ABSTRACT
Sodium valproate acts as an anticonvulsant drug and is used for treatment of bipolar disorder. it has been reported that sodium valproate has effects on the cardiovascular system. Due to changes in blood pressure in epileptic patients, the aim of this study was to evaluate the effect of sodium valproate on blood pressure in epileptic rats. Fifty rats were divided into ten groups (5 per each group) in the following order: The groups 1-3 received saline (5, 10 and 15 times) every other day. Groups 4-6 were injected pentylenetetrazole (PTZ) (5, 10 and 15 times) thirty minutes after saline. Groups 7 to 9 were injected 30 minutes before PTZ valproate similar to groups 4-6. The tenth group received losartan (10 times) thirty minutes before PTZ every other day. All injections were intraperitoneal. Cardiovascular parameters were recorded in three time periods of fifth, tenth and fifteenth injections. The results showed that PTZ increased systolic and mean arterial blood pressure and sodium valproate returned these parameters to normal levels. Losartan (as an angiotensin II antagonist) reduced these parameters. The results showed that sodium valproate may reduce blood pressure in epileptics and its mechanisms may be related to reducing phosphorylation of the ERK (interfering with the angiotensin II receptor signaling pathway) However, more investigations on this topic are needed to clarify the mechanisms.
Keywords: Valproate; Kindling; Cardiovascular Function; High blood pressure
Introduction
Hypertension is one of the main causes of mortality and affects the financial burden of the health system in the world. Although the prevalence of epilepsy is not more than one to three percent, since one of the problems of people with epilepsy is high blood pressure (Gaitatzis, et al. [1]); Therefore, addressing this issue can be one of the priorities. In those people with epilepsy with high blood pressure (due to or unrelated to epilepsy), the simultaneous use of antihypertensive and anticonvulsant drugs is mandatory (Lukawski, et al. [2]). Epileptic seizures are associated with changes in the function of the cardiovascular system (Reeves, et al. [3]). During seizures caused by amygdala kindling, an increase in blood pressure has been reported along with a significant decrease in heart rate (Crawford, et al. [4,5]). Although there have been limited reports of ystolic and diastolic hypertension and heart rate in penicillin-induced seizures (Mameli, et al [6]), there have been numerous reports of hypertension and decreased heart rate after seizure (Lathers, et al. [7-10]). A study by Goodman et al. showed that blood pressure increased, and heart rate decreased significantly up to 30 minutes after amygdala kindling (Goodman, et al. [11]).
Valproic acid (VPA), a short-chain fatty acid extracted from Valerian officinalis, has been used as an anticonvulsant for decades around the world (Rajeshwari, et al. [12]). Valproic acid has been shown to lower blood pressure in spontaneusly hypertensive rats Cardinale et al. [13] and prevented high fat diet-induced hypertension (Choi, et al. [14]). On the other hand, it has been reported that one of the side effects of taking valproate is increased blood pressure and high heart rate (Sivananthan, et al. [15]). Due to the contradictory results of the effect of sodium valproate (VPA) on blood pressure; In this study, the role of VPA on the hypertension of epileptic rats was investigated by the kindling method (Figure 1).
Figure 1: Timeline diagram of experimental groups. Means penteylenetetrazol (PTZ) injection (all groups except groups
1-3).
Means penteylenetetrazol (PTZ) injection (all groups except groups
1-3). Means vehicle (groups 1-3) or valproate (groups 7-9) injection. The animals in groups 10 received Losartan (10 mg/kg)
30 min before PTZ injection. Groups 1, 4 and 7 received 5, groups 2, 5, 8, and 10 received 10 and groups 3, 6 and 9 received 15
PTZ injection.
Means vehicle (groups 1-3) or valproate (groups 7-9) injection. The animals in groups 10 received Losartan (10 mg/kg)
30 min before PTZ injection. Groups 1, 4 and 7 received 5, groups 2, 5, 8, and 10 received 10 and groups 3, 6 and 9 received 15
PTZ injection.
