ABSTRACT
Background: In recent times, product quality has been a primary focus in the pharmaceutical industry. The quality by design (QbD) strategy contributes to higher productivity through the application of several approaches, such as process analytical technology (PAT), analytical target profiling (ATP), critical quality attribute (CQA) assessment, and quality target product profiling (QTPP) (QTPP).
Objectives: To summarize the quantitative applications of HPLC, HPTLC, UPLC and UHPLC by applying Analytical Quality by Design.
Methods: This article shows current quantitative applications of Analytical QbD in analytical method development like HPTLC (High-Performance Thin Layer Chromatography), HPLC (High-Performance Liquid Chromatography), UPLC (Ultra- Performance Liquid Chromatography) and UHPLC (Ultra-High-Performance Liquid Chromatography) for more than 60 drugs.
Result: To demonstrate drug quality, the ICH recommendations Q8, Q9, and Q10 for pharmaceutical development, quality risk management, and pharmaceutical quality management, respectively, have been produced.
Conclusion: All found methods required very less time for detection and it improves the quality by applied all analytical methods.
Keywords: Analytical Quality by Design; Pharmaceuticals; HPLC; HPTLC; UPLC; UHPLC
Abbreviations: QBD: Quality by Design; PAT: Process Analytical Technology, ATP: Analytical Target Profiling, CQA: Critical Quality Attribute, QTPP: Quality Target Product Profiling; CMC: Chemical, Manufacturing, and Control; TPQP: Target Product Quality Profile; CPP: Critical Process Parameters; RPN: Risk Priority Number; HPTLC: High-Performance Thin-Layer Chromatography; HPLC: High-Performance Liquid Chromatography; UHPLC: Ultra-High-Performance Liquid Chromatography; RP-HPLC: Reversed-Phase High Performance Liquid Chromatographic Method; API: Active Pharmaceutical Ingredient
Introduction to Quality by Design (QBD)
The pharmaceutical industry develops a high-quality product that is both safe and efficient. Not only does QbD improve the quality of the final product, but it also improves the quality of the entire production process. Finally, the product must satisfy patient’s demands as well as experimental results [1]. Various regulatory bodies are concerned with product safety. Manufacturing costs can be reduced by adopting PAT, CQA, and ATP. The strategies for developing goods vary from industry to industry. The substance is of high quality since it is contamination-free, resulting in a proper therapeutic effect [2]. For the development of a product, either an empirical or a systematic method, or both, can be employed; however, when implementing QbD, the systematic approach is used more [3]. A systematic approach facilitates designing the production process and accelerates formulation development, which results in a product worthy of being evaluated in a clinical trial. For goods with minimal risk of chemical, manufacturing, and control (CMC) modifications in post-approval manufacture, the FDA wants to reduce the regulatory filing requirement [4].
Design
i. Product should fulfil a patient’s demands.
ii. Product should be of good quality.
iii. The starting unprocessed materials will affect the product.
Definition of Quality by Design (QBD)
A systemic approach to development begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.
Benefits of QBD
i. It improves the Quality of Product.
ii. It provides a better understanding of manufacturing.
iii. It increases production.
iv. Chances of batch failure are less.
v. The investigations are not costly [5-7].
vi. It reduces the time for manufacturing.
vii. The deviation is minimized.
viii. Less time is required.
ix. It gives a better understanding of manufacturing processes.
x. It reduces the time for testing.
xi. It builds patient’s confidence [8,9].
xii. It provides a positive environment.
xiii. The economic rate is high.
It is for better development.
Opportunities
i. It is efficient and flexible.
ii. It validates the process.
iii. It increases efficiency and potency.
iv. It reduces costs [10].
v. The speed for production is more.
vi. It reduces batch rejections.
vii. Scientific knowledge is more for all products.
viii. For various issues, better interact with industry,
ix. Chance of risk management is less [11].
Steps involved in QbD
The Target Product Quality Profile (TPQP): TPQP has been intended for quality of a drug product that will reach to secure the good product TPQP is defined as ,“prospective and dynamic summary of the quality characteristics of a drug product that ideally will be achieved to ensure that the desired quality, and thus the safety and efficacy, of a drug product is realized”. Thus safety, quality and efficacy of a drug is perceived. It cover strength of dosage form (s), route of administration of drug, therapeutic index , and pharmacokinetic parameters such as aerodynamic performance , Drug dissolution , relevant quality product its dosage form has been developed and product quality criteria such as purity and sterility suitable for the design of sells product [12].
