ABSTRACT
Cutaneous melanoma (cM) is the deadliest of all primary skin cancers. Its prognosis is strongly influenced by the stage at diagnosis, with early stages having a good prognosis and potentially treatable with surgery alone, and advanced stages displaying a much worse prognosis with a high rate of recurrence and metastasis. For this reason, the accurate and early diagnosis of cM is crucial, with misdiagnosis that may have extremely dangerous consequences for the patient and drastically reduce its chances of survival. Although the histological exam remains the “gold standard” for the diagnosis of cM, a continuously increasing number of immunohistochemical markers that could help in diagnosis, prognostic characterization and appropriate therapeutical choices are identified every day, with some of them becoming part of the routine practice. This review aims to discuss and summarize all the data related to the immunohistochemical analyses potentially useful for the diagnosis of cM, thus rendering it easier to appropriately applicate to routine practice. We will discuss these topics and the potential impact on diagnosis and treatment of cM, integrating the literature data with the experience of our surgical pathology department.
Keywords: Cutaneous Melanoma; Skin Melanoma; Melanoma; Immunohistochemistry; Immunohistochemical Markers; Diagnosis
Introduction
Cutaneous melanoma (cM) is a malignant and potentially lethal tumor developing from the transformation of melanocytes normally resident in the basal layer of the skin epidermis and forming with the keratinocytes the epidermal-melanin unit [1,2]. The annual incidence and morbidity of cM are constantly increasing worldwide (the number of newly diagnosed cases has more than doubled since 1973), probably due to population aging, the increase of risk factors as chronic sun damage and the improvement of diagnostic tools; besides, unlike other malignancies, cM affects a higher proportion of younger patients (median age: 57 years), with the sex preponderance that varies in different age groups [female preponderance in younger age groups (4:10 in 20-30-year-olds); male preponderance (16:10 in >85-year-olds)] [3,4]. cM is also the most lethal cutaneous tumor, with mortality rates ranging between 3.5/100,000 in Australia and 1.7/100,000 in Europe [3,4]. This review aims to present and summarize all the data related to the immunohistochemistry of cM, discussing its application for diagnosis, prognostic characterization and treatment of this deadly disease.
Diagnosis
Histological Exam
Despite an everyday increasing understanding of molecular biology and the etiology of cM, the diagnosis of cM is mainly performed by the pathologists with the histological exam rendered on hematoxylin and eosin (H&E) slides [5,6]. The differential diagnosis between cM and cutaneous nevus (cN) is based on the identification and the assessment of numerous morphological criteria. Nevertheless, none criteria are completely specific of cM and all of them could be potentially found also in cN, some criteria are found only in specific cN and cM subtypes, and others are qualitatively assessed and so suffer from low interobserver agreement [5,6]. Besides, new histological entities of cN and cM are identified every day based on different clinical-pathological and molecular backgrounds [5-8]. As result, the diagnosis of cM remains one of the most difficult of the surgical pathology and it should be rendered by only dedicated dermatopathologists that integrate the histological exam rendered on H&E slides, with available clinical, immunohistochemical and molecular data [5,6,9,10].
Immunohistochemistry
Despite the continuous development of molecular-genetic diagnostic techniques, immunohistochemistry remains the most frequently performed and cost-effective tool to implement the histological exam for the diagnosis of cM. In this review, we analyzed the immunohistochemical markers preferentially adopted by us and the other surgical pathology laboratories for the diagnosis of melanocytic lesions, along with their expression profile, the routinary use and clones, the potential diagnostic pitfalls and the ongoing research topics. For a more practical purpose, we divided them into four major classes (in italic, we reported the markers subsequently described):
• Melanocytic differentiation markers (S100, SOX10, HMB- 45, Melan A/MART-1, MITF, Tyrosinase, KBA 6.2, NKI/beteb, PNL2, MC1R, CD146/Mel-CAM, NKI/C3, p75NGFR)
• Markers useful for the differential diagnosis between CN and CM (HMB-45, Ki67, p16, p21, p53, PRAME, NKI/beteb, 5-hmC, PTEN, PHH3, H3KT and H3KS)
• Markers useful for the identification of specific histological subtypes of CN and CM (BRAF V600E, c-Kit/CD117, ROS1, ALK, pan-TRK, BAP-1, β-catenin, PRKAR1A, NF1, IDH1)
• Double stains (DS) (HMB-45/Ki67, MART-1/Ki67, D2-40/ MITF, D2-40/S-100, D2-40/SOX10, D2-40/MART-1, CD34/ SOX10, HMB-45/PRAME, MART-1/PRAME, MART-1/PHH3)
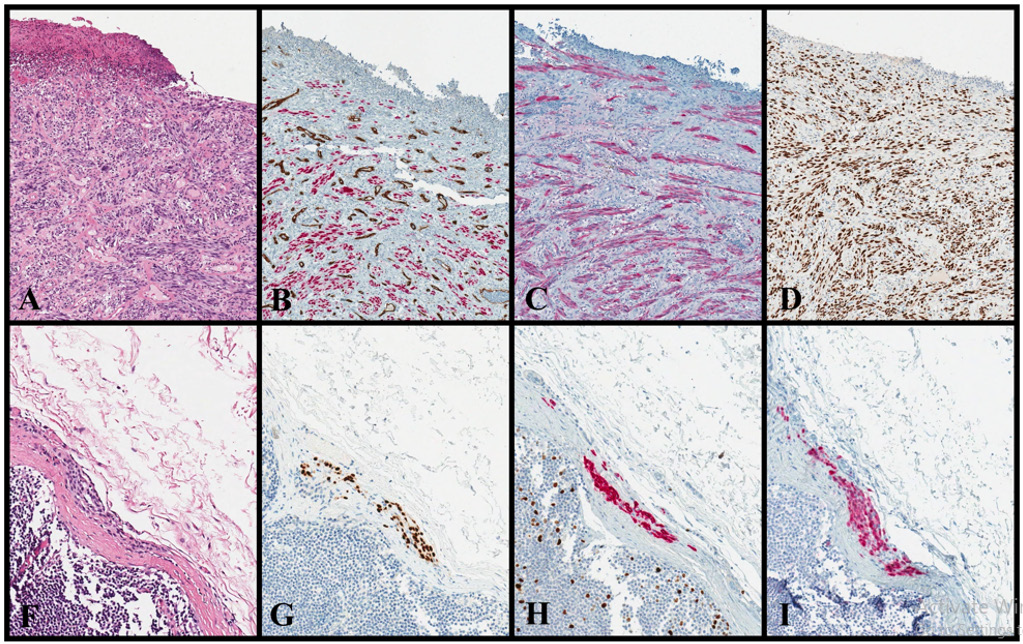
Some of these markers could belong to more than one class (HMB-45) and have been discussed only in one of them. A summary of the main application fields of the immunohistochemical markers most frequently adopted for the diagnosis of cM is presented in (Table 1). Illustrative examples of the immunohistochemical markers adopted in complex routine diagnostic cases are shown in Figure 1.
Table 1: Summary of the main application fields for the immunohistochemical markers most frequently adopted for the diagnosis of cM.
Figure 1: cM: cutaneous melanoma; DS: double staining; NN: nodal nevus.