Results
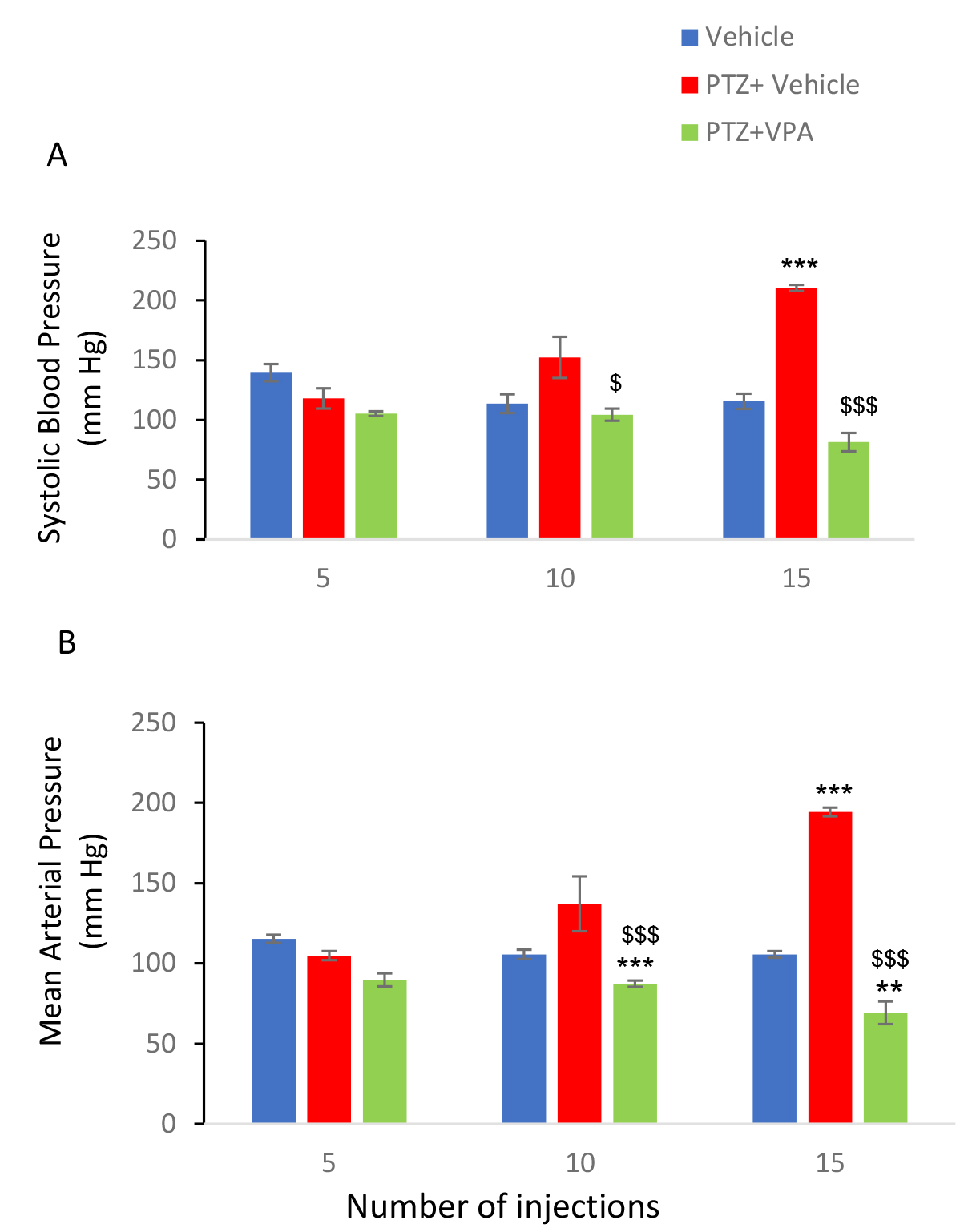
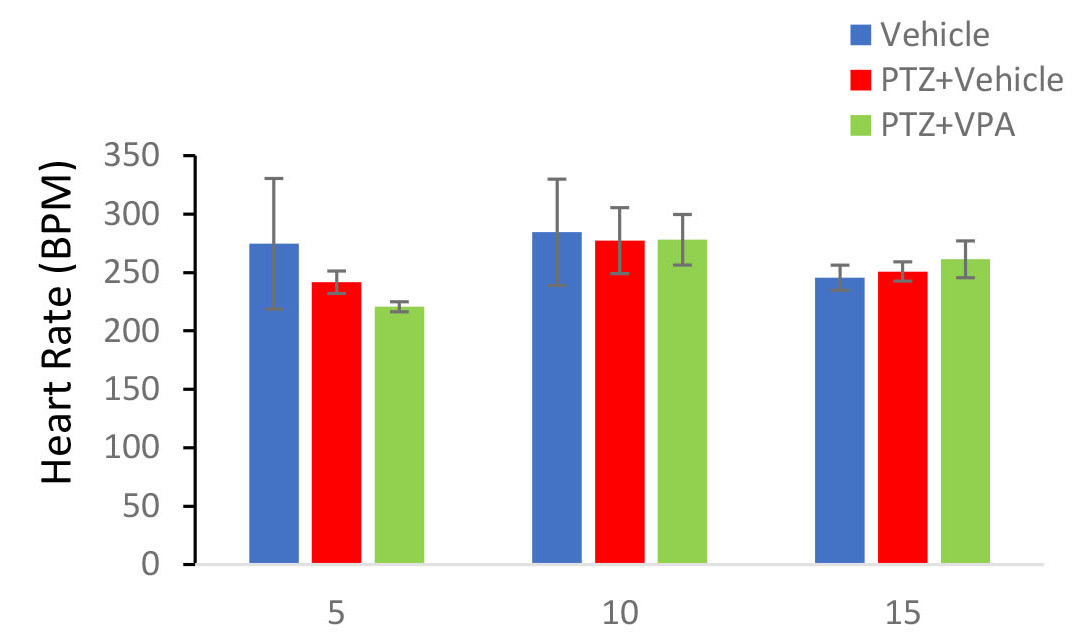
Data analysis demonstrated that systolic mean arterial pressure increased over time following PTZ injection (Figure 2A) [F (9, 38) = 17.20; P <0.001] was also drug-dependent, meaning that PTZ-induced hypertension was significantly reduced by VPA. Mean arterial pressure was increased significantly over time (Figure 2B) [F (9, 38) = 41.67; P <0.001] and as in systolic pressure under the influence of VPA this increase returned to the initial level and decreased significantly [F (9, 38) = 17.28; P <0.001]. Heart rate was another quantity that was evaluated in this study. Heart rate was recorded for 10 minutes in anesthetized animals after receiving the fifth, tenth and fifteenth drug / vehicle injections. One-way analysis of variance showed that this variable did not change significantly under the influence of pentylenetetrazol [F (9,38) = 0.6; P = 0.79]. VPA also failed to significantly change this parameter (Figure 3).