Critical Quality Attribute: After the TPQP the next step involved is Critical quality attributes. CQA has been defined as “a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distributed to ensure the desired product quality”. As per the ICH Q9 [12]. Guidelines identification of CQAs is done by risk assessment. For making risk assessments the knowledge of product required such as accumulated laboratory, clinical and non-clinical experience with certain product-quality attribute.
Critical Process Parameter: “Parameters whose variability have an impact on a CQA and therefore should be monitored or controlled to ensure the process produces the desired quality” are defined as Critical process parameters (CPPs). The ability of a process to demonstrate acceptable quality and performance and tolerate variability in inputs at the same time is defined as Process robustness. Process capability Should be considered. To indicate the uniformity and reproducibility of a process. The Six Sigma is popularly accepted for process capability. Process capability index is the statistical a process ability to build output within specific range [12]. Process capability index (CpK) = Upper limit of specification - Lower limit of specification / 6 standard deviation.
Risk Assessment: Quality risk management is a systemic approach for a control, communication and risk of a drug product quality of a lifecycle. The parameter that can affect CQAs can be reduced by QRA (Quality risk assessment. QRA is a process that can recognizes CPPs and lead to remove risk. Thus, Quality product achieved. By using QRA many parameters can remove such as FMEA (Failure mode effect analysis) and Ishikawa diagrams. FMEA lead to identified a failure occur in a pharmaceutical product, detectability, Risk priority number (RPN). Ishikawa diagrams segregate risks into different categories [12].
Design Space: The ICH Q8(R2) States that the design space is multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality. chance is not mean by doing work within a design. chance is considered as doing work out of design .and would begin a change in post approval process. Design space is implemented for approval and regularly assessment. It is necessary to verified a Design space at commercial scale unless it is independent. Because Design space is dependent on equipment so it may vary from lab level to industry.
Control Strategy: The capability to assess and secure the quality in-process and in final product. ICH Q8 (R2). Control strategy is defined as “a planned set of controls, derived from current product and process understanding that assures process performance and product quality”. In QbD the control strategy is established with risk assessment the criticality of the CQA is taken into consideration. The control strategy include various element such as process monitoring, characterization testing, procedural controls , in process controls , lot releasing testing , stability testing . It may incorporate with raw material attributes. environmental condition and operating systems that may impact on downstream process. e.g. Degradation is may affected by drying parameters [12].
Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing
The product Quality has been taken consideration in pharmaceutical industry. For increasing Product quality there are various tools such as PAT. The growth in manufacturing is necessary to have a Scientific knowledge. It will decrease the risk which leads to increasing in the productivity and quality. Applying the Principle of QbD to Analytical Method Which reduce the change of poor method robustness and thereby ensure that product meet its performance requirement. The Knowledge obtain during development which will support process control and design space. Thus, same method of QbD is apply to analytical and as “Analytical QbD”. AQbD consist of various tools like CQA, ATP (Analytical Target Profile) [11,12].
ATP (Analytical Target Profile)
ATP is for development process of an analytical Method. The purpose is for Design Method selection and development activities. ATP leads an improvement in Analytical method. The method is selected based on targeted analytes like API, products and impurities. To apply AQbD analytical methods has to be selected like HPLC, GC and HPTLC [11,12].
