ABSTRACT
A Chimeric antigen receptor (CAR) T-cell therapy is an effective new treatment for hematologic malignancies. The U.S. FDA has approved the anti-CD19 CAR T-cell product tisagenlecleucel for multiply relapsed or refractory adult diffuse large B-cell lymphoma (DLBCL). There are many adverse effects after CAR T-cell therapy; primarily reported cytokine release syndrome (CRS) and neurologic toxicity, including fevers, hypotension, hypoxia, end organ dysfunction, cytopenias, coagulopathy, hemophagocytic lymphohistiocytosis, encephalopathy, cognitive defects, dysphasias, seizures and cerebral edema. Some adverse effects are rare, eg, fibroma. We report a new case of a 62-year-old female who experienced fibroma after CAR T-cell therapy.
Keywords: Chimeric Antigen Receptor (Car) T Cells; Diffuse Large B-Cell Lymphoma; Fibroma
Abbreviations: CAR: Chimeric Antigen Receptor; DLBCL: Diffuse Large B-Cell Lymphoma; CRS: Ytokine Release Syndrome; ALL: Acute Lymphocytic Leukemia; CLL: Chronic Lymphocytic Leukemia; NHL: Non-Hodgkin Lymphoma; FC: Fludarabine and Cyclophosphamide; TLS: Tumor Lysis Syndrome
Introduction
A chimeric antigen receptor (CAR) is a fusion protein comprised of an antigen recognition moiety and T-cell signaling domains [1]. CAR T-cell therapy has led to significant improvements in treating multiple hematologic malignancies, including acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL) and diffuse large B-cell lymphoma (DLBCL) [2,3]. Tisagenlecleucel and axicabtagene ciloleucel are both FDA approved for DLBCL following 2 or more prior lines of therapy [4]. With further approvals of CAR T-cell products expected for use in DLBCL, CAR T cells are used in a growing number of patients. However, adverse effects after treating patients with CAR T cells have been reported. Commonly observed adverse events include fevers, hypotension, hypoxia, end organ dysfunction, cytopenias, coagulopathy, hemophagocytic lymphohistiocytosis, encephalopathy, cognitive defects, dysphasias, seizures and cerebral edema [5,6]. So far, it has not been reported that patients suffered fibroma as a side effect after CAR T-cell therapy. Here, we show a fibroma case presentation in a patient treated with CAR T-cell for DLBCL in Hematology Department Ward at Fujian Medical University Affiliated Union Hospital.
Case Presentation
Briefly, A 62-year-old woman, diagnosed as NHL in 2013, performed a cycle-therapy of FC (fludarabine and cyclophosphamide). In Nov 2019, she relapsed again with regions of bilateral cervical, supraclavicular, axillary and inguinal lymph nodes most of which manifested as oval and smooth mass with medial hardness and mobility in the transversal plane. A tumor with tenacious texture and diameter of 6×7 cm was observed in the left chest of this patient. The patient was diagnosed as multiply relapsed and refractory adult DLBCL (Follicular lymphocyte transformed, GCB type, IVA phase, IPI score 4) with twice bone marrow aspiration. After a following FC lympho-depletion chemotherapy (cyclophosphamide 1.2g qd d1-3 combined with fludarabine 40mg qd d1-3), the patient received autologous CAR-T19 expressing murine anti-CD19 scFv costimulatory activation domains (CAR-T measurement: 2×106/kg; co-stimulatory: CD20; Duration: 9m). The analysis by flow cytometry on anti-CD19 CAR-T cell percentage showed a sudden drop from 1.10% to 0.30%. Meanwhile, she suffered fever, cough, expectoration and shortness of breath 15 days following CAR-T infusion and was diagnosed as a grade 3 CRS, pulmonary infection and tumor lysis syndrome (TLS) according to the results of inflammatory index, blood uric acid, blood phosphorus, AST increased, blood oxygen, blood calcium decreased and lung CT display.
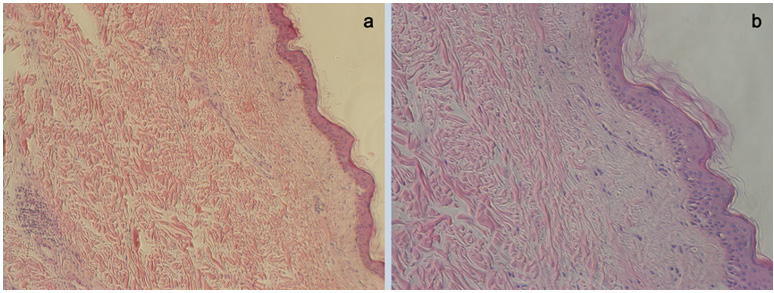
This patient obtained rapid remission after receiving tocilizumab, glucocorticoid and anti-inflammatory treatment. In the end, the patient was discharged with no compliment. About one year after CAR-T treatment, the patient came to dermatology clinic at Fujian Medical University Affiliated Union Hospital and complained about nodular in the same region of her left chest. It presents a smooth surface, hard consistency and a sessile base. Color is similar to skin, measuring up to 3 cm in diameter, and displaying slow growth due to low mitotic index. Finally, the patient was diagnosed as fibroma by pathological biopsy showing that mild keratinization of epidermis, hyperplasia of dermal collagen fibers and some lymphocytes infiltrated around blood vessels (Supplementary Figure 1).