• Desmoplastic cM (1A-1D):
A case of ulcerated desmoplastic cM with marked desmoplasia, atypical spindle cells and rare mitoses (1A: H&E, original magnification x100). This case turns out positive for SOX10 (1B: CD34/SOX10, original magnification x100; CD34: brown, SOX10: red), S100 (1C: S100, original magnification x100) and p53 (1D: p53, original magnification x100). Note as DS CD34/ SOX100 shows the absence of lympho-vascular invasion (1B), without SOX10(+) cells inside the vessels (labeled with CD34). • NN (1E-1H):
A small intra-capsular NN that histologically resembles cN, with bland nuclei and absence of mitoses (1E: H&E, original magnification x200). This NN is positive for SOX10 (1F: SOX10, original magnification x200), MART-1 (1G: MART-1/Ki67, original magnification x200; MART-1: red, Ki67: brown) and p16 (1H: p16, original magnification x200). Note as DS MART- 1/Ki67 shows the absence of proliferating melanocytic cells (1G), without MART-1(+)/Ki67(+) cells; by contrast, it shows proliferating lymphocytes MART-1(-)/Ki67(+) within the lymphoid follicles.
Melanocytic Differentiation Markers
S100: The S100 protein family comprises about 25 members encoded by different genes located on chromosome 1q21 and involved in a wide variety of cellular processes (cell growth, cell cycle regulation, protein secretion, etc.) [11-14]. The most commonly used antibodies against S100 in routine practice are mouse and rabbit monoclonal antibodies [clones SHB1, 9A11B9 and SP127 (used in our laboratory)] direct against the S100B protein subtype [15,16]. S100 is probably the most historically known and commonly used melanocytic differentiation marker in surgical pathology laboratories, being expressed in almost all cN and cM (also desmoplastic cM) [17-20]. Its sensitivity ranges between 93% and 100% in the published series, with a characteristic staining pattern in both the nucleus and the cell cytoplasm; however, S100 is not highly specific being also expressed by several soft tissue tumors (nerve sheath tumors, adipocytic tumors, chondroid tumors, notochordal tumors and many others), hematopoietic disorders (Langerhans cell histiocytosis) and others tumors (glial tumors, sex cord-stromal tumors, myoepithelial carcinoma and other salivary gland tumors) [17-25]. For this reason, we always recommend using S100 in conjunction with other melanocytic (HMB-45, MART-1) and case-by-case selected immunohistochemical markers, in specific diagnostic settings (metastasis of unknown primary, primary cutaneous tumors with undifferentiated morphology). On the other hand, taking into account the high sensibility of S100, this marker has been largely used for the detection of melanoma metastases (MMs) in sentinel lymph node biopsy (SLNB) [26,27]. However, as S100 could label histiocytic and dendritic cells in lymph nodes, in the last years we always added HMB-45 and recently started to substitute it with SOX10.
SOX10: The sex determinant region Y box 10 (SOX10) is a member of a family of approximately 20 transcription factors encoded by a gene located on chromosome 22q13.1 and involved in the development of neural crest, peripheral nervous system and melanocytes [28,29]. At present, several antibodies anti-SOX10 are commercially available, among which clones 1E6 (used in our laboratory) and A-2 [30-35]. SOX10 is universally accepted as the most sensitive marker for cN and cM (98%-10 in metastatic CM, 78%-100% in desmoplastic CM) with the advantage of not staining dendritic cells and/or histiocytic cells in lymph nodes; as result, it is largely preferred to S100 in for the evaluation of SLNB with the updated EORTC protocol and the characterization of unknown primary metastatic and/or primary cutaneous undifferentiated tumor [27,30-33]. However, similarly to S100, SOX10 exhibits a low specificity being potentially expressed by a large number of tumors (carcinomas and soft tissue tumors) and it should be always used in conjunction with other immunohistochemical markers depending on the diagnostic scenarios [32,34-35].
The staining pattern of SOX10 is nuclear and provides a cleaner signal compared to cytoplasmatic (HMB-45, MART-1) and cytoplasmatic/nuclear (S100) melanocytic markers; for this reason, in our personal experience, it results more appropriate for the highly pigmented lesions, the evaluation of the nuclear profile (useful for the assignment of melanocytic dysplasia according to WHO 2018 criteria) and the correct estimation of intra-epithelial pagetoid spreading. An additional advantage of SOX10 is the potential application for the differential diagnosis between proliferating fibroblasts of scar [SOX10 (-)] and the residual component of desmoplastic cM [SOX10(+)] in excisional enlargements [36].
HMB-45: The name HMB-45 (human melanoma black) originated to indicate the immunogen associated with the monoclonal antibody and targeting PMEL17/gp100, which is a membrane-bound melanosomal protein encoded by a gene located on chromosome 22q13.1 and involved in the intracellular organization of melanosomes [37,38]. The most frequently adopted antibody (also in our laboratory) to detect HMB-45 in routine practice is the monoclonal mouse antibody, clone HMB- 45 [38]. HMB-45 has a lower sensibility as melanocytic marker if compared to S-100 and SOX-10 (73%-100% in primary cutaneous cM, 58%-95% for metastatic cM and only 9-15% in desmoplastic cM), so the latter should be preferred for the immunohistochemical characterization of unknown primary metastatic and/or primary cutaneous undifferentiated tumor [38-40]. Nevertheless, HMB-45 is negative in most of the tumors that could histologically mimic cM and be positive for S-100 and SOX-10, so we often add it to the immunohistochemical panels adopted in these diagnostic settings [32,41]. HMB-45 could turn out positive in PEComa and related tumors, melanotic schwannoma, clear cell sarcoma, sex cordstromal tumors, MiT family translocation renal cell carcinomas, pheochromocytoma and rare cases of salivary gland tumors (it reacts with the fibrillar matrix in stage II melanocytes and should be more appropriately considered an organelle-specific marker rather than a lineage-specific marker) [42-45].
In the melanocytic lesions, HMB-45 strongly reacts with the junctional and intraepidermal melanocytes and, in our experience, it is the best marker for the evaluation of the junctional component, with the intensity that correlates with the grade of the dysplasia in dysplastic cN [46,47]. By contrast, the dermal component of cN is completely negative for HMB-45 and/or tends to retain it only in the superficial portion and loses it with maturation, differently from the dermal component of cM (mainly nevoid cM) that retains the stain (diffusely or patchy/focal with isolated and/ or clustered cells in both superficial and deep parts of the lesion) [46,47]. However, dermatopathologists are aware that this axiom has several exceptions in routinary diagnostic practice: 1) blue cN, deep-penetrating cN and other benign dermal melanocytosis are usually HMB-45(+); 2) nevoid cM could be completely HMB- 45(-) in the dermal component exhibiting the so-called “pseudomaturation” [46-50]. An additional diagnostic field for HMB-45 is the differential diagnosis between nodal nevi (NN) [HMB45(-)] and MM [HMB45(+)] in the pathological evaluation of SLNB [51]. Nevertheless, according to the literature data and also in our experience, p16 and PRAME [NN: p16(+) and PRAME (-); MM: p16(-) and PRAME (+)] have much more sensibility and specificity than HMB-45 in this specific diagnostic set [51,52].