Quantitative Applications of Analytical Quality by Design
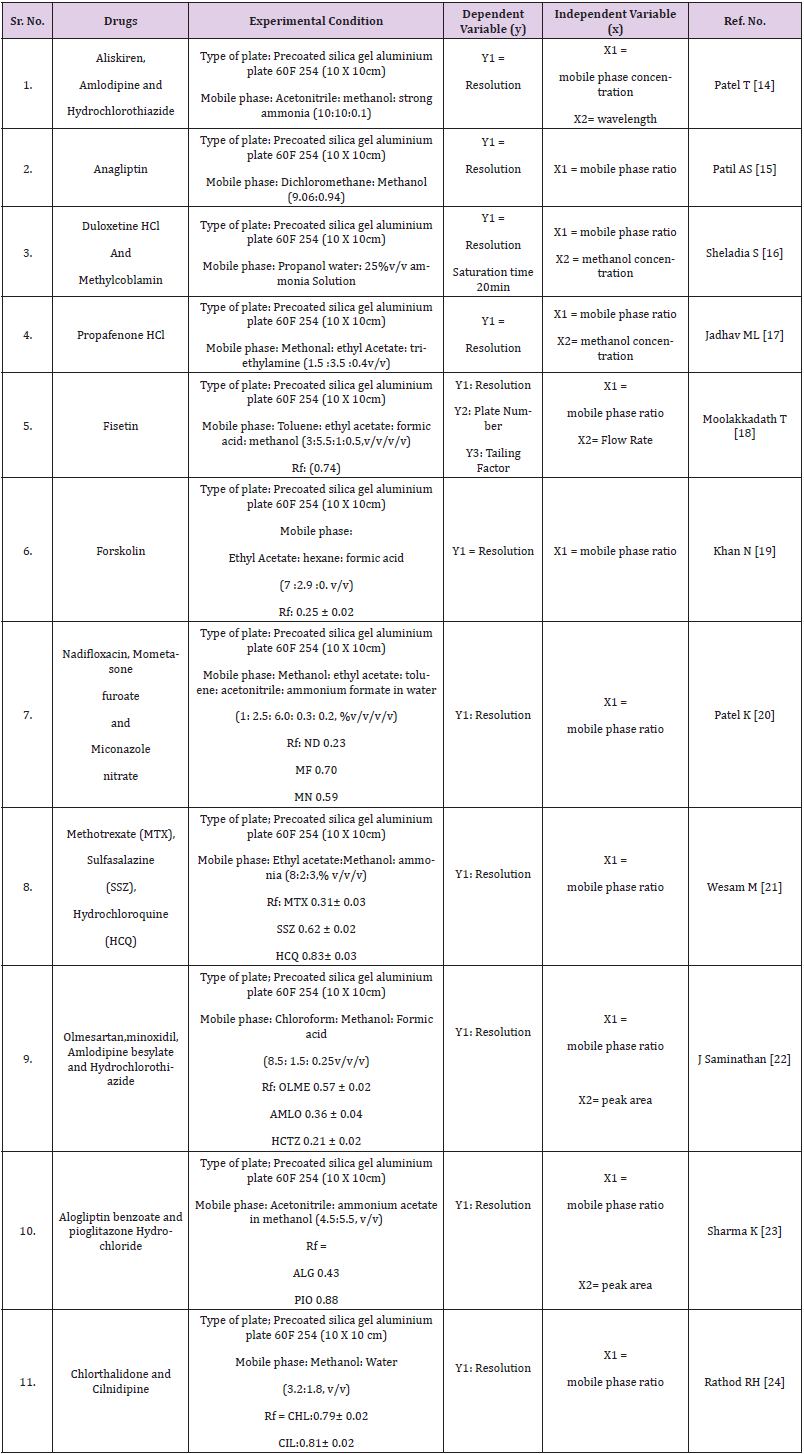
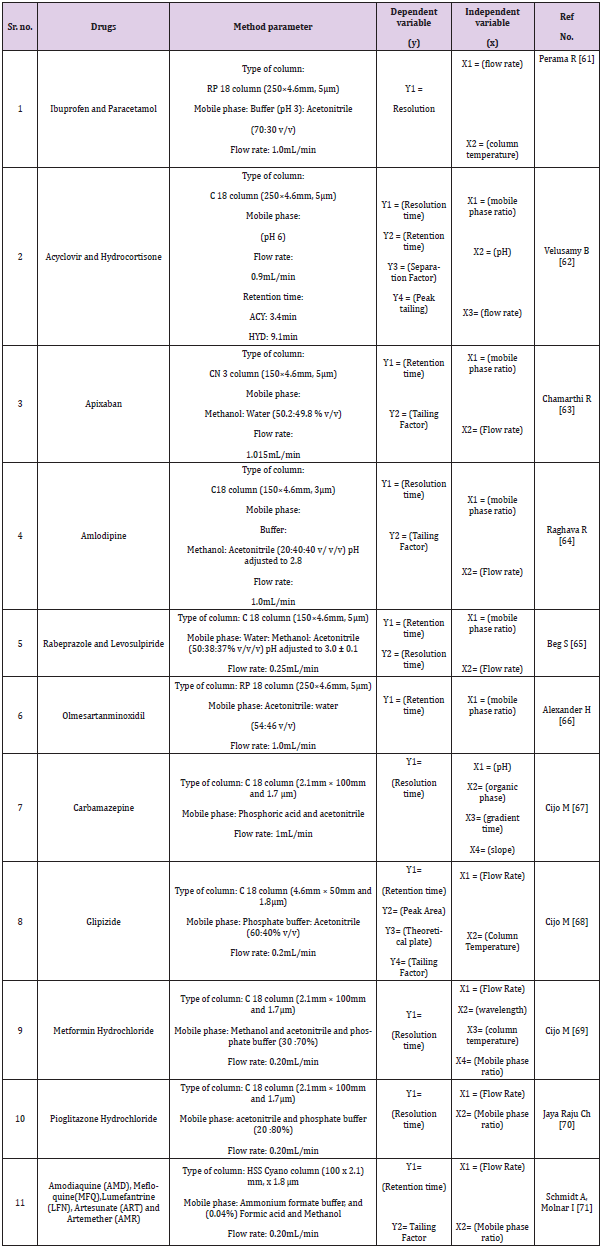
Quantitative applications by HPTLC, HPLC, UPLC and UHPLC method are given in Tables 1-4 respectively.
