Abstract
Numerous studies have confirmed a link between aluminium (Al) and neurodegenerative disorders. In this study, the toxic effects of aluminium ions on the brain were investigated after a 14-day sub-acute exposure of Balb/c mice to 0.1 LD50 Al3+. The exposure to Al3+ caused an increase in malondialdehyde, a marker of lipid peroxidation, and a decrease in total glutathione, a marker of intracellular redox status, concentrations in the brain. To elucidate the reason of oxidative stress in the brain, concentrations of Al ions and biomarkers of redox status and iron homeostasis were measured in the blood serum of mice. Exposure to AlCl3 caused an increase in Al3+ concentration in the blood serum and thus disturbed iron homeostasis by displacing iron from the iron transport protein transferrin. Consequently, Al3+ increased production of serum reactive oxygen metabolites, which is a marker of oxidative stress. Since the total amount of aluminium and iron in the brain did not change, it was proposed that oxidative stress in brain tissue is probably due to the indirect effect of aluminium ions, which caused systemic oxidative stress through the iron mediated mechanism.
Keywords: Aluminium; Oxidative Stress; Iron Status; Neurotoxicity; Mice
Abbreviations: AI: Aluminium; TTL: Total Serum Thiol Levels; ALP: Activity of Alkaline Phosphatase; GSSSG: Convert Oxidized Glutathione
Introduction
Aluminium (Al) is an abundant element in the environment, but it does not perform any known physiological function in the human body (Exley, et al. [1]). Al is associated with toxic effects on various body systems (Pogue, et al. [2,3]), and especially with neurotoxicity (Exley, et al. [4,5]). Some studies have linked increased levels of Al in the brain to Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative disorders (Virk, et al. [6-9]). Al interferes with many physical and cellular processes by disturbing the redox status (Morris, et al. [5,10]). Although Al is a non-redox metal and occurs only in one oxidation state – Al3+, its strong pro-oxidant activity can cause oxidative damage through multiple mechanisms (Mujika, et al. [11]). As the brain is a target tissue for AI intoxication, a likely mechanism of its toxicity can be the generation of oxidative stress in the brain. To gain more knowledge about this complex mechanism, this study investigated Al-induced oxidative stress by analyzing biomarkers in the blood and brain tissue.
Materials and Methods
Experimental Animals and Exposure Protocol
Experiments were performed with 4 to 6-week-old male Balb/c mice weighing 20-25g. The mice were housed in a conventional area of animal housing facility at 22 °C and 50% humidity, with a 12-hour light/dark cycle. The mice were exposed to Al3+ by an intraperitoneal (i.p.) injection of AlCl3 solution daily for 14 days. AlCl3 was dissolved in deionized water. The dose was 5 mg (0.185 mmol) Al3+/kg body weight, corresponding to 0.1 LD50. Control animals were injected with the same volume of physiological solution (0.9% NaCl). Each group consisted of eight animals. After 14 days the mice were anesthetized and terminated. All procedures were performed according to the rules of the European Convention for the Protection of Vertebrates used for experimental and other scientific purposes (Republic of Lithuania, License of the State Veterinary Service for Work with Laboratory Animals No. 0221).
Preparation of Samples
The serum samples were prepared as follows. Whole blood was collected in Eppendorf test tubes after the decapitation of mice. The blood was centrifuged at 4000 rpm × 10 min (1600 × g) in a Savant centrifuge. The serum samples were immediately frozen at -80°C and shipped on dry ice from Kaunas to Bilthoven. The frozen samples were received and stored at -80°C until analysis. The brain was removed, chilled rapidly on ice, and homogenized in 3 volumes (e.g., 1g of tissue plus 3 ml of buffer) of homogenization buffer (50 mM Tris-HCl, pH 7.6; 5 mM MgCl2; 60 mM KCl; 25 mM sucrose). The tissue homogenate was centrifuged at 3000 rpm x 10 min (1000 × g) at 4°C in a Beckman J2-21 centrifuge. The first supernatant was poured into another reaction tube and the pellet was discarded. The supernatant was centrifuged at 10,000 rpm × 15 min (12,000 × g) at 4°C in a Beckman J2-21 centrifuge. The entire volume of the second (post-mitochondrial) supernatant was immediately frozen at -80°C. All the tissue data was adjusted and standardized for protein content.