Melan A/MART-1: Melan A/MART-1 is a melanoma-associated antigen recognized by autologous cytotoxic T lymphocytes, encoded by the MLANA gene located on chromosome 9p24.1 and involved in the formation and trafficking of melanosomes [53]. At present, several antibodies anti-MART-1 are commercially available, but the most commonly used in routine practice and for research purposes are the mouse monoclonal antibodies clone M2-7C10 and A103 (used in our laboratory) [54]. Like HMB-45, also MART- 1 shows a lower sensibility compared to S-100 and SOX-10 (85%- 97% in primary cM, 57%-86% in metastatic cM and only 0-7% in desmoplastic cM) and it is negative in the majority of tumors that could be immune-histologically be confounded with cM; as result, in our daily practice routine, we often use MART-1 alone and/or in combination with HMB-45 (and obviously with S-100 and SOX-10) in the above-mentioned diagnostic settings [49,50,55,56]. MART- 1 strongly reacts with the junctional, intraepidermal and also dermal melanocytes in both cN and cM and, we always performed it in conjunction with HMB-45 to evaluate the silhouette of the melanocytic lesion (symmetry/asymmetry), estimate the depth of invasion in cM, and assess the lympho-vascular invasion, the adnexal involvement and the peri-adnexal extension [46,49,50,54].
However, dermatopathologists should be aware that:
1) cN with neurotization and/or stromal metaplasia, congenital cN and hyper-maturating cN could completely lose or show a gradual diminishing of expression of MART-1;
2) MART-1 could be expressed by adrenal cortical tumors, PEComa and related tumors, mesotheliomas, salivary gland tumors and sex cord-stromal tumors (interestingly, some authors showed as these tumors do not produce MART-1 RNA and so concluded that this “apparently paradoxical” positivity is related to an immunologically cross-reaction with unrelated antigens) [45,50,54,57,58].
Because of its high sensitivity for melanocytic lesions, MART- 1 is a useful marker for the pathological evaluation of SLNB, to identify but not to differentiate, NN and MM [both MART-1(+)] [59,60]. Besides, MART-1 has the advantage (over S-100 and HMB-45) to not be expressed in histiocytes and dendritic cells and, as result, it is frequently used in association with the other immunohistochemical markers for the evaluation of SLNB [59,60].
Markers Useful for the Differential Diagnosis between cN and cM
Ki67: Ki67 is a protein associated with cell proliferation and encoded by the MKI67 gene located on chromosome 10q26.2 [61]. It is expressed during all active phases of the cell cycle (late G1, S, G2, and mitosis, but not in G0 and early G1) and it is a reliable tool to evaluate the growth fraction of a cell population [61]. At present, the antibody adopted in the vast majority of laboratories (and also in our) do detect Ki67 is the mouse monoclonal antibody, clone MIB1 (it is often used as a synonym of Ki67, sometimes creating linguistic confusion) [62,63]. Several authors showed as Ki67 shows significant differences between cN and cM [49,50,62-65]. Specifically, conventional, Spitz, congenital, blue and dysplastic cN exhibit positivity in about 1-3% of cells, usually disposed at the dermal-epidermal junction with no/scattered positive cells in the deep part of the lesion (“dermal hot-spot” with Ki67<5%) [62-65]. By contrast, cM shows a higher percentage of positive cells (>15%) and a different staining pattern, with clustered positive cells in the deeper part of the lesion (“dermal hot-spot” with Ki67>5%) and/or a random pattern of staining [62-65].
Although in 2018 WHO Classification of Skin Tumors, Ki67 is strongly recommended for the differential diagnosis between dysplastic cN (<5%) and superficial spreading cM (>30%), in our personal experience it is quite impossible to find “early” superficial spreading cM (those that raise more diagnostic problems with dysplastic cN) with a so high Ki67. Besides, the pathologists should be aware of several diagnostic pitfalls in the application of Ki67 to the diagnosis of melanocytic neoplasms; namely, cN with a high Ki67 index (recurrent/persistent cN, traumatized cN, proliferative nodules in congenital cN, etc.), cM that could display a Ki67 similar to that of cN (especially nevoid cM), and cN for which it is difficult to evaluate Ki67 only in the melanocytic component (cN with a high inflammatory component as halo cN, Meyerson cN, regressed cN) [62-67]. To reduce these pitfalls, several authors elaborated “combined scoring systems” (integrating Ki67 with other markers to obtain a predictive score) and/or DS (Chapters 2.2.4) that allow evaluating Ki67 only in the melanocytic component [68,69]. In our laboratory, we adopt DS (MART-1/Ki67 and HMB-45/Ki67) and we found that more than the absolute value of Ki67, should be taken into account:
1) unusual, deep and/or asymmetrical staining pattern of the dermal component;
2) Ki67(+) deep dermal cells with pleomorphism atypical nuclei;
3) Ki67(+) intraepithelial cells exhibiting pagetoid spreading (personal observation, data unpublished).
p16, p21 and p53: p16/INK4a (p16), p21/WAF-1 (p21) and p53 are all proteins involved in the regulation of the cell cycle and encoded by CDKN2A, CDKN1A and TP53 genes, located on chromosomes 9p21.3, 6p21.2, and 17p13.1, respectively [70,71]. p16 and p21 belong to the CIP/KIP family of kinase inhibitors and play a critical role in cell cycle progression and senescence, mainly cooperating with Rb (“p16/Rb pathway”) and p53 (“p53/ p21 pathway”); p53 is a master regulator of the cell cycle, apoptosis and genomic stability through several mechanisms (activation of DNA repair proteins, arrest of the cell cycle at the G1/S, initiation of the apoptosis and senescence response to short telomeres) [70,71]. Several antibody clones (E6H4, JC8 and G175-405) have been developed for the detection of p16 but the most commonly used in surgical pathology laboratories (and in our laboratory), is the mouse monoclonal E6H4 [49,50,72,73]. p16 attracted great interest in the field of melanocytic pathology since it has been shown that the biallelic/homozygotic inactivation of CDKN2A gene and the corresponding loss of immunohistochemical expression is a molecular step able to distinguish cM [p16 (-)] from cN [p16(+)] [72-75]. Numerous studies showed that almost cN stain (61%- 100%) for p16 with a typical “mosaic/puzzle” staining; by contrast, only 12-80% of cM are p16(+) [72-75].
Nevertheless, the major limits of these studies are the criteria used to define p16 positivity (nuclear, cytoplasmatic, or both; percentage of positivity; the pattern of staining) and the differences between the cohorts (different histotypes of cN and cM, different stages of cM, primary VS metastatic cM; etc.) [72-78]. It is well known that specific histotypes of cN and cM preferentially show loss of p16, due to the relevance of the biallelic inactivation of CDKN2A for their oncogenesis process [78]. Besides, CDKN2A biallelic inactivation is recognized to be a late molecular step in in the oncogenesis of cM, mainly involved in the advanced/metastatic stages (the percentage of metastatic cM p16(+) ranges between 0% and 41%); as result, p16 is not useful for the differential diagnosis of superficial lesions (superficial spreading cM and dysplastic cN), which represent the majority of routine diagnostic dilemmas [77,78]. In our experience and in line with most of the literature data, the diagnostic scenarios in which p16 is mainly useful are the followings: 1) the evaluation of dermal and/or nodular atypical melanocytic lesions/ melanocytomas (atypical Spitz tumor, atypical cellular blue tumor, atypical proliferative nodule arising in congenital cN), where p16 loss reflects the biallelic inactivation of CDKN2A (also proved by molecular techniques) and represents a strong criterion of malignancy; 2) the identification of a more aggressive phenotype acquired by the primary cM, as p16 loss is characteristic of the advanced/metastatic cM; 3) differential diagnosis between NN and MM in the evaluation of SLNB [26,27,51,52,72-79].