Result and Discussion
As we know, quality by design is an approach to implement quality in pharmaceutical but analytical QbD is a new approach to implement quality and reduce cost in pharmaceutical analysis. In this review many chromatographic methods have been covered like HPLC, UPLC, UHPLC and impurity profiling. For chromatographic methods QbD has been implemented by design expert software using different designs like central composite design (CCD), fractional factorial design (FFD), box banchan design. Gundala A [13] has developed high performance liquid chromatographic method to estimate saxagliptin (SAXA) and dapagliflozin (DAPA), to determine the essential method parameters, a risk assessment was conducted. The mathematical models were created using three independent factors: mobile phase composition, flow rate, and column temperature. The response surface methodology and the results of these independent factors were studied using a central composite design (CCD), method was optimized by mobile phase ratio, flow rate and temperature considering as an independent variable. Another study was performed to develop a new responsive and robust stability indicating HPLC method based on a fractional factorial design (FFD) approach for the simultaneous estimation of gliclazide and metformin hydrochloride in tablets without prior separation. Preliminary tests were carried out to determine the essential attribute variables, with the Taguchi screening approach being used to a large extent. two retention time (Y1) and separation factor (Y2) models were obtained and statistically interpreted. Study of constructed models and contour plots yielded the chromatographic optimum range for each input variable (Xn). The predicted data for resolution time (Y1) and separation factor (Y2) from response models were statistically important [14-72].
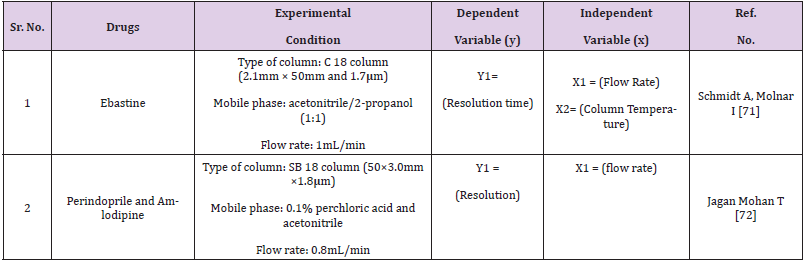
For the simultaneous determination of relevant organic impurities of Ibuprofen and paracetamol in a combination solid oral dosage form by reverse phase high performance liquid chromatography, a stability suggesting QbD dependent gradient method was developed and validated using the principle of consistency by design (QbD) and the design of experiments (DoE) tool (RP-HPLC). Using the “Design-Expert® 8” software tool with a quadratic mode of central composite design, the most important critical quality attributes (CQA) of the established test method were chosen and evaluated (CCD). For purity testing of ebastine and its pharmaceutical formulations, a TA stability-indicating ultra-high-performance liquid chromatographic (UHPLC) method has been developed. The robustness of the established method was investigated by varying six parameters at three levels (+1, 0, 1): gradient time, temperature, ternary composition of the eluent, flow rate, and gradient start and end concentration. The 729 experiments that resulted were carried out in silico using the previously collected data.
Conclusion
Quality by Design (QbD) is an approach to design and develop predefined product quality. QbD is a method that will improve the consistency, protection and effectiveness of products. QbD would increase the output rate; in other words, by using QbD, the risk is reduced while the input is maximised. Analytical Quality by Design applies the same concepts of QbD to analytical processes. In the pharmaceutical industry, Analytical Quality by Design (AQbD) is critical for improving product quality. It is implemented in order to reduce the number of defective products. Also, increase production. The risk can be identified early on, resulting in a highquality finished product. The various Tools are PAT, CMC, Critical Quality Attributes (CQA), Quality target Product Profile, Continuous Method Monitoring, Method optimization, and development with DOE, MODR (Method Operable Design Region), and continuous improvement. AQbD requires the right ATP and Risk Assessment of correct tools for a suitable quantity of work within proper timelines. By application of AQbd, the analytical technique makes it easier to obtain the optimum values, which makes it easier for further analysis of the drug.