Biochemical Analysis
The concentrations of Al and iron in the whole blood and brain were evaluated using an inductively coupled plasma mass spectrometer NexION 300 D (Perkin Elmer, USA). Blood and brain tissue samples were digested with 0.125 M NaOH at 90°C and diluted to an appropriate volume to be analyzed according to the manufacturer's recommendations. To ensure the accuracy of the analysis, we followed internal and external quality control procedures, including the use of analytically pure water, reagents, (Merck, Sigma-Aldrich) and certified reference materials ClinCheck® Whole Blood Controls Level (Recipe, Germany). Standard Reference Material®-1577c bovine liver (NIST-1577c, Gaithersburg, USA) was used as a standard reference material when determining iron in tissue samples. Also, we checked the laboratory equipment for trace element contamination.
ROS-derived hydroperoxides, as an indicator of ROS production, were measured using the Diacron test for reactive oxygen metabolites (d-ROMs kit, No. MC-003 from Diacron, Grosseto, Italy). The test is based on the estimation of the amount of organic hydroperoxides formed in blood serum caused by the presence of free radicals. In practice, a small amount of serum is diluted in an acidic solution (pH 4.8). In these conditions, iron ions become available to catalyze the breakdown of hydroperoxides to alkoxyl and peroxyl radicals. The chromogenic substrate is N,N-dimethylparaphenylenediamine transformed into a pink to red colored radical cation. Quantitation is possible with the use of a photometer (wavelength 505 or 546 nm). The concentration of colored complex is directly related to the levels of hydroperoxides in the tested biological sample. The levels of hydroperoxides were quantified in Carratelli Units (1CARR.U. = 0.08 mg H2O2 / 100 ml) (Schöttker, et al. [12]).
Total serum thiol levels (TTL) were measured with a kit from Rel Assay (Gyantzip, Turkey) (TTL kit No. RL0178) and expressed in µmol/l. Both ROM and TTL assays are adapted for automatic use on an LX-20 Pro autoanalyzer (Beckman-Coulter, Woerden, The Netherlands) (Leufkens, et al. [13,12]). Serum iron bound to transferrin (TrF-Fe) expressed in µmol/l was determined on the same autoanalyzer LX-20 Pro (kit No. 467910 from Beckman-Coulter). All the intra assay coefficients of variation for these assays were between 1.5 and 4.3%. Mouse transferrin (TrF) expressed in g/l (kit No. E-90TX), and mouse ferritin (FER) expressed in µg/L (kit No. E-90F), were determined by ELISA obtained from ICL (Portland, OR, USA). The intra-assay coefficients of variation were less than 5%, according to the manufacturer's data. Activity of alkaline phosphatase (ALP) (kit No. 476821 form Beckman-Coulter) and the concentration of total protein (TP) (kit No. 465986 from Beckman-Coulter) in the brain tissue were measured using the autoanalyzer LX-20 Pro (Beckman-Coulter, Mijdrecht, The Netherlands). The intra assay coefficients of variation were less than 5%.
Lipid peroxidation in the brain tissue was estimated by measuring the absorption of thiobarbituric acid reactants using UV/Vis spectrophotometer LAMBDA 35 (Perkin Elmer, USA) and expressed as malondialdehyde content in nmol/g wet organ weight. The mice brains were homogenized in cold 1.15% KCl solution to make 10% homogenate. To 0.5 ml of this homogenate, 3 ml of 1% H3PO4 and 1 ml of 0.6% thiobarbituric acid (Serva, Germany) aqueous solutions were added. The reaction mixture was placed in a boiling water bath for 45 min. After cooling, 4 ml of n-butanol was added and mixed vigorously. The butanol phase was separated by centrifugation and the light absorption measured at wavelength 535 and 520 nm (Uchiyama, et al. [14]).