Although some studies showed that PRAME is superior to p16 to discriminate NN from MM, in our experience p16 remains a reliable diagnostic tool in this diagnostic setting [51,52,78,79]. Interestingly, we found very exceptional cases of cM that show a “paradoxical” diffuse and/or clonal overexpression of p16, representing a potential diagnostic pitfall and reflecting complex cell cycle deregulation that results in the intracellular accumulation of p16 protein [80]. p21 protein exhibits an opposite pattern compared to p16, with over-expression observed in cM and noor hypo-expression in cN [81,82]. However, this molecule and the underlying molecular mechanisms are less known compared to p16 and the immunohistochemistry for p21 is not frequently adopted in routine practice but mainly for research purposes [81,82]. At present, we use p21 (clone 4D10, mouse monoclonal) in our daily routine as an additional diagnostic tool only in selected scenarios for which the literature data are more substantial, such as Spitz lesions (especially in acral sites) and mucosal melanocytic lesions [81-85]. Although the alterations of the TP53 pathway are very frequent in cM, from a molecular point of view these could underlie numerous genetic, epigenetic and post-translational alterations, whose effects on the protein production (and therefore on our capability to immunohistochemically detect it) are very complex to predict [6,49,50,86,87].
Furthermore, the alterations of the TP53 pathway are a late event in the carcinogenesis of cM (therefore not so useful in the routine practice for the diagnosis of the most problematic cases) and rarely could also affect cN and melanocytic lesions with unpredictable biological potential [6,49,50,73,80,86-88]. At present, we use p53 (clone DO-7, mouse monoclonal) in our daily routine as an additional diagnostic tool only in the context of desmoplastic melanoma, especially for the differential diagnosis between neurofibroma-like desmoplastic cM and neurofibroma [89].
PRAME: PRAME (PReferentially expressed Antigen in MElanoma) is a tumor-associated antigen identified through T-cell clones obtained from a patient with metastatic CM and encoded by the PRAME gene located on chromosome 21q11.22 [90]. PRAME is expressed in several normal tissues and tumors, with a large variety of functions in oncogenesis, immune response, apoptosis and metastases [91-94]. It became of great interest in the field of melanocytic tumors as it proved to be expressed (and so immunohistochemically detectable) in cM but not in cN, so potentially being the marker able to solve one of the most problematic issues of the surgical pathology [95]. Over the last years, several antibodies against PRAME have been developed, but the most commonly used in routine practice and for the evaluation of melanocytic tumors is the rabbit monoclonal, clone EPR20330 [95]. Lezcano et al. developed a score based on tumor cells with nuclear stain (0: 0%, 1+: 1-25%, 2+: 26-50%, 3+: 51-75%, 4+: ≥ 76%) and showed that it has a high sensibility and specificity in distinguishing cM and cN (4+: 87% of metastatic cM, 83.2% of primary cM, 93.8% of in situ cM, 94.4% of acral cM, 92.5% of superficial spreading cM, 90% of nodular cM, 88.6% of lentigo maligna melanomas, 35% of desmoplastic cM and only 1 case of Spitz cN; 0-1%: 86.4% of all cN, 100% of NN, 100% of solar lentigo) [95]. The same authors found a 90% of concordance between PRAME score and cytogenetic tests results, supporting this marker as an important ancillary test (cheaper and faster but not completely interchangeable with cytogenetic tests) for the diagnosis of complex melanocytic lesions [96].
Subsequently, other authors tested this antibody in the most problematic areas of the melanocytic pathology (atypical Spitz tumors, pauci-cellular lentigo maligna, nevus-associated cM, resections margins of lentigo maligna, NN and MM, etc.) and found very promising results; however, they adopted different cut-offs and raised the problem to correctly identify the exact percentage of positive cells able to differentiate cN from cM and whether different percentages need to be adopted for different melanocytic lesions [97-102]. Besides, these results need to be validated in large case series with long-term follow-up able to prove the real nature of ambiguous melanocytic tumors and many other aspects have to be clarified before the adoption of this marker as the “answer to all our problems” (how to interpret “intermediate” results? How to interpret PRAME results in cases of a discordant molecular test?). Besides, PRAME is expressed in many other tumors (germ cell tumors of the testis, lymphomas, peripheral nerve sheath tumors, ovarian carcinomas, etc.) but not in the majority of desmoplastic cM (one of the most challenging melanocytic lesions), and we already suggested great caution before the adoption of PRAME as “panmelanoma” marker [91-95,103]. We recommend using PRAME in conjunction and/or with DS adopting a melanocytic marker (HMB- 45 or MART-1), only in appropriately selected diagnostic settings, and integrating this result with the histologic exam, the other immunohistochemical analyses and the molecular techniques in “really-difficult-to-diagnose” melanocytic lesions. In our practice, we adopt this marker as an adjunctive diagnostic tool especially for
1) Ambiguous melanocytic lesions (atypical Spitz tumors VS Spitz cM, high-grade dysplastic cN VS early cM in situ, etc.);
2) Differential diagnosis between NN and MM in selected difficult cases;
3) More accurate evaluation of surgical resection margins in lentigo maligna;
4) Distinction between the dermal “nevoid” component of nevoid cM and dermal cN in nevus-associated cM.
