ABSTRACT
Background: There is great need for and concern over vaccines against influenza, measles, mumps, rubella, varicella, and COVID-19. Immediate reactions to gelatincontaining vaccines in two of our patients with IgE-alpha-gal-meat allergy prompted the question of safety of such vaccines in patients sensitized to galactose-α-1,3-galactose (alpha-gal) or in regions endemic to the Lone Star tick.
Objective: To evaluate the prevalence of IgE-mediated allergic reactions to alpha-gal (gelatin) containing vaccines.
Design: To assess the magnitude of the problem, a search of the Vaccine Adverse Event Reporting System (VAERS) database, our own database of 650 alpha-gal IgE allergic patients, the literature, and a physician survey in an endemic area for the Lone Star tick were employed.
Setting: This study was conducted as a retrospective observational analysis of the VAERS database, our own database, and via a physician survey.
Results: Results from the VAERS database were not statistically analyzable. No physicians surveyed had observed IgE-mediated allergic reactions in their alpha-gal allergic patients following vaccination with gelatin containing vaccines. Limitations: Data obtained from the VAERS database did not hold statistical significance.
Conclusion: Data obtained in this study did not support a markedly increased risk of IgE-mediated hypersensitivity reactions in alpha-gal sensitized patients which is consistent with the observation that many such patients have received gelatin-based vaccines without incident [1].
Introduction
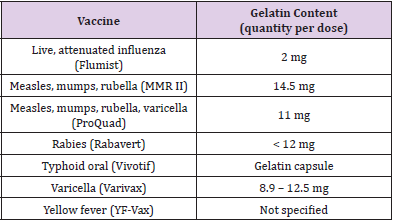
Alpha-gal syndrome is a recently discovered disorder of sensitization to the carbohydrate galactose-α-1,3-galactose (alphagal) following a bite from the Lone Star tick. This can result in an IgE-mediated hypersensitivity reaction to non-primate mammalian meat [2,3] typically developing 3-8 hours following red meat ingestion. Alpha-gal can also be found in gelatin products [1], which gives rise to the question of whether gelatin-containing vaccines, such as the MMR, Varivax, Zostavax, and some influenza (Table 1), can be safely administered to patients sensitized to alpha-gal. Primary care physicians have been observed to be hesitant to administer gelatin-based vaccines to patients with diagnosed alpha-gal meat allergy. Concerns for adverse reactions to vaccines, in general, have resulted in a disconcerting 30% of the US population who have refused vaccination for themselves or their family members.
Methods
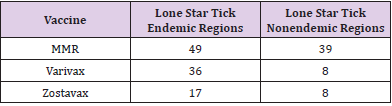
Our own records were first studied. Both a search of the Vaccine Adverse Event Reporting System (VAERS) database and a physician survey in an area endemic to the Lone Star tick were employed to access the magnitude of the problem afterwards. The VAERS database was utilized to study the analyzable incidence of hypersensitivity reactions to the MMR, Varivax, and Zostavax vaccines in reference to geographic location from 2000-2020. Pediatricians from an endemic area of Long Island, NY area were questioned regarding the incidence of allergic reactions observed to the gelatin-containing vaccines observed in more than 650 patients known to be sensitized to alpha-gal.
Results
The VAERS results (Table 2) show more vaccine reactions in states endemic to the Lone Star tick, but insufficient information did not permit statistical analysis. Among pediatricians surveyed from the endemic area with a patient population of more than 650 patients sensitized to alpha-gal, there were no noted reactions to the vaccines in the sensitized patients other than the 2 following cases. An observation of an immediate IgE reaction to gelatin-containing vaccines was observed in two patients. The first patient was an adult male who gave a longstanding history of suspected allergic reactions to meat and dairy and required an MMR vaccine at 38 years of age. Within 40 minutes of vaccination, he developed acute onset of cough, chest and throat tightness, and difficulty breathing and speaking. He required emergency room management and 2 doses of epinephrine for resolution. His alpha-gal IgE was noted to be 57.4 kU/L. The second patient had a history of severe gastrointestinal symptoms with dairy and meat, dating back to around 5 years of age. While in the pediatrician’s office following vaccination with Varivax, she developed acute anaphylaxis requiring treatment with epinephrine. She was subsequently tested for alpha-gal allergy and her alpha-gal IgE level was noted to be 10.5 kU/L.
Discussion
Data obtained in this study did not support a markedly increased risk of IgE-mediated hypersensitivity reactions in alphagal sensitized patients which is consistent with the observation that many such patients have received gelatin-based vaccines without incident [1]. There is often hesitancy to give gelatin-containing vaccines (Table 1) due to concern for allergic reactions, however the risk of not receiving a particular vaccine may outweigh the possible risk of allergic reaction which is amenable to mitigation. As the world is presented with the development of the COVID-19 vaccines, there is significant scrutiny on every individual aspect of the various vaccines. As more pharmaceutical companies formulate their own versions, some may contain gelatin and the question of whether it is safe to administer these vaccines in alpha-gal sensitized patients will likely arise. The risks of measles, influenza, and COVID-19 infection likely outweigh the possible risk of a controllable IgE-mediated hypersensitivity reaction to a gelatincontaining COVID-19 vaccine. Vaccines should be considered by their physicians in individuals at-risk or hesitant patients, with practiced precautionary measures taken when indicated [4].
Funding Source
None.
Conflict of Interest
No conflict of interest with any institution/organization.
References
- Commins SP (2020) Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol 16: 667-677.
- Platts-Mills TA, Schuyler AJ, Hoyt AE, Commins SP (2015) Delayed Anaphylaxis Involving IgE to Galactose-alpha-1,3-galactose. Curr Allergy Asthma Rep 15: 512.
- Mullins RJ, James H, Platts-Mills TA, Commins S (2012) Relationship between red meat allergy and sensitization to gelatin and galactose-alpha-1,3-galactose. J Allergy Clin Immunol 129: 1334-1342 e1.
- Stone CA, Commins SP, Choudhary S, Vethody C, Heavrin JL, et al. (2019) Anaphylaxis after vaccination in a pediatric patient: further implicating alpha-gal allergy. J Allergy Clin Immunol Pract 7: 322-324 e2.

 Research Article
Research Article