The most critical process is analytical in drug production and the development of a product.
In the whole process of all the stages of the drug product life cycle, it plays a major role in development. An Analytical Method should be accurate, precise, and reliable for the intended purpose. Among all Liquid Chromatography techniques are most commonly Utilized an Overview of this article using the development of a High-performance thin-layer chromatography (HPTLC), High-performance Liquid Chromatography (HPLC), Ultra-highperformance liquid chromatography (UHPLC), reversed-phase high performance liquid chromatographic method (R-HPLC). The separation of analytes present in a sample is the key concept of an analytical method in the chromatographic method, which is developed using the QbD approach growth, which results in a highquality product. The most popular application is the assay of an active pharmaceutical ingredient (API) or determined degradation products.
References
- Nishendu P, Nadpara Rakshit V, Thumar Vidhi N, Patel PB (2012) Qbd: a complete review. Int J Pharm Sci Rev 17(2): 20-28.
- Gandhi Ar, Chandrani R (2016) Qbd in Pharmaceutical Industry: Tools, Perspectives and Challenges. pharma Tutor 4(11):12-20.
- Jadhav JB, Girawale NN (2014) QbD approach used in development of pharmaceutical. Int J of Pure and Applied Biosci 2(5): 214-223.
- Ashrani S, Goyal A, Rajat V (2018) QbD and process analytical technology: Important Tools for Building quality in pharmaceutical Products. Journal of Scientific & Technical Research 2(1): 2408-2412.
- Janet W (2004) The concept of pharmaceutical quality. American Pharmaceutical Review 7(6): 10-15.
- (2015) ICH. Q9: Quality Risk Management. ICH Harmonized Tripartite Guidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. European Medicines Agency Science Medicines Health, p. 1-20.
- (2008) ICH. Q10: Pharmaceutical Quality System, ICH Tripartite Guidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use 4: 1-21.
- (2007) A Guidance for Industry and Review Staff: Target Product Profile – A Strategic Development Process Tool, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER).
- (2009) ICH. Q8 (R1): Pharmaceutical Development, Revision 1, ICH Harmonized Tripartite Guidelines, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
- Callis JB, Illman DL, Kowalski BR (1987) Process analytical chemistry. Analytical Chemistry 59(9): 624A-637A.
- Lawrence, X Yu (2008) Pharmaceutical quality by design: Product and process development, understanding, and control. Pharmaceutical Research 25(4): 781-791.
- Balasaheb J, Jadhav N (2014) Quality by Design (QBD) Approach used in Development of Pharmaceuticals. J Pure App Biosci 2(5): 214-223.
- Gundala A, Prasad K, Koganti, B (2019) Application of QbD approach in RP-HPLC method development for simultaneous estimation of Saxagliptin and Dapagliflozin in tablet dosage form. Brazilian Journal of Pharmaceutical Sciences 11(1): 59-63.
- Patel T, Shaj S (2015) HPTLC Method for Simultaneous Estimation of Aliskiren, Amlodipine, and Hydrochlorothiazide in Synthetic Mixture Using Quality by Design Approach. J of Liquid Chrom. and Related Tech 38(16): 1546-1554.
- Patil AS, Shirkhedkar AA (2017) Application of Quality by Design in the Development of HPTC Method for Estimation of Anagliptin in Bulk and in-house Tablets. Eurasian J Anal Chem 12(5): 443-458.
- Sheladia S, Patel B (2016) Implementation of QBD approach to develop and validate analytical method for simultaneous 13. estimation of duloxetine hydrochloride and methylcobalamin in pharmaceutical dosage form by HPTLC method. Int J of Pharm And Pharm Sci 8(7): 105-111.
- Jadhav ML, Tambe SR (2013) Implementation of QbD Approach to the Analytical Method development and Validation for the Estimation of Propafenone Hydrochloride in Tablet Dosage Form. chromatography Research International, p. 1-9.
- Moolakkadath T, Mohd A (2020) Analytical Quality By Design (AQBD) approach based HPTLC Method for Quantification of Fisetin with superior recovery in Formulation. Current Analytical Chemistry 16(2): 149-157.