Markers Useful for The Identification of Specific Histological Subtypes of cN and cM (BRAF V600E, c-Kit/CD117, ROS1, ALK, pan-TRK, BAP-1, β-catenin, PRKAR1A, NF1, IDH1): Over the last years, the growing research in the field of molecular biology made it possible to identify as specific clinical-pathological entities are characterized by specific molecular alterations and 2018 WHO classification of melanocytic lesions is mainly based on their molecular background and its correlation with the entity of UVdamage. As result, the search of these genetic alterations has become fundamental to identify and characterize these new histological entities, thus allowing a more detailed diagnosis and prognostictherapeutic stratification (many of these molecular alterations identify potentially targetable therapeutic targets). Since these genetic alterations lead to an over- and/or aberrant expression of specific molecules and these latter are associated with welldefined histological features of the melanocytic lesion, an expert dermatopathologist could suspect a specific genetic alteration just from the H&E exam and prove it with the immunohistochemistry [104-117]. In our routine practice, we do not use standard panels but the choice of the immunohistochemical panels is performed case-by-case based on the H&E exam. Specifically, the antibodies we use in our laboratory and the specific histological entities related to their over and/or aberrant expression are the following:
- BRAF V600E: melanocytic lesions in intermittently sunexposed skin (superficial spreading cM, simple lentigo, conventional and/or lentiginous cN, dysplastic cN), deep-penetrating cN, BAP1-inactivated melanocytic lesions, pigmented epithelioid melanocytoma (PEM), acral melanocytic lesions (especially cM), nodular cM (less frequent), nevoid cM (less frequent) • c-Kit/CD117: acral melanocytic lesions (especially cM), lentigo maligna
• ALK, ROS1, pan-TRK (NTRK1, NTRK2, NTRK3), RET, MET: Spitz lesions (also Reed cN), acral melanocytic lesions (especially cM)
• β-catenin: deep-penetrating cN
• PRKAR1A: PEM
• BAP-1: BAP1-inactivated melanocytic lesions, cM arising in blue cN and atypical cellular blue tumor (rare cases)
• NF1: lentigo maligna, desmoplastic cM, acral melanocytic lesions (especially cM)
• IDH1: recently introduced category of melanocytoma
Double Stains (DS)
(HMB-45/Ki67, MART-1/Ki67, CD34/SOX10, HMB-45/ PRAME, MART-1/PRAME): Over the last years, the development and application of DS have greatly increased in surgical pathology, due to the more detailed assessment of specific histopathological features (compared to the respective single stains) and the saving of time, money and histological material [118]. Specifically, in the field of melanocytic pathology, the most commonly used DS are those combining Ki67 with cytoplasmic melanocytic markers (HMB-45 and MART-1), thus allowing to more correctly assess the proliferation index only in the melanocytes (ignoring lymphocytes, keratinocytes and endothelial cells) [119]. In our experience, these DS (HMB-45/Ki67 and MART-1/Ki67) are particularly useful in lesions almost exclusively junctional/intraepithelial and in lesions with a high inflammatory infiltrate (halo cN, highly regressed cM, etc.). Other promising DS are those that allow to correctly evaluate the presence of lympho-vascular invasion (D2-40/MITF, D2-40/ SOX10, D2-40/S-100, D2-40/MART-1), even if the obtained results and the superiority compared to single stains and H&E are partially discordant [120-122]. We are currently leading a study aimed to evaluate the accuracy of the DS CD34/SOX10 (“pan-vascular marker” and “pan-melanocytic” marker) to identify the lymphovascular invasion and predict survival compared to H&E [123]. As just clarified (Chapter 2.2.2.3), our working group has recently developed two DS combining PRAME (nuclear) with HMB-45 and MART-1 (cytoplasmatic) that showed very encouraging results and become part of the immunohistochemical panels used routinely in our laboratory [123]. In our experience, these DS (HMB-45/PRAME and MART-1/PRAME) are particularly useful in the following diagnostic scenarios:
a. Lesions almost exclusively junctional/intraepithelial (allowing not to evaluate keratinocytes)
b. Lesions with a high inflammatory infiltrate (allowing not to evaluate lymphocytes)
c. Differential diagnosis between NN and MM, especially in SLNB
d. Metastasis of unknown primary tumor and/or primary
cutaneous tumor with undifferentiated morphology, especially with limited available histological material.
Conclusion
Here we summarize the current concepts and advances on the application of immunohistochemistry in the diagnosis of cN and cM. Despite continuous progress in the genetic classification of melanocytic lesions, there is still a need for improvements in the correct immunohistochemical characterization and diagnosis of this deadly disease. Hopefully, this diagnostic progress could result in the improvement of the therapeutic choices and the reduction of mortality and morbidity by cM.
Author Contributions
Conceptualization, C.R. and B.C.; writing-original draft preparation, C.R., B.C., F.A., M.L., E.D.; writing-review and editing, C.R., B.C., F.A., M.L., E.D.; visualization, C.R., F.A.; supervision, B.C., E.D.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Please refer to suggested Data Availability Statements in section “MDPI Research Data Policies” at https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgment
No acknowledgments for this study.
References
- Regad T (2013) Molecular and cellular pathogenesis of melanoma initiation and progression. Cell Mol Life Sci 70(21): 4055-4065.
- Strashilov S, Yordanov A (2021) Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int J Mol Sci 22(12): 6395.
- Karimkhani C, Green A, Nijsten T, Weinstock M, Dellavalle R, et al. (2017) The global burden of melanoma: Results from the Global Burden of Disease Study 2015. Br J Dermatol 177(1): 134-140.
- Carr S, Smith C, Wernberg J (2020) Epidemiology and Risk Factors of Melanoma. Surg Clin North Am 100(1): 1-12.
- Massi G, LeBoit PE (2014) Histological Diagnosis of Nevi and Melanoma. Berlin, Germany: Steinfopgg Verlag Darmstadt.
- Elder DE, Massi D, Scolyer RA, Willemze R (2018) World Health Organization Classification of Skin Tumours. Lyon, France: IARC Press.
- Elder D E, Bastian BC, Cree IA, Massi D, Scolyer RA, et al. (2020) The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch Pathol Lab Med 144(4): 500-522.
- Cabrera R, Recule F (2018) Unusual Clinical Presentations of Malignant Melanoma: A Review of Clinical and Histologic Features with Special Emphasis on Dermatoscopic Findings. Am J Clin Dermatol 19(1): 15-23.
- Zarabi SK, Azzato EM, Tu ZJ, Ni Y, Billings SD, et al. (2020) Targeted next generation sequencing (NGS) to classify melanocytic neoplasms. J Cutan Pathol 47(8): 691-704.
- Gaiser T, Kutzner H, Palmedo G, Siegelin, Wiesner T, et al. (2010) Classifying ambiguous melanocytic lesions with FISH and correlation with clinical long-term follow up. Mod Pathol 23(3): 413-419.
- Isobe T, Okuyama T (1978) The amino-acid sequence of S-100 protein (PAP I-b protein) and its relation to the calcium-binding proteins. Eur J Biochem 89(2): 379-388.
- Isobe T, Okuyama T (1981) The amino-acid sequence of the alpha subunit in bovine brain S-100a protein. Eur J Biochem 116(1): 79-86.
- Heizmann CW, Fritz G Schäfer BW (2002) S100 proteins: structure, functions and pathology. Front Biosci 7: d1356-d1368.
- Zimmer DB, Wright Sadosky P, Weber DJ (2003) Molecular mechanisms of S100-target protein interactions. Microsc Res Tech 60(6): 552-559.
- Heizmann CW (2004) S100B protein in clinical diagnostics: assay specificity Clin Chem 50(1): 249-251.
- Tímár J, Udvarhelyi N, Bánfalvi T, Gilde K, Orosz Z, et al. (2004) Accuracy of the determination of S100B protein expression in malignant melanoma using polyclonal or monoclonal antibodies. Histopathology 44(2): 180-184.
- Gaynor R, Herschman HR, Irie R, Jones P, Morton D, et al. (1981) S100 protein: a marker for human malignant melanomas? Lancet 1(8225): 869-871.
- Palazzo J, Duray PH (1989) Typical, dysplastic, congenital, and Spitz nevi: a comparative immunohistochemical study. Hum Pathol 20(4): 341-346.
- Yaziji H, Gown AM (2003) Immunohistochemical markers of melanocytic tumors. Int J Surg Pathol 11(1): 11-15.
- Busam KJ, Iversen K, Coplan KC, Jungbluth AA (2001) Analysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanoma. Am J Surg Pathol 25(2): 197-204.
- Nonaka D, Chiriboga L, Rubin BP (2008) Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol 35(11): 1014-1019.
- Coindre JM, de Mascarel A, Trojani M, de Mascarel I, Pages A, et al. (1988) Immunohistochemical study of rhabdomyosarcoma. Unexpected staining with S100 protein and cytokeratin. J Pathol 155(2): 127-132.
- Redd L, Schmelz M, Burack WR, Cook JR, Day AW, et al. (2016) Langerhans Cell Histiocytosis Shows Distinct Cytoplasmic Expression of Major Histocompatibility Class II Antigens. J Hematop 9(3): 107-112.
- Meyer MT, Watermann C, Dreyer T, Ergün S, Karnati S, et al. (2021) Update on Diagnostic Markers and Translocation in Salivary Gland Tumors. Int J Mol Sci 22(13): 6771.