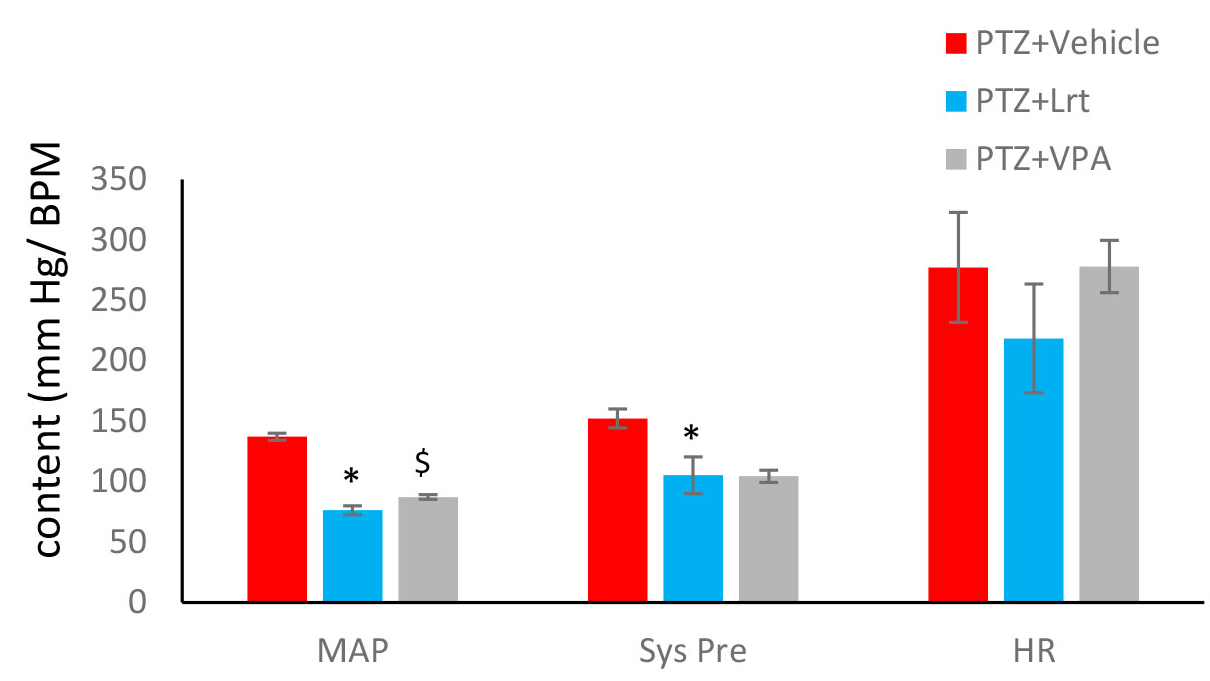
In another part of our study, losartan was used as a blood pressure lowering (positive control). The desired quantities (Mean arterial pressure, Systolic pressure and Heart rate) under the influence of this drug in a period of 10 injections statistical analyzed. Our data showed that: systolic pressure from 152.3 ± 7.8 in PTZ + vehicle group to 105.3±15.2 significantly decreased in PTZ + LRT group (P <0.05). Mean arterial pressure also decreased significantly from 137.1 ± 2.9 in PTZ + vehicle group to 76.3 ± 3.6 in PTZ + LRT group (P <0.05). But the heart rate did not change significantly (Figure 4).
Figure 2: A. Effect of PTZ and VPA on systolic blood pressure and
B. Mean arterial pressure during kindling acquisition.
The quantities were measured after the fifth, tenth and fifteenth PTZ injection. PTZ administration increased systolic blood pressure and mean arterial pressure time dependently and VPA reduce them to normal values. *** P<0.001 compared to respective vehicle group and $$$ P<0.001 when compared to respective PTZ group.
Figure 3: Effect of PTZ and VPA on heart rate during kindling acquisition. The quantities were measured after the fifth, tenth and fifteenth PTZ injection. PTZ administration and VPA injection did not change them relative to vehicle group.
Figure 4: Effect of PTZ, VPA and losartan (Lrt) on systolic blood pressure (Sys Pre), mean arterial pressure (MAP) and heart rate (HR) during kindling acquisition. The quantities were measured after the tenth PTZ injection. Losartan decrease MAP and systolic pressure but did not change heart rate. * P<0.05 compared to PTZ+vehicle group and $ P<0.05 when compared to PTZ+VPA group.