- Khan N, Ameeduzzafar A (2018) A Novel Validation Stability –indicating HPTLC Method to Quantitate Forskolin as a Bulk Drug and in a Nanosuspension. Indian J of Pharma sci 80(5): P820-826.
- Patel K, Patel P, Shah P (2016) Validation high Performance thin layer chromatographic (HPTLC) Method for simultaneous determination of Nadifloxacin, mometasone furoate, and miconazole nitrate cream using fractional factorial design. J of food and drug ana 24(3): 610-619.
- Wesam M, Noha N, Gamal S (2019) Innovative HPTLC method for simultaneous determination of ternary mirture of certain DMARDS in real samples of rheumatoid arthritis patients: an application of quality by design approach. J of chrom B 11(24): 135-145.
- J Saminathan, T Vetrichelvan (2013) HPTLC method development and validation for simultaneous estimation of Olmesartan medoximil, Amlodipine besylate and Hydrochlorthiazide in bulk drug and formulation. Indian of Pharm Sci 13(9): 325-331.
- Sharma K, Parle A (2015) Development and Validation of HPTLC Method for Simultaneous estimation of alogliptin Benzoate and Pioglitazone Hydrochloride in Bulk Drug and Combined Dosage forms. Int J of Pharma Res. and rev 4(11): 35-42.
- Rathod RH, Amod S (2018) Novel NP and RP-HPTLC in Praxis for Simultaneous Estimation of chlorthalidone and cilnidipine in Bulk and pharmaceutical Formulation. Journal Analytical Chemistry Letters 8(6): 862-871.
- Chandarana C, Kapupara P (2019) Quantitative simultaneous estimation of aspirin and omeprazole by RP-HPLC method implementing AQbD approach in pharmaceutical dosage form. Int J of Pharma Sci and Res 10(8): 3777-3784.
- Singh P, Maurya J (2017) QbD approach for stability indicating HPLC method for determination of Artemether and Lumefantrine in combined dosage form. International J of Drug Regulatory Affairs 5(4): 44-59.
- Jayaprakash J, Amirtharaj V (2018) Quality by Design approach to analytical method development for simultaneous estimation of ibuprofen and famotidine in their combined dosage form by RP-HPLC method. International Journal of Research in Pharmacology & Pharmacotherapeutics 7(3): 291-298.
- Thakur D, Kaur A (2017) Application of QbD based approach in method development of RP-HPLC for simultaneous estimation of antidiabetic drugs in pharmaceutical dosage form. Journal of Pharmaceutical Investigation 47(3): 229-239.
- Manikandan K, Lakshmi K (2019) Qbd Approach in RP-HPLC Method development for the Assay of Benzocaine and Diclofenac in dosage forms. AIP Conference proceeding 2112(1): 0200831-0200839.
- Roma N, Trivedi P (2017) Implementation of Box-Behnken Experimental Design for the Robustness Study and Its Comprehensive Approach in Development and Validation of RP-HPLC Method for Simultaneous Estimation of Rifampicin and Levofloxacin. 4th International Conference on Multidisciplinary Research & Practice (41CMRP), pp. 191-199.
- Raja T, Rao A (2012) Development and validation of a reversed phase HPLC Method for Simultaneous determination of levocetirizine and montelukast sodium in tablet dosage form., International journal of Research in pharmacy and chemistry 2(4): 2231-2781.
- Karode K, Dharbale N (2019) Qbd approach to method development and validation of Secnidazole. Innovation in Pharmceuticals and Pharmacotherapy 7(2): 45-60.
- Shah A, Patel C (2020) Implementation of QbD Approach to the RP-HPLC Method Development and Validation of Roflumilast in bulk and Tablet Dosage Form: An Application in Degradation Study. World Journal of Pharmacy and Pharm Sci 3(6): 2281-2307.
- Chaphekarm M, Hamrapurkar P (2016) Development and Validation of RP-HPLC Assay Method for Vildagliptin Using Qbd Approach and Its Application to Forced Degradation Studies. Int J of Pharm Sci & Drug Research 8(3): 157-165.
- Singh v, Daharwal S (2017) Optimization of RP-HPLC Method for Simultaneous Estimation of Lamivudine and Raltegravir in Binary Mixture by Using Design of Experiment. Eurasian J of Anal Chem 12(3): 179-195.
- Gholve S, Gandge NV, Giram P (2017) Analytical Method Development and Validation of Melatonin by QbD Approach Form. International J of Pharm & Pharm Res 10(1): 28-54.