- Chen H, Xu C, Jin O, Liu Z (2014) S100 protein family in human cancer. Am J Cancer Res 4(2): 89-115.
- Cook MG, Massi D, Szumera-Ciećkiewicz A, Joost Van den Oord J, Blokx W, et al. (2019) An updated European Organisation for Research and Treatment of Cancer (EORTC) protocol for pathological evaluation of sentinel lymph nodes for melanoma. Eur J Cancer 114: 1-7.
- Szumera-Ciećkiewicz A, Bosisio F, Teterycz P, Antoranz A, Delogu F, et al. (2020) SOX10 is as specific as S100 protein in detecting metastases of melanoma in lymph nodes and is recommended for sentinel lymph node assessment. Eur J Cancer 137: 175-182.
- Potterf SB, Mollaaghababa R, Hou L, Southard-Smith EM, Hornyak TJ, et al. (2001) Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev Biol 237(2): 245-257.
- Kelsh RN (2006) Sorting out Sox10 functions in neural crest development. Bioessays 28(8): 788-798.
- Agnarsdóttir M, Sooman L, Bolander A, Strömberg S, Rexhepaj E, et al. (2010) SOX10 expression in superficial spreading and nodular malignant melanomas. Melanoma. Res 20(6): 468-478.
- Jeonghyun S, Vincent JG, Cuda JD, Xu H, Kang S, et al. (2012) Sox10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytosis. J Am Acad Dermatol 67(4): 717-726.
- Nonaka D, Chiriboga L, Rubin BP (2008) Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 32(9): 1291-1298.
- Jennings C, Kim J (2011) Identification of nodal metastases in melanoma using sox-10. Am J Dermatopathol 33(5): 474-482.
- Karamchandani JR, Nielsen TO van de Rijn M, West RB (2012) Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol 20(5): 445-450.
- Lee JH, Kang HJ, Yoo CW, Park WS, Ryu JS, et al. (2019) PLAG1, SOX10, and Myb Expression in Benign and Malignant Salivary Gland Neoplasms. J. Pathol. Transl Med 53(1): 23-30.
- Ramos-Herberth FI, Karamchandani J, Kim J, Dadras SS (2010) SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar. J Cutan Pathol 37(9): 944-952.
- Gown AM, Vogel AM, Hoak D, Gough F, McNutt MA, et al. (1986) Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol 123(2): 195-203.
- Bacchi CE, Bonetti F, Pea M, Martignoni G, Gown AM, et al. (1996) HMB-45: a review. Appl Immunohistochem 4: 73-85.
- Orchard GE (2000) Comparison of immunohistochemical labelling of melanocyte differentiation antibodies melan-A, tyrosinase and HMB 45 with NKIC3 and S100 protein in the evaluation of benign naevi and malignant melanoma. Histochem J 32(8): 475-481.
- Skelton HG, Smith KJ, Barrett TL, Lupton GP, Graham JH, et al. (1991) HMB-45 staining in benign and malignant melanocytic lesions. A reflection of cellular activation. Am J Dermatopathol 13(6): 543-550.
- El-Naggar AK, Ordóñez NG, Sara A, McLemore D, Batsakis JG, et al. (1991) Clear cell sarcomas and metastatic soft tissue melanomas. A flow cytometric comparison and prognostic implications. Cancer 67(8): 2173-2179.
- Tazelaar HD, Batts KP, Srigley JR (2001) Primary extrapulmonary sugar tumor (PEST): a report of four cases. Mod Pathol 14: 615-622.
- Vang R, Kempson RL (2002) Perivascular epithelioid cell tumor (‘PEComa’) of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol 26(1): 1-13.
- Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, et al. (2005) Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 29(12): 1558-1575.
- Ricci C, Chiarucci F, Ambrosi F, Balbi T, Barbara Corti, et al. (2021) Co-expression of Myoepithelial and Melanocytic Features in Carcinoma Ex Pleomorphic Adenoma. Head Neck Pathol 15(4): 1385-1390.
- Dean NR, Brennan J, Haynes J, Goddard C, Cooter RD, et al. (2002) Immunohistochemical labeling of normal melanocytes. Appl Immunohistochem Mol Morphol 10(3): 199-204.
- Smoller BR, McNutt NS, Hsu A (1989) HMB-45 staining of dysplastic nevi. Support for a spectrum of progression toward melanoma. Am J Surg Pathol 13(8): 680-684.
- Magro CM, Crowson AN, Mihm MC (2006) Unusual variants of malignant melanoma. Mod Pathol 2: S41-70.
- Prieto VG, Shea CR (2008) Use of immunohistochemistry in melanocytic lesions. J Cutan Pathol 35: 1-10.
- Prieto VG, Shea CR (2011) Immunohistochemistry of melanocytic proliferations. Arch Pathol Lab Med 135(7): 853-859.
- Lezcano C, Pulitzer M, Moy AP, Hollmann TJ, Jungbluth AA, et al. (2020) Immunohistochemistry for PRAME in the Distinction of Nodal Nevi from Metastatic Melanoma. Am J Surg Pathol 44(4): 503-508.
- See SHC, Finkelman BS, Yeldandi AV (2020) The diagnostic utility of PRAME and p16 in distinguishing nodal nevi from nodal metastatic melanoma. Pathol Res Pract 216(9): 153105.
- Fetsch PA, Marincola FM, Filie A, Hijazi YM, Kleiner DE, et al. (1999) Melanoma-associated antigen recognized by T cells (MART-1): the advent of a preferred immunocytochemical antibody for the diagnosis of metastatic malignant melanoma with fine-needle aspiration. Cancer 87(1): 37-42.
- Bergman R, Azzam H, Sprecher E, Manov L, Munichor M, et al. (2000) Friedman-Birnbaum, R.; Ben-Itzhak, O. A comparative immunohistochemical study of MART-1 expression in Spitz nevi, ordinary melanocytic nevi, and malignant melanomas. J Am Acad Dermatol 42(3): 496-500.
- Sundram U, Harvell JD, Rouse RV, Natkunam Y (2003) Expression of the B-cell proliferation marker MUM1 by melanocytic lesions and comparison with S100, gp100 (HMB45), and MelanA. Mod Pathol 16(8): 802-810.
- Xu X, Chu AY, Pasha TL, Elder DE, Zhang PJ (2002) Immunoprofile of MITF, tyrosinase, melan-A, and MAGE-1 in HMB45-negative melanomas. Am J Surg Pathol 26(1): 82-87.
- Zhao C, Vinh TN, McManus K, Dabbs D, Barner R, et al. (2009) Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. Am J Surg Pathol 33(3): 354-366.
- Loy TS, Phillips RW, Linder CL (2002) A103 immunostaining in the diagnosis of adrenal cortical tumors: an immunohistochemical study of 316 cases. Arch Pathol Lab Med 126(2): 170-172.
- Shidham VB, Qi DY, Acker S, Kampalath B, Chang CC, et al. (2001) Evaluation of micrometastases in sentinel lymph nodes of cutaneous melanoma: higher diagnostic accuracy with Melan-A and MART-1 compared with S-100 protein and HMB-45. Am J Surg Pathol 25(8): 1039-1046.
- Shidham VB, Qi D, Rao RN, Acker SM, Chang CC, et al. (2003) Improved immunohistochemical evaluation of micrometastases in sentinel lymph nodes of cutaneous melanoma with 'MCW melanoma cocktail'--a mixture of monoclonal antibodies to MART-1, Melan-A, and tyrosinase. BMC Cancer 3: 15.