Supplementary Figure 1: Pathological biopsy vertified that the patient suffered fibroma after CAR T therapy.
a. Pathological image under microscope (×10).
b. Pathological image under microscope (×20).
Discussion and Conclusion
Currently, the U.S. FDA has approved the anti-CD19 CAR T-cell treatment for multiply relapsed or refractory adult DLBCL not well controlled with topical chemotherapy or when other therapies are inadvisable [7,8]. In the case report, we administrated CAR-T therapy for this relapsed and refractory adult DLBCL patient after FC chemotherapy. As a noval immunotherapy, CAR-T cell treatment is more effective as was established in sevaral placebocontrolled clinical trials a total of 488 adult participants with relapsed or refractory adult DLBCL not adequately controlled by topical chemotherapies [9-11]. Overall, participants who received CAR-T immunotherapy achieved greater response, defined as the objective response rate. Moreover, a systematic review was performed in Jan 2019 included 13 English literatures and 263 cases which showed that patients treated with CAR-T experienced a higher complete-remission rate (46.8%) than the patients treated with placebo, and they also found that age, hematopoietic stem cell transplantation administration, CAR-T cell counts, and drug pretreatment also affected immunotherapy on CAR-T on relapsed or refractory DLBCL [12]. Unfortunately, a considerable proportion of the patients enrolled in the meta-analysis experienced ≥3 grade CRS or/both neurotoxicity. Regarding the adverse events, limitations to widespread use of CAR T-cell therapy may be toxicity, primarily CRS and neurologic toxicity. Manifestations of CRS include fevers, hypotension, hypoxia, coagulopathy, cytopenias, end organ dysfunction, and hemophagocytic lymphohistiocytosis. Neurotoxicities are diverse and include seizures, encephalopathy, dysphasias, cognitive defects, and cerebral edema [5,6].
Nowadays, it has not been reported that patients suffered fibroma as a side effect after CAR T-cell therapy. In this case, the 62-year-old patient experienced fibroma 1 year following CAR-T treatment, presenting a smooth surface, hard consistency and a sessile base in her left chest and the pathological biopsy verified the result. We proposed a hypothesis that fibroma occurred because of local cytokine abnormalities induced by CAR T-cell therapy. However, the mechanism is still unclear and needs to be further research. To our knowledge, immune agents, such as CAR-T cells, are still included in the current guidelines for the treatment of relapsed or refractory adult DLBCL, when topical medications are ineffective. Although the therapeutic effect of different types of CAR-T cells for the treatment of DLBCL was confirmed in recent clinical trials, there were frequent CRS and neurologic toxicity characterized by high fevers, hypotension, hypoxia, sinus tachycardia, depressed cardiac function, and another organ dysfunction were restricted. Our findings provide evidence that fibroma is a newfound side effect of CAR T-cell immunotherapy in a patient with DLBCL. Further studies should be conducted to assess the long-term stability and safety of CAR-T therapy for treatment of relapsed or refractory adult DLBCL.
Ethics
Written informed consent has been provided by the patient to have the case details and any accompanying images published. No institutional approval was required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
Funding Sources
Fujian Provincial Health Technology Project (2020GGA030) and Fujian Talent Introduction Project (2020XH003).
References
- Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, et al. (2019) Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci 20(6): 1283.
- Feins S, Kong W, Williams EF, Milone MC, Fraietta JA (2019) An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol 94(1): 3-9.
- Roddie C, O Reilly M, Dias Alves Pinto J, Vispute K, Lowdell M (2019) Manufacturing chimeric antigen receptor T cells issues and challenges. Cytotherapy 21(3): 327-340.
- Zhao Z, Xiao X, Saw PE, Wu W, Huang H, et al. (2020) Chimeric antigen receptor T cells in solid tumors: a war against the tumor microenvironment. Sci China Life Sci 63(2): 180-205.
- Yu S, Yi M, Qin S, Wu K (2019) Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer 18(1): 125.
- Salter AI, Pont MJ, Riddell SR (2018) Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood 131(24): 2621-2629.
- Mohty M, Gautier J, Malard F, Aljurf M, Bazarbachi A, et al. (2019) CD19 chimeric antigen receptor-T cells in B-cell leukemia and lymphoma: current status and perspectives. Leukemia 33(12): 2767-2778.
- Springuel L, Lonez C, Alexandre B, Van Cutsem E, Machiels JH, et al. (2019) Chimeric antigen receptor-T cells for targeting solid tumors: current challenges and existing strategies. BioDrugs 33(5): 515-537.
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, et al. (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas TRANSCEND NHL 001 a multicentre seamless design study. Lancet 396(10254): 839-852.
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, et al. (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma ZUMA-1 a single arm multicentre phase 1-2 trial. Lancet Oncol 20(1): 31-42.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, et al. (2017) Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 377(26): 2531-2544.
- Cao HH, Wang LL, Geng CK, Mao WW, Yang LL, et al. (2020) Therapeutic effects of chimeric antigen receptor T cells CAR-T on relapse refractory diffuse large B-cell lymphoma R R DLBCL a meta-analysis. Eur Rev Med Pharmacol Sci 24(9): 4921-4930.

 Case Report
Case Report