The total brain glutathione concentration (totGSH) (expressed in µmol/g protein) was measured in the post-mitochondrial supernatant after deproteinization with an equal volume of 10% metaphosphoric acid at 20°C for 5 min. The mixture was centrifuged at 2000 x g for 2 min and the supernatant was incubated with glutathione reductase solution (2500 U/6 ml) at 25 °C for 60 min to convert oxidized glutathione (GSSG) to GSH. The GR kit No. G9297 was obtained from Sigma-Aldrich, Zwijdrecht, The Netherlands. The total GSH was determined after derivatization with 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB), kit No. D8130 from Sigma-Aldrich at 37°C for 5 min. The light absorption of reaction product was measured at 412 nm with the autoanalyzer LX-20 Pro from Beckman-Coulter, Woerden, The Netherlands. The intra assay variation was 6.7%.
Statistical Analysis
The results were expressed as the mean ± standard error of mean. The data was analyzed with ANOVA using Microsoft Excel. Statistical significance was set at p<0.05. In addition, the statistical significances in the serum biomarkers and brain biomarkers were checked separately to counteract the problem of multiple using the Bonferroni method.
Results
Systemic Biomarkers
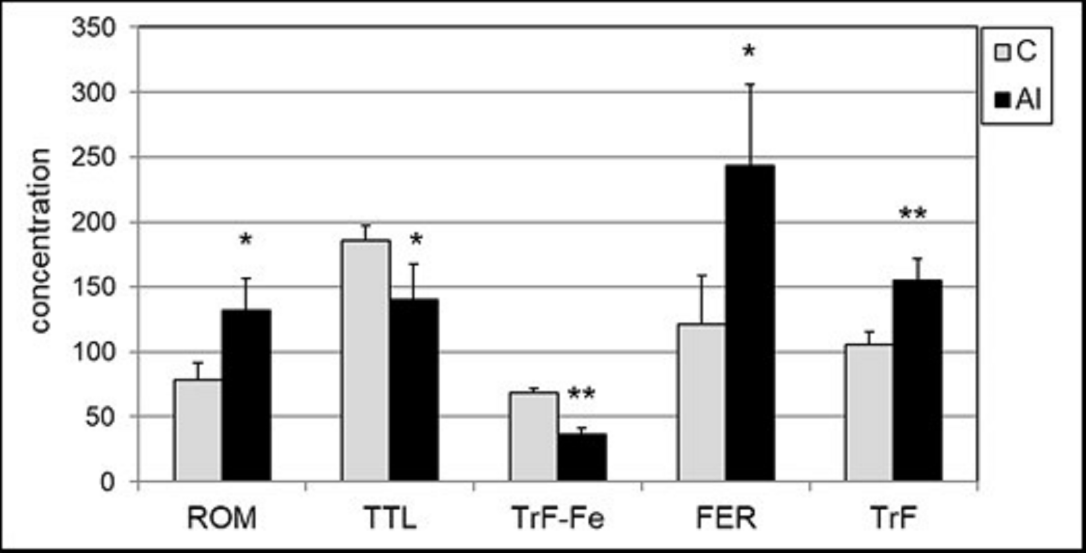
Concentration of Al and iron after a 14-day intraperitoneal (i.p.) exposure of mice to Al3+ was determined in the whole blood. Al concentration increased sevenfold, from 74.0±13 µg/l to 535±77 µg/l (p <0.001), and the total iron concentration of decreased by 27%, from 208±18 mg/l to 153±28 mg/l (p <0.001). To evaluate the effect of Al on iron homeostasis, the biomarkers of iron status in the serum were measured: total content of iron bound to transferrin (TrF-Fe), transferrin (transport protein of Fe) and ferritin (indicating iron status in tissues). Several changes in iron status were observed after the exposure of mice to Al3+: an increased concentration of TrF from 2.10 to 2.52 g/l, and a decreased concentration of TrF-Fe from 68.2 to 36.5 µmol Fe/l. Ferritin concentration increased from 1.09 to 2.44 µg/l (Figure 1). Oxidative stress biomarkers were estimated in the blood serum of Al-treated mice. Al caused a statistically significant increase (almost 60%) in the concentration of lipid peroxides, measured by ROM assay from 83.7 to 133.7 CARR.U (p=0.0003) (Figure 1). Total redox status measured by TTL assay decreased statistically significantly from 371 to 280 µmol/l (24%) (p<0.004).