Discussion
Current findings demonstrated that in addition to causing seizures pentylenetetrazol increases the mean arterial pressure and systolic pressure. Valproate (VPA) as an anticonvulsant significantly reduces these two quantities. But the heart rate was not affected by pentylenetetrazol and VPA. Clinical seizures are associated with changes in the function of the cardiovascular system (Reeves, et al. [3]). During seizures caused by amygdala kindling, an increase in blood pressure has been reported along with a significant decrease in heart rate (Crawford, et al. [4,5]). Although there have been limited reports of systolic and diastolic hypertension and heart rate in penicillin-induced seizures (Mameli, et al. [6]), there have been numerous reports of hypertension and decreased heart rate after seizures (in different animal models of epilepsy) (Lathers, et al. [7-10]). A study by Goodman et al. showed increasing in blood pressure and significantly decreasing of heart rate up to 30 minutes after amygdala kindling. They showed that decreased heart rate was prevented by pretreatment with atropine and that hypertension was due to activation of alpha-adrenoceptors (Crawford IL, et al. [11]). The results of our study also showed that the mean arterial pressure and systolic pressure increase under the influence of pentylenetetrazol.
VPA through different ways has anticonvulsant effects. Delay in the return of voltage-dependent sodium channels to rest is one of these mechanisms. VPA reduces the synaptic transmission of glutamate (Zeise, et al. [16]) and increases GABA levels at the corresponding synapses by inhibiting GABA transaminase (Deckers, et al. [17]). On the other hand, GABA has been observed to play a protective role in hypertensive mice and significantly reduce blood pressure (Hayakawa, et al [18-20]). A clinical study also showed that sodium glutamate increased blood pressure (Shi, et al. [21]). In other words, lowering glutamate levels (by VPA) may lower blood pressure. In the present study, VPA probably significantly reduced mean arterial pressure and systolic pressure through one of these mechanisms. The lack of change in heart rate in this study as a result of PTZ and VPA injections may be due to the fact that the values were recorded in an unconscious state, in which case a change in the activity of the autonomic system could possibly affect the heart rate. Due to the fact that losartan in this study result in decrease in blood pressure caused by PTZ administration; it can be concluded that angiotensin 2 receptors may also be involved in increasing the blood pressure induced by PTZ.
Angiotensin leads to an increase in blood pressure by activating the ERK pathway in vascular smooth muscle cells (Sousa Lopes, et al. [22]). On the other hand, it has been shown that phosphorylation and ERK activation occur by angiotensin. Since the valproate cause reduction of ERK phosphorylation, it can be followed by a reduction in cardiac fibrosis caused by angiotensin (Zhang, et al. [23]). Therefore, it is possible that in the present study, valproate also reduced blood pressure by reducing ERK phosphorylation. However, measuring and determining the amount of ERK can confirm this claim and it is suggested that a study in this field to determine the exact mechanism of action of this anticonvulsant drug on blood pressure.
Acknowledgment
This work was done entirely at the Department of Physiology and was supported by a grant from Deputy of Research and Technology of Mashhahd University of Medical Sciences (#970139).
Conflict of Interests
None of the authors has any conflict of interest to disclose.
References
- Gaitatzis A, Carroll K, Majeed A, Josemir W Sander (2004) The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia 45(12): 1613-1622.
- Lukawski K, Swiderska G, Czuczwar SJ (2012) Effect of hydrochlorothiazide on the anticonvulsant action of antiepileptic drugs against maximal electroshock-induced seizures in mice. Pharmacol Rep 64: 315-320.
- AL Reeves, KE Nollet, DW Klass, FW Sharbrough, EL So (1996) The ictal bradycardia syndrome. Epilepsia 37: 983-987.
- Crawford IL (2010) Acute Cardiovascular Response during Kindled Seizures. Sudden Death in Epilepsy. CRC Press, pp. 681-694.
- Goodman JH, Homan RW, Crawford IL (1990) Kindled seizures elevate blood pressure and induce cardiac arrhythmias. Epilepsia 31(5): 489-495.
- P Mameli, O Mameli, E Tolu, G Padua, D Giraudi, et al. (1988) Neurogenic myocardial arrhythmias in experimental focal epilepsy. Epilepsia 29(1): 74-82.