- Dalal A, Anees M, Sangshetti J (2019) HPLC method development for determination of pyrazinamide and related substances by using qbd approach. European Chemical Bulletin 8(10): 328-334.
- Awotwe Otoo D, Agarabi C, Habib M, Awotwe otoo D, Agarabi C, et al. (2012) Application of QbD elements for the development and optimization of an analytical method for protamine sulphate. J of Pharm and Biom Anal 62: 61-67.
- Puranpole A, Chavan V (2016) Analytical Quality by design (QbD) Approach to RP-HPLC Method Development and Validation of Meloxicam. Asian J Pharm Tech & Inno 04(20): 1-10.
- Yadav N, Raghuvanshi A, Gajanand Sharma, Sarwar Beg, Om P Katare, et al. (2015) QbD-Based Development and Validation of a Stability-Indicating HPLC Method for Estimating Ketoprofen in Bulk Drug and Proniosomal Vesicular System. J of Chrom Sci 54(3): 377-389.
- Jain A, Sharma T, Prakash O (2019) QbD-Driven Analytical Method Development and Validation for Raloxifene Hydrochloride in Pure Drug and solid Oral Dosage Form 9(0): 463-477.
- Singh P, Sarwar Beg, O P Katare, Singh B (2016) QbD-Driven Development and Validation of a HPLC Method for Estimation of Estimation of Tamoxifen Citrate with Improved Performance. J of Chrom Sci 54(8): 1373-1384.
- Maria RM, Najla M, Erika RM (2006) Quantitative determination of gatifloxacin, levofloxacin, lomefloxacin and pefloxacin fluoroquinolonic antibiotics in pharmaceutical preparations by High Performance Liquid Chromatography. Journal of pharm and biom anal 40(1): 179-184.
- E Calleri, Lorenzi E, Furlanetto S, Caccialanza G (2002) Validation of a RP-HPLC method for the simultaneous determination of Isoniazid, Pyrazinamide and Rifampicin in a pharmaceutical formulation. J of pharm and Biom Anal 29(6): 1089-1096.
- Peraman R, Bhadraya K, Reddy Y, C Surayaprakash Reddy, Lokesh T (2015) Analytical Quality By Design approach in RP-HPLC Method Development for the Assay of Etofenamate in Dosage Forms. Indian Journal of pharmaceutical Science 77(6): 751-757.
- Bhusnure O, Shinde N (2015) QbD approach for analytical method development of Anti-pschotic drug. Der Pharmacia Lettre 12(7): 62-70.
- Patel K, patel A, Shah P (2017) Multivariate optimization for simultaneous determination of Aspirin and simvastatin by reverse phase liquid chromatographic Method using AQbD approach. Bulletin of Faculty of Pharmacy, Cairo University 55(2): 293-301.
- Ashu M, Parmar S, Gilani S (2017) Optimization and Validation for Simultaneous Estimation of citicoline and piracetam in bulk and tablet formulations using RP-HPLC Method Analytical quality by design Approach. Asian J of Res in chem 10(2):198-205.
- Peraman R, Kalva BY, Y Padmanabha Reddy, Hemraj Sharma (2016) Analytical Quality By Design Approach in Selection of Method variables for Simultaneous Analysis of Ciprofloxacin and Hydrocortisone by LC Method Using Taguchi Method. Analytical Chemistry Letter 6(1): 1-12.
- Alexander H Schmidt, Imre Molnar (2013) Using an innovate Quality-by-Design approach for development of a stability imdicating HPLC method for ebastine In the API and pharmaceutical formulations, Journal of Pharmaceutical and Biomedical Analysis 78(79): 65-74.
- Bondea S, Bondea C (2019) Quality by Design Based Development and Validation of HPLC Method For Simultaneous Estimation of Paclitaxel and Vinorelbine tartrate in dual drug loaded liposomes. Microchemical Journal 149: 1-9.
- Shah P, Pandya T, Gohel M, Vaishali Thakkar (2019) Development and Validation of HPLC method for simultaneous estimation of Rifampicin and Ofloxacin using experimental design. J of Taibhah University for Sci 13(1): 146-154.
- Sistla R, Kashyap Y, Chandrasekar D (2005) Development and Validation of a reversed-phase HPLC method for the determination of ezetimibe in pharmaceutical dosage forms, Journal of Pharmaceutical and Biomedical Analysis 39(3-4): 517-522.
- Mohammadi A, Rezanour N, Walker R (2007) A Stability Indicating HPLC assay for the simultaneous determination of Atorvastatin and Amlodipine in commercial tablets. J of chrom B 846(1): 215-221.