Biomarkers of Neurotoxicity
Exposure to Al3+ did not affect Al and iron levels in the mice brain. In the control and Al-treated groups, Al concentration was 11.3±2.6 and 11.7±1.6 µg/l, and Fe concentration was 18.6±1.6 and 18.5±1.6 mg/l, respectively. However, concentration of oxidative stress biomarker MDA, increased statistically significantly from 109±19.7 to 131±16.5 nmol/g (20.5%), (p=0.003). Also, concentration of totGSH, which reflects brain redox status, decreased from 15.0±3.3 to 8.3±2.7 μmol/g protein (64.7%) (p=0.0011) (Figure 2). In addition, we measured the enzymatic activity of ALP due to its possible relation with neurodegenerative, e.g. Alzheimer, diseases. ALP activity increased substantially in the brain of Al-treated mice from 6.64±1.07 to 9.97±1.69 U/g protein; ** p <0.0005 vs. control group.
Figure 1: Serum concentrations of oxidative stress biomarkers and iron status biomarkers: ROM (expressed in CARR.U), TTL (expressed in μmol/l), serum iron (TrF-Fe) (expressed in μmol Fe/l), ferritin (FER) (expressed in μg/l) and transferrin (TrF) (expressed in g/l) in control mice (C) and after Al exposure (Al). Statistics: * p<0.01; ** p<0.001 vs. control group.
Figure 2: Concentrations of MDA (expressed in nmol/g tissue), totGSH (expressed in μmol/g protein) and ALP (expressed in U/g protein*10) in brain post-mitochondrial supernatant in control and Al-treated mice. Statistics: * p<0.01 vs. control group; ** p<0.001 vs. control group.
Discussion
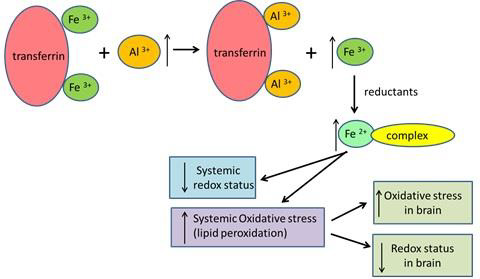
In this study, mice were exposed to Al for 14 days. It was determined that even low doses of Al3+ corresponding to 0.1 LD50 caused a sevenfold increase in Al concentration in the blood, while there were no changes in Al concentration in the brain. As Al is known to affect iron homeostasis (Peto, et al. [15,16]), we used this knowledge to explain the generation of oxidative stress in the blood and possibly in the brain. The serum iron status was measured because free iron is one of the major factors associated with the production of reactive oxygen species in vivo (Bresgen, et al. [17,18]), including the brain (Piloni, et al. [19,20]). Indeed, the data of this study showed that exposure to low Al3+ doses corresponding to 0.1 LD50 significantly affected serum iron status. It was demonstrated that Al3+ can displace iron ions from the iron-binding protein transferrin, which was reflected by a lower amount of bound iron. The transferrin saturation, which is the percentage of TrF that has been occupied by iron, decreased from 41.4% to 18.4% after exposure to Al. Our results are in agreement with the data of other authors (Jacobs, et al. [21]).