- Uxa S, Castillo-Binder P, Kohler R, Stangner K, Müller GA, et al. (2021) Ki-67 gene expression. Cell Death. Differ 28: 3357-3370.
- Talve LA, Collan YU, Ekfors TO (1996) Nuclear morphometry, immunohistochemical staining with Ki-67 antibody and mitotic index in the assessment of proliferative activity and prognosis of primary malignant melanomas of the skin. J Cutan Pathol 23(4): 335-343.
- Niemann TH, Argenyi ZB (1993) Immunohistochemical study of Spitz nevi and malignant melanoma with use of antibody to proliferating cell nuclear antigen. Am J Dermatopathol 15(5): 441-445.
- Tu P, Miyauchi S, Miki Y (1993) Age-related proliferative activity in dermal melanocytic naevi detected by PCNA ⁄ cyclin expression. Br J Dermatol 129(1): 65-68.
- Sparrow LE, English DR, Taran JM, Heenan PJ (1998) Prognostic significance of MIB-1 proliferative activity in thin melanomas and immunohistochemical analysis of MIB-1 proliferative activity in melanocytic tumors. Am J Dermatopathol 20(1): 12-16.
- Bergman R, Malkin L, Sabo E, Kerner H (2001) MIB-1 monoclonal antibody to determine proliferative activity of Ki-67 antigen as an adjunct to the histopathologic differential diagnosis of Spitz nevi. J Am Acad Dermatol 44(3): 500-504.
- Nguyen TL, Theos A, Kelly DR, Busam K, Andea AA, et al. (2013) Mitotically active proliferative nodule arising in a giant congenital melanocytic nevus: a diagnostic pitfall. Am J Dermatopathol 35(1): e16-21.
- Uguen A, Talagas M, Costa S, Duigou S, Bouvier S, et al. (2015) A p16-Ki-67-HMB45 immunohistochemistry scoring system as an ancillary diagnostic tool in the diagnosis of melanoma. Diagn Pathol 10: 195.
- Nielsen PS, Riber-Hansen R, Steiniche T (2011) Immunohistochemical double stains against Ki67/MART1 and HMB45/MITF: promising diagnostic tools in melanocytic lesions. Am J Dermatopathol 33(4): 361-370.
- Terzi MY, Izmirli M, Gogebakan B (2016) The cell fate: senescence or quiescence. Mol Biol Rep 43(11): 1213-1220.
- Al-Khalaf HH, Nallar SC, Kalvakolanu DV, Aboussekhra A (2017) p16(INK4A) enhances the transcriptional and the apoptotic functions of p53 through DNA-dependent interaction. Mol Carcinog 56(7): 1687-1702.
- Reed JA, Loganzo F Jr, Shea CR, Walker GJ, Flores JF, et al. (1995) Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res 55(13): 2713-2718.
- Al Dhaybi R, Agoumi M, Gagne I, McCuaig C, Powell J, et al. (2011) p16 expression: a marker of differentiation between childhood malignant melanomas and Spitz nevi. J Am Acad Dermatol 65(2): 357-363.
- George E, Polissar NL, Wick M (2010) Immunohistochemical evaluation of p16INK4A, E-cadherin, and cyclin D1 expression in melanoma and Spitz tumors. Am J Clin Pathol 133(3): 370-379.
- Bartkova J, Lukas J, Guldberg P, Alsner J, Kirkin AF, et al. (1996) The p16-cyclin D ⁄ Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res 56(23): 5475-5483.
- Scurr LL, McKenzie HA, Becker TM, Irvine M, Lai K, et al. (2011) Selective loss of wild type p16(INK4a) expression in human nevi. J Invest Dermatol 131(11): 2329-2332.
- Straume O, Sviland L, Akslen L (2000) Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and prognosis in patients with vertical growth phase melanoma. Clin. Cancer Res 6(5): 1845-1853.
- Alonso SR, Ortiz P, Pollan M, Pérez-Gómez B, Sánchez L, et al. (2004) Progression in cutaneous malignant melanoma is associated with distinct expression profiles. Am J Pathol 164(1): 193-203.
- Mihic-Probst D, Saremaslani P, Komminoth P, Heitz PU (2003) Immunostaining for the tumour suppressor gene p16 product is a useful marker to differentiate melanoma metastasis from lymph-node nevus. Virchows Arch 443(6): 745-751.
- Ricci C, Ambrosi F, Grillini M, Serra M, Melotti B, et al. (2020) Next-generation sequencing revealing TP53 mutation as potential genetic driver in dermal deep-seated melanoma arising in giant congenital nevus in adult patients: A unique case report and review of the literature. J Cutan Pathol 47(12): 1164-1169.
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, et al. (1993) p21 is a universal inhibitor of cyclin kinases. Nature 366(6456): 701-704.
- Karjalainen JM, Eskelinen MJ, Kellokoski JK, Reinikainen M, Alhava EM, et al. (1999) P21WAF1/CIP1 expression in stage I cutaneous malignant melanoma: its relationship with p53, cell proliferation and survival. Br J Cancer 79(5-6): 895-902.
- Wiedemeyer K, Guadagno A, Davey J, Thomas Brenn T (2018) Acral Spitz Nevi: A Clinicopathologic Study of 50 Cases with Immunohistochemical Analysis of P16 and P21 Expression. Am J Surg Pathol 42(6): 821-827.
- De Andrade BAB, León JE, Carlos R, Delgado-Azañero W, Mosqueda-Taylor A, et al. (2012) Immunohistochemical Expression of p16, p21, p27 and Cyclin D1 in Oral Nevi and Melanoma. Head Neck Pathol 6(3): 297-304.
- Dika E, Lambertini M, Pellegrini C, Veronesi G, Melotti B, et al. (2021) Cutaneous and Mucosal Melanomas of Uncommon Sites: Where Do We Stand Now? J Clin Med 10(3): 478.
- Soto JS, Cabrera CM, Serrano S, López-Nevot MA (2005) Mutation analysis of genes that control the G1/S cell cycle in melanoma: TP53, CDKN1A, CDKN2A, and CDKN2B. BMC Cancer 5: 36.
- Ragnarsson-Olding B, Platz A, Olding L, Ringborg U (2004) p53 protein expression and TP53 mutations in malignant melanomas of sun-sheltered mucosal membranes versus chronically sun-exposed skin. Melanoma. Res 14(5): 395-401.
- Levin DB, Wilson K, Valadares de Amorim G, Webber J, Kenny P, et al. (1995) Detection of p53 mutations in benign and dysplastic nevi. Cancer Res 55(19): 4278-4282.
- Gerami P, Kim D, Zhang B, Compres EV, Khan AU, et al. (2020) Desmoplastic Melanomas Mimicking Neurofibromas. Am J Dermatopathol 42(12): 916-922.
- Ikeda H, Lethe B, Lehmann F, Van Baren N, Baurain JF, et al. (1997) Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 6(2): 199-208.
- Hemminger JA, Toland AE, Scharschmidt TJ, Mayerson JL, Guttridge DC, et al. (2014) Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod Pathol 27(9): 1238-1245.
- Ricci C, Franceschini T, Giunchi F, Grillini M, Ambrosi F, et al. (2021) Immunohistochemical Expression of Preferentially Expressed Antigen in Melanoma (PRAME) in the Uninvolved Background Testis, Germ Cell Neoplasia in Situ, and Germ Cell Tumors of the Testis. Am J Clin Pathol.
