removal of amniotic fluid, especially in polyhydramnios [5]. Therefore, isolated prolapse of the umbilical cord, without combination with other abnormalities of the umbilical cord, we considered as an independent cause of acute intranatal fetal asphyxia, separately from other abnormalities of the umbilical cord. The results of the analysis of the structure of umbilical cord abnormalities in acute intranatal fetal asphyxia are presented in Table 3. The most common probable causes of acute intrapartum fetal asphyxia were a long umbilical cord and an umbilical cord entanglement around fetus neck and (or) body parts (10.5% and 9.5%, respectively, pχ2<0.05). Somewhat less often, acute asphyxia was recorded with the marginal attachment of the umbilical cord (8.5%), pathology of Wharton’s jelly (7.5%) and umbilical cord vessels (6.5%), an excessive number of umbilical cord coils (6.5%). A short umbilical cord and sheathing of the umbilical cord (3.5% each, pχ2<0.05), as well as an insufficient number of cord coils (2.5%, respectively, pχ2<0.05), were significantly less likely among the probable causes (pχ2>0.05).
Table 3: The structure of umbilical cord abnormalities in acute intrapartum fetal asphyxia.
Note: n (%) – absolute number of cases and their proportion; * - statistically significant differences between the proportion of single and multiple umbilical cord abnormalities according to the χ2 criterion (p<0.05).
It is possible that such a rating of probable causes of acute fetal asphyxia among umbilical cord abnormalities is due to their prevalence. Multiple abnormalities of the umbilical cord accounted for 34 (72.3%) cases and occurred 2.6 times significantly more often than single ones (13 (27.7%), px2<0.05). The number of multiple abnormalities in one umbilical cord ranged from 2 to 5, on average 3.1 ± 0.11. Many researchers also point to the predominance of multiple umbilical cord abnormalities over single ones [3- 6,8,10,17]. In our sample, an insufficient number of umbilical cord coils and a deficiency of Wharton’s jelly were always combined with each other (100%), as well as with the sheath or marginal attachment of the umbilical cord. The frequent combination of these abnormalities of the umbilical cord is noted by many authors, who conclude that there is an extremely high risk of acute intrapartum asphyxia of the fetus with a low location of the placenta along the posterior wall of the uterus [3,4,16].
In all 8 cases of intranatal losses, the pathology of Wharton’s jelly was revealed, in 4 of them - its deficiency in combination with an insufficient number of umbilical cord coils and its entanglement. In three cases, the combination of marginal or sheath attachment of the umbilical cord, insufficient number of coils, deficiency of Wharton’s jelly and low location of the placenta on the posterior wall of the uterus turned out to be fatal. Of all the existing pathologies, only the low location of the placenta was diagnosed on ultrasound before delivery. However, it can be assumed that the marginal and sheath attachment of the umbilical cord was localized along the lower edge of the placenta. With such localization, the insertion of the head instantly blocked the umbilical blood flow, which led to acute intrapartum asphyxia and fetal death. The inevitability of intranatal asphyxia of the fetus when a pathologically attached umbilical cord between the head and the sacrum is compressed is indicated in their works by J.H. Collins (2014), I. A. Hammad et al. (2020), M. Arizawa (2021) [3,4,16]. In one case, the fatal combination was the twisting of the umbilical cord around the body of the fetus, an excessive number of spirals, a false node of the umbilical vein, and edema of Wharton’s jelly. Of all the combined pathology, ultrasound diagnosed only a false umbilical vein node. Many authors point to obstructed blood flow with an excess of umbilical cord coils and its rapid decompensation with the addition of additional complications [17-22]. Of all 164 umbilical cord abnormalities registered during histopathological examination, only 29 (17.7%) were diagnosed with ultrasound. The pathology of the number of umbilical cord vessels was always detected. The umbilical cord entanglement around fetus neck and (or) body parts was partly diagnosed. Sometimes the marginal and meningeal attachment of the umbilical cord, false nodes of the umbilical vein were diagnosed. Not diagnosed before delivery - pathology of the length of the umbilical cord and the number of coils of the umbilical cord, aneurysm of the umbilical artery, pathology of Wharton’s jelly.
Conclusion
The Results of the Study lead to the following Conclusions
1. Umbilical cord abnormalities accounted for 23.5% in the structure of probable causes of acute intrapartum asphyxia of the fetus and took third place after the use of oxytocin in childbirth and premature detachment of a normally located placenta (35.5% and 31% respectively, pχ2<0.05).
2. The highest specific gravity of severe intrapartum fetal asphyxia was found in cases of umbilical cord abnormalities (40.4%, pχ2<0.05). The largest share of perinatal losses was recorded in cases of umbilical cord prolapse (18.2%), umbilical cord abnormalities (10.6%) and premature detachment of a normally located placenta (8%), in comparison with other possible causes (pχ2<0.05).
3. Out of 11 cases of umbilical cord prolapse in 7 (63.6%) cases, other abnormalities of the umbilical cord were absent, in 6 (54.6%) - it was impossible to exclude an iatrogenic cause, which made it possible to consider this pathology as an independent cause of acute intrapartum fetal asphyxia, separately from umbilical cord anomalies.
4. Multiple umbilical cord abnormalities were detected 2.6 times significantly more often than single ones (72.3%, pχ2<0.05) during postmortem examination. All perinatal deaths from umbilical cord abnormalities were associated with multiple anomalies.