The release of iron from the serum transferrin was also confirmed by a higher level of serum ferritin, reflecting the increase in iron stores in tissues. The higher content of unbound iron in the serum and tissues may explain the development of oxidative stress (Figure 3). Exposure to Al3+ resulted in a significant decrease in total iron concentration in the blood by 27% but caused no changes in the iron levels of the brain. The blood-brain barrier is believed to protect the brain from the transport and accumulation of Al and iron. To assess the increased oxidative stress in serum, we measured ROM and TTL as biomarkers of lipid peroxidation and redox status. It was shown that ROM concentration increased, while TTL level decreased after exposure to Al3+. The importance of ROM and TTL as biomarkers of oxidative stress was proved in several large cohort studies on cancer (Leufkens, et al. [12,13,22]), cardiovascular diseases (Vassalle, et al. [23-25]), and aging (Schöttker, et al. [26,27]). The stability of serum samples for the ROM and TTL assays have been tested during short-term and long-term storage (Jansen, et al. [28,29]).
For the evaluation of oxidative stress in the brain tissue we used biomarkers MDA and totGSH. Due to technical reasons, the ROM test could not be performed on tissue extracts. The increased concentration of MDA and decreased concentration of totGSH indicated oxidative stress in brain tissue. In addition, the activity of ALP increased in the brain of Al-treated mice. Our results are in agreement with the data of other studies, in which higher activity of ALP in the brain of Alzheimer patients (Karabulut-Bulan, et al. [30,31]) was demonstrated. Increased enzymatic activity may be one of the indicators of increased oxidative stress in the brain. The observation that exposure to Al3+ did not change Al and iron levels in the brain implies the indirect effect of Al3+ on the brain via reactive oxygen species originating in the circulatory system. It was concluded that oxidative stress in the brain tissue is a result of systemic oxidative stress induced by aluminium through the iron mediated mechanism.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
References
- Exley C, Mold MJ (2015) The binding, transport and fate of aluminium in biological cells. J Trace Elem Med Biol 30: 90-95.
- Pogue AI, Lukiw WJ (2016) Aluminum, the genetic apparatus of the human CNS and Alzheimer's disease (AD). Morphologie 100: 56-64.
- Geyikoglu F, Türkez H, Bakir TO, Cicek M (2013) The genotoxic, hepatotoxic, nephrotoxic, haematotoxic and histopathological effects in rats after aluminium chronic intoxication. Toxicol Ind Health 29: 780-791.
- Exley C (2014) What is the risk of aluminium as a neurotoxin? Expert Rev Neurother 14: 589-591.
- Morris G, Puri BK, Frye RE (2017) The putative role of environmental aluminium in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved? Metab Brain Dis 32: 1335-1355.
- Virk SA, Eslick GD (2015) Aluminum levels in brain, serum, and cerebrospinal fluid are higher in Alzheimer's disease cases than in controls: a series of meta-analyses. J Alzheimers Dis 47: 629-638.
- Walton JR (2013) Aluminum involvement in the progression of Alzheimer's disease. J Alzheimers Dis 35: 7-43.
- Inan-Eroglu E, Ayaz A (2018) Is aluminum exposure a risk factor for neurological disorders? J Res Med Sci 23: 51.
- Exley C, Clarkson E (2020) Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci Rep 10: 7770.
- Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41: 154-166.
- Mujika JI, Ruipérez F, Infante I, Ugalde JM, Exley C, et al. (2011) Pro-oxidant activity of aluminum: stabilization of the aluminum superoxide radical ion. J Phys Chem 115: 6717-6723.
- Schöttker B, Saum KU, Jansen EH, Holleczek B, Brenner H (2016) Associations of metabolic, inflammatory and oxidative stress markers with total morbidity and multi-morbidity in a large cohort of older German adults. Age Ageing 45: 127-135.