- Lathers CM, Schraeder PL (1982) Autonomic dysfunction in epilepsy: characterization of autonomic cardiac neural discharge associated with pentylenetetrazol-induced epileptogenic activity. Epilepsia 23(6): 633-647.
- Plum F, Posner JB, Troy B (1968) Cerebral metabolic and circulatory responses to induced convulsions in animals. Archives of Neurology 18(1): 1-13.
- Wasterlain CG (1974) Mortality and morbidity from serial seizures. An experimental study. Epilepsia 15: 155-176.
- Westergaard E, Hertz MM, Bolwig TG (1978) Increased permeability to horseradish peroxidase across cerebral vessels, evoked by electrically induced seizures in the rat. Acta Neuropathol 41(1): 73-80.
- Goodman JH, Homan RW, Crawford IL (1999) Kindled seizures activate both branches of the autonomic nervous system. Epilepsy research 34(3): 169-176.
- Rajeshwari T, Raja B, Manivannan J, Thangarasu Silambarasan (2014) Valproic acid attenuates blood pressure, vascular remodeling and modulates ET-1 expression in L-NAME induced hypertensive rats. Biomedicine & Preventive Nutrition 4: 195-202.
- Cardinale JP, Sriramula S, Pariaut R, Anuradha Guggilam, Nithya Mariappan, et al. (2010) HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension 56(3): 437-444.
- Choi J, Park S, Kwon TK, KM Park, JI Kim, et al. (2017) Role of the histone deacetylase inhibitor valproic acid in high-fat diet-induced hypertension via inhibition of HDAC1/angiotensin II axis. Int J Obes (Lond) 41: 1702-1709.
- Sivananthan M, Mohiuddin S (2016) Valproate Induced Hypertensive Urgency. Case Rep Psychiatry, pp. 1458548.
- Zeise ML, Kasparow S, Zieglgansberger W (1991) Valproate suppresses N-methyl-D-aspartate-evoked, transient depolarizations in the rat neocortex in vitro. Brain Res 544: 345-348.
- Deckers CL, Czuczwar SJ, Hekster YA, PN Patsalos, WO Renier, et al. (2000) Selection of antiepileptic drug polytherapy based on mechanisms of action: the evidence reviewed. Epilepsia 41(11): 1364-1374.
- Hayakawa K, Kimura M, Kasaha K, Keisuke Matsumoto, Hiroshi Sansawa, et al. (2004) Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr 92(3): 411-417.
- Hayakawa K, Kimura M, Yamori Y (2005) Role of the renal nerves in gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur J Pharmacol 524(1-3): 120-125.
- Kawakami K, Yamada K, Yamada T, Toru Nabika, Masato Nomura (2018) Antihypertensive Effect of gamma-Aminobutyric Acid-Enriched Brown Rice on Spontaneously Hypertensive Rats. J Nutr Sci Vitaminol (Tokyo) 64(1): 56-62.
- Zumin Shi, Baojun Yuan, Anne W Taylor, Yue Dai, Xiaoqun Pan, et al. (2011) Monosodium glutamate is related to a higher increase in blood pressure over 5 years: findings from the Jiangsu Nutrition Study of Chinese adults. Journal of hypertension 29: 846-853.
- Sousa-Lopes A, De Freitas RA, Carneiro FS, Kenia Pedrosa Nunes, Kyan James Allahdadi, et al. (2020) Angiotensin (1-7) Inhibits Ang II-mediated ERK1/2 Activation by Stimulating MKP-1 Activation in Vascular Smooth Muscle Cells. Int J Mol Cell Med 9: 50-61.
- Zhang Y, Gao F, Tang Y, Jinwen Xiao, Chuanchuan Li, et al. (2018) Valproic acid regulates Ang II-induced pericyte-myofibroblast trans-differentiation via MAPK/ERK pathway. Am J Transl Res 10: 1976-1989.

 Review Article
Review Article