5. Prenatal diagnosis of umbilical cord abnormalities using ultrasound was 17.7%. Increasing the efficiency of prenatal diagnosis of umbilical cord abnormalities will make it possible to correctly determine the obstetric tactics of delivery, which will help reduce the likelihood of developing acute intranatal fetal asphyxia, severe neonatal asphyxia, stillbirth and perinatal mortality.
References
- FG Cunningham, KJ Leveno, SL Bloom, JS Dashe, CY Spong, et al. (2018) Williams Obstetrics (25th)., McGraw-Hill Education Medical, pp. 1344.
- ГМ Савельева, Р И Шалина, ЛГ Сичинава, ОБ Панина, МА Курцер (2020) Obstetrics (2nd)., ГЭОТАР Медиа, pp. 576.
- Collins JH (2014) Silent risk Issues about the Human Umbilical Cord. Xlibris LLC, pp. 236.
- IA Hammad, NR Blue, AA Allshouse, Silver RM, Gibbins KJ, et al. (2020) Umbilical Cord Abnormalities and Stillbirth. Obstetrics & Gynecology 135(3): 644-652.
- Mac Dorman MF, Gregory EC (2015) Fetal and perinatal mortality: United States 2013. Natl Vital Stat Rep 64: 1-24.
- P Tantbirojn, A Saleemuddin, K Sirois, Crum CP, Boyd TK, et al. (2009) Gross abnormalities of the umbilical cord: related placental histology and clinical significance. Placenta 30: 1083-1088.
- R Bukowski, NI Hansen, Н Pinar, Willinger M, Reddy UM, et al. (2017) Altered fetal growth, placental abnormalities, and stillbirth. PLoS One 10: 1371 journal pone0182874.
- Х Mantakas, I Dalivigkas, L Aravantinos, Goutas N, Goudeli C, et al. (2018) Placenta and umbilical cord cause in antepartum deaths Cureus 10(11): e3556 cureus 3556.
- L Zahedi Spung, N Raghuraman, EB Carter (2021) 382 Umbilical cord gas abnormalities in the presence of a nuchal cord. Am J Obstet Gynecol 224(2): 248.
- Y Narang, NB Vaid, S Jain (2014) Is nuchal cord justified as a cause of obstetrician anxiety? Arch Gynecol Obstet 289: 795-801.
- Jauniaux E, Savvidou MD (2016) Vasa praevia: more than 100 years in preventing unnecessary fetal deaths. 123: 1287.
- Е Jauniaux, Z Alfirevic, AG Bhide, GJ Burton, SL Collins (2018) Vasa Praevia: Diagnosis and Management: Green-top Guideline Royal College of Obstetricians and Gynaecologists. 126(1): 49-61.
- ЕА Sullivan, N Javid, G Duncombe, Li Z, Safi N, et al. (2017) Vasa previa diagnosis, clinical practice, and outcomes in Australia. Obstet Gynecol 130: 591-598.
- А Kulkarni, J Powel, М Aziz, Shah L, Lashley S, et al. (2018) Vasa previa: prenatal diagnosis and outcomes: thirty-five cases from a single maternal-fetal medicine practice. Ultrasound Med 37: 1017-1024.
- Arisaka М, Hannigan A, O Donoghue K, Cotter A (2021) Abnormal insertion of umbilical cord: Marginal insertion and velamentous insertion Placenta. 103: 257.
- Alsatou А. Umbilical cord coiling abnormality as a predictor of maternal and fetal outcomes. Moldovan Medical Journal 63(1): 29-32.
- Dutman AC, Nikkels PG J (2015) Umbilical hypercoiling in 2nd- and 3rd trimester intrauterine fetal death Pediatr Develop. Pathol p. 10-16.
- М Maayeh, Е McClennen, D Chamchad J Perinat (2018) Hypercoiling of the umbilical cord in uncomplicated singleton pregnancies. Med 46: 593-598.
- А Mittal, S Nanda, J Sen (2015) Antenatal umbilical coiling index as a predictor of perinatal outcome. Arch Gynecol Obstet 291: 763-768.
- Y Ohno, М Terauchi, К Tamakoshi J (2016) Perinatal outcomes of abnormal umbilical coiling according to a modified umbilical coiling index. Obstet Gynaecol Res 42: 1457-1463.
- NS Patil, SR Kulkarni, R Lohitashwa (2013) Umbilical Cord Coiling Index and Perinatal Outcome. J Clinical & Diagnostic Reserch 7(8): 1675-1677.
- JE Lawn, Н Blencowe, Peter Waiswa, Agbessi Amouzou, et al. (2016) Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 387(10018): 587-603.
- MV Ordoñez, G Biglino, М Caput, Kelly B, Mohan, Colin Mathers, et al. (2021) Case of placental insufficiency and premature delivery in a Fontan pregnancy: physiological insights and considerations on risk stratification. Open Heart 10: 1136.
- N Melamedab, L Hierschab, A Aviram (2021) Diagnostic accuracy of fetal growth charts for placenta-related fetal growth restriction. Placenta 105: 70-77.
- Н Pinar, М Koch, Н Hawkins, Heim-Hall J, Abramowsky CR (2012) The stillbirth collaborative Research Network postmortem examination protocol. Am J Perinatology 29: 187-202.

 Research Article
Research Article