- Leufkens AM, van Duijnhoven FJ, Woudt SH, Siersema PD, Jenab M, et al. (2012) Biomarkers of oxidative stress and risk of developing colorectal cancer: a cohort-nested case-control study in the EPIC study. Amer J Epidemiol 175: 653-663.
- Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituris acid test. Anal Biochem 86: 271-278.
- Peto MV (2010) Aluminium and iron in humans: bioaccumulation, pathology, and removal. Rejuvenation Res 13: 589-598.
- Zhang L, Li X, Gu Q, Zhu Y, Zhao H, et al. (2011) Effects of subchronic aluminum exposure on serum concentrations of iron and iron-associated proteins in rats. Biol Trace Elem Res 141: 246-253.
- Bresgen N, Eckl PM (2015) Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 5: 808-847.
- Jansen EH, RH van den Berg, JJ Bergman (1989) Effect of iron-chelates on luminol chemiluminescence in the presence of xanthine oxidase. Anal Chim Acta 227: 57-63.
- Piloni NE, Fermandez V, Videla LA, Puntarulo S (2013) Acute iron overload and oxidative stress in brain. Toxicology 314: 174-182.
- Farina M, Avila DS, da Rocha JB, Aschner M (2013) Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int 62: 575-594.
- Jacobs EMG, Hendriks JCM, Tits van B, Evans PJ, Breuer W, et al. (2005) Results of an international round robin for the quantification of serum non-transferrin-bound iron: Need for defining standardization and a clinically relevant isoform. Anal Bioch 341: 241-250.
- Gào X, Wilsgaard T, Jansen EH, Holleczek B, Zhang Y, et al. (2019) Pre-diagnostic derivatives of reactive oxygen metabolites and the occurrence of lung, colorectal, breast and prostate cancer: an individual participant data meta-analysis of two large population-based studies. Int J Cancer 145: 49-57.
- Vassalle C, Bianchi S, Battaglia D, Landi P, Bianchi F, Carpeggiani C (2012) Elevated levels of oxidative stress as a prognostic predictor of major adverse cardiovascular events in patients with coronary artery disease. J Atheroscler Thromb 19: 712-717.
- Schöttker B, Saum KU, Jansen EH, Boffetta P, Trichopoulou A, et al. (2015b) Oxidative stress markers and all-cause mortality at older age: a population-based cohort study. J Gerontol A Biol Sci Med Sci 70: 518-524.
- Xuan Y, Bobak M, Anusruti A, HJM Jansen E, Pająk A, et al. (2019) Association of serum markers of oxidative stress with myocardial infarction and stroke: pooled results from four large European cohort studies. Eur J Epidemiol 34: 471-481.
- Schöttker B, Brenner H, HJM Jansen E, Gardiner J, Peasey A, et al. (2015a) Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Medicine 13: 300.
- Saum KU, Dieffenbach AK, Jansen EH, Schöttker B, Holleczek B, et al. (2015) Association between oxidative stress and frailty in an elderly German population: results from the ESTHER cohort study. Gerontol 61: 407-415.
- Jansen EH, Beekhof PK, Cremers JW, Viezeliene D, Muzakova V, et al. (2013) Short-term stability of biomarkers of oxidative stress and antioxidant status in human serum. ISRN Biomarkers ID 316528, p. 5.
- Jansen EH, Beekhof PK, Viezeliene D, Muzakova V, Skalicky J (2015) Long term stability of cancer biomarkers of oxidative stress, redox status, homocysteine, CRP and the enzymes ALT and GGT. Biomark Med 9: 425-432.
- Karabulut-Bulan Ö, Bayrak BB, Sarıkaya-Ünal G, Yanardağ R (2019) The influence of melatonin supplementation against aluminum-induced toxicity in brains of male rats. J Res Pharm 23: 275-283.
- Vardy ERLC, Kellett KAB, Cocklin SLC, Hooper NMC (2012) Alkaline phosphatase is increased in both brain and plasma in Alzheimer’s disease. Neurodegener Dis 9: 31-37.

 Research Article
Research Article