- Steger B, Floro L, Amberger DC, Kroell T, Tischer J, et al. (2020) WT1, PRAME, and PR3 mRNA Expression in Acute Myeloid Leukemia (AML). J Immunother 43(6): 204-215.
- Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini Bereshneh A, et al. (2016) Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers. Immunol Invest 45(7): 619-640.
- Lezcano C, Jungbluth AA, Nehal KS, Hollmann TJ, Busam KJ, et al. (2018) PRAME Expression in Melanocytic Tumors. Am J Surg Pathol 42(11): 1456-1465.
- Lezcano C, Jungbluth AA, Busam KJ (2020) Comparison of Immunohistochemistry for PRAME With Cytogenetic Test Results in the Evaluation of Challenging Melanocytic Tumors. Am J Surg Pathol 44(7): 893-900.
- Raghavan SS, Wang JY, Kwok S, Rieger KE, Novoa RA, et al. (2020) PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol 47(12): 1123-1131.
- Gradecki SE, Slingluff CLJr, Gru AA (2021) PRAME expression in 155 cases of metastatic melanoma. J Cutan Pathol 48(4): 479-485.
- Ruby KN, Li Z, Yan S (2021) Aberrant expression of HMB45 and negative PRAME expression in halo nevi. J Cutan Pathol 48(4): 519-525.
- Lezcano C, Pulitzer M, Moy AP, Hollmann TJ, Jungbluth AA, et al. (2020) Immunohistochemistry for PRAME in the Distinction of Nodal Nevi from Metastatic Melanoma. Am J Surg Pathol 44(4): 503-508.
- Gradecki SE, Valdes-Rodriguez R, Wick MR, Gru AA (2021) PRAME immunohistochemistry as an adjunct for diagnosis and histological margin assessment in lentigo maligna. Histopathology 78(7): 1000-1008.
- Lohman ME, Steen AJ, Grekin RC, North JP (2021) The utility of PRAME staining in identifying malignant transformation of melanocytic nevi. J Cutan Pathol 48(7): 856-862.
- Grillini M, Ricci C, Pino V, Pedrini S, Fiorentino M, et al. (2022) HMB45/PRAME, a Novel Double Staining for the Diagnosis of Melanocytic Neoplasms: Technical Aspects, Results, and Comparison with Other Commercially Available Staining (PRAME and Melan A/PRAME). Appl Immunohistochem. Mol Morphol 30(1): 14-18.
- Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, et al. (2017) Whole-genome landscapes of major melanoma subtypes. Nature 545: 175-180.
- Mehnert JM, Kluger HM (2012) Driver Mutations in Melanoma: Lessons Learned from Bench-to-Bedside Studies. Curr Oncol Rep 14(5): 449-457.
- Greenwald HS, Friedman EB, Osman I (2012) Superficial spreading and nodular melanoma are distinct biological entities: a challenge to the linear progression model. Melanoma Res 22(1): 1-8.
- Dika E, Veronesi G, Altimari A, Riefolo M, Ravaioli GM, et al. (2020) BRAF, KIT, and NRAS Mutations of Acral Melanoma in White Patients. Am J Clin Pathol 153(5): 664-671.
- Moon KR, Choi YD, Kim JM, Jin S, Shin MH, et al. (2018) Genetic Alterations in Primary Acral Melanoma and Acral Melanocytic Nevus in Korea: Common Mutated Genes Show Distinct Cytomorphological Features. J Invest Dermatol 138(4): 933-945.
- Wiesner T, Kutzner H, Cerroni L, Mihm MCJr, Busam KJ, et al. (2016) Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy. Pathology 48(2): 113-131.
- Kervarrec T, Pissaloux D, Tirode F, Samimi M, Jacquemus J, et al. (2021) Morphologic features in a series of 352 Spitz melanocytic proliferations help predict their oncogenic drivers. Virchows Arch.
- Yeh I, de la Fouchardiere A, Pissaloux D, Mully TW, Garrido MC, et al. (2015) Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am J Surg Pathol 39(5): 581-591.
- Cappellesso R, Nozzoli F, Zito Marino F, Simi S, Castiglione F, et al. (2021) NTRK Gene Fusion Detection in Atypical Spitz Tumors. Int J Mol Sci 22(22): 12332.
- Yeh I, Lang UE, Durieux E, Tee MK, Jorapur A, et al. (2017) Combined activation of MAP kinase pathway and β-catenin signaling cause deep penetrating nevi. Nat Commun 8(1): 644.
- Llamas-Velasco M, Pérez-Gónzalez YC, Requena L, Kutzner H (2014) Histopathologic clues for the diagnosis of Wiesner nevus. J Am Acad Dermatol 70(3): 549-554.
- Cohen JC, Joseph NM, North JP, Onodera C, Zembowicz A, et al. (2017) Genomic Analysis of Pigmented Epithelioid Melanocytomas Reveals Recurrent Alterations in PRKAR1A, and PRKCA Genes. Am J Surg Pathol 41(10): 1333-1346.
- Kadokura A, Frydenlund N, Leone DA, Yang S, Hoang MP, et al. (2016) Neurofibromin protein loss in desmoplastic melanoma subtypes: implicating NF1 allelic loss as a distinct genetic driver? Hum Pathol 53: 82-90.
- Macagno N, Pissaloux D, Etchevers H, Haddad V, Vergier B, et al. (2020) Cutaneous Melanocytic Tumors with Concomitant NRASQ61R and IDH1R132C Mutations: A Report of 6 Cases. Am J Surg Pathol 44(10): 1398-1405.
- Idikio HA (2010) Immunohistochemistry in diagnostic surgical pathology: contributions of protein life cycle, use of evidence-based methods and data normalization on interpretation of immunohistochemical stains. Int J Clin Exp Pathol 3(2): 169-176.
- Nielsen PS, Riber-Hansen R, Steiniche T (2011) Immunohistochemical double stains against Ki67/MART1 and HMB45/MITF: promising diagnostic tools in melanocytic lesions. Am J Dermatopathol 33(4): 361-370.
- Petitt M, Allison A, Shimoni T, Uchida T, Raimer S, et al. (2009) Lymphatic invasion detected by D2-40/S-100 dual immunohistochemistry does not predict sentinel lymph node status in melanoma. J Am Acad Dermatol 61(5): 819-828.
- Feldmeyer F, Tetzlaff M, Fox P, Nagarajan P, Curry J, et al. (2016) Prognostic Implication of Lymphovascular Invasion Detected by Double Immunostaining for D2-40 and MITF1 in Primary Cutaneous Melanoma. Am J Dermatopathol 38(7): 484-491.
- Straker RJ, Taylor LA, Neuwirth MG, Sinnamon AJ, Shannon AB, et al. (2022) Optimizing Detection of Lymphatic Invasion in Primary Cutaneous Melanoma with the Use of D2-40 and a Paired Melanocytic Marker. Am J Dermatopathol 44(1): 21-27.
- Ricci C, Chillotti S, Ambrosi F, Corradini C, Lambertini M, et al. (2021) Novel Double Immunohistochemistry (CD34/SOX10) for the Detection of Lymphovascular Invasion in Cutaneous Melanoma. Clinical-pathological Evidence Emerging from a Routine Set Mod Pathol 34: 312-313.

 Review Article
Review Article