- Franeta J, Agbaba D, Vladimirov S (2002) HPLC Assay of Acetylsalicyclic acid, paracetamol, caffeine, and phenobarbital in Tablets, II Farmaco 57(9): 709-713.
- Dongre V, Shah B, Karmuse P (2008) Simultaneous determination of metoprolol succinate and amlodipine besylate in pharmaceutical dosage form by HPLC. J of BioPharm And Biomed Anal 46(3): 583-586.
- Wankhede S, Tajne M, Gupta K (2007) RP-HPLC Method For Simultaneous estimation of Telmisartan and Hydrochlorotiazide in tablet dosage form. Univ. Indian J Pharm Sci 69(2): 298-300.
- Rathinavel G, Mukherjee P, J Valarmathy, L Samueljoshua, Saravanan T, et al. (2006) A Validated RP-HPLC Method for Simultaneous Estimation of Cefixime and Cloxacillin in Tablets. E- Journal of Chemistry 5(3): 648-651.
- Singh V, Singh R, Baghel R (2018) RP-HPLC Method development and validation for Simultaneous estimation of Cilnidipine, Atenolol and chlorthalidone. Journal of drug Delivery & Therapeutics 8(6): 78-82.
- Kumar P, Thirupathi D (2017) Simultaneous determination of related organic impurities of ibuprofen and paracetamol in combination solid dosage form by RP-HPLC with Qbd approach. Oriental journal of chemistry 33(3): 1461-1468.
- Perama R, Kalva B (2014) Analytical Quality By Design (AQBD) Approach to liquid Chromatographic Method for Quantification of Acyclovir and Hydrocortisone in dosage forms. Analytical Chemistry Letters 4(5): 329-342.
- Velusamy B, Katari N, Thirupathi Dongala, Sreekantha B Jonnalagadda (2019) Stability –indicating RP-HPLC method development and validation for determination of nine impurities in apixaban tablet dosage forms. Robustness study by Qbd approach. Biomedical Chromatography 34(1): 1-8.
- Chamarthi R, Godavarthi K (2016) Development and validation of amlodipine impurities in amlodipine tablet using design space computer modelling. American Journal of Analytical chemistry 12(7): 918-926.
- Raghava R, Rao I, Seshadri R (2014) Development and Validation of a UPLC Method by the QbD-Approach for the Estimation of Rabeprazole and Levosulpiride from Capsule. Scientia Pharmaceutica 82(2): 307-326.
- Beg S, Jain A, Singh B (2016) QbD-driven development and validation of an efficient bioanalytical UPLC method method for estimation of olmesartan medoxomil. journal of liquid chrom & Related Tech 39(13): 587-597.
- Alexander H, Schmidt WA (2014) QBD With design-of-experiment of a state-of-the-art UPLC Purity Method of Carbamazepine. J of Liq And Rel Tech 37(18): 2653-2666.
- Cijo M, Kanakapura B, KB Vinay, N Swamy (2013) Quality by Design Approach for the Development and Validation of Glipizide, an Antidiabetic Drug, by RP-UPLC with Application to Formulated Forms and Urine. International Scholarly Research Notice. Isrn Chrom, p. 1-10.
- Cijo M, kanakapura Basavaiah (2015) RP-UPLC Development and Validation of Metformin Hydrochloride in pure drug and pharmaceutical drug. World J of pharm and pharm Sci 4(4): 1649-1668.
- Cijo M, kanakapura B (2012) Implementation of QbD for the development and validation of pioglitazone Hydrochloride by RP-UPLC with application to Formulated forms. International Scholarly Research notice.
- Jaya Raju Ch, Bhaskara Rao T, Sanath Kumar Goud P, Satish J, Rajashekar K (2018) Development and Validation of liquid chromatography method using the principles of QbD for antimalarials used in Artemisinin based combination therapy. Journal of Liquid chromatography & Related Technologies 41(17-18).
- Schmidt A, Molnar I (2013) Using an innovate Quality-by-Design approach for development of a stability imdicating UHPLC method for ebastine In the API and pharmaceutical formulations. Journal of Pharmaceutical and Biomedical Analysis 78: 65-74.
- Jagan Mohan T, Hitesh A, K Mukkanti (2020) Novel Stability Indicating UHPLC Method Development and Validation for the Quantification of Perindopril, Amlodipine and Their Impurities in Pharmaceutica. Chromatographia 16.

 Review Article
Review Article