Abstract
In a continuously growing elderly population, subjective memory complaints and mild cognitive impairment are increasing in prevalence worldwide. Omega-3 fatty acids, especially docosahexaenoic acid, are integral part of neural membrane and positively impact on brain structure and function. We have performed a narrative review on preclinical and clinical (normal ageing, subjective memory complaints, mild cognitive impairment and even Alzheimer´s disease) relevant scientific evidence on the eventual benefits on cognition that omega-3 fatty acids nutritional supplementation could provide. Although the available evidence to date is not conclusive, we have found some epidemiological studies and clinical trials which point to a positive effect of omega-3 fatty acids on cognition, mainly in memory functions. Additional longterm intervention clinical trials examining the role of omega-3 fatty acids and trying to ascertain which dosage and time of supplementation should be more efficient are needed.
Keywords: Omega-3 Fatty Acids; Docosahexaenoic Acid; Cognition; Memory; Cognitive Impairment; Dementia
Introduction
Today we know quite well that a healthy lifestyle that includes
physical activity, healthy eating or moderating toxic habits such as
alcohol and tobacco are associated with a lower risk of suffering
from some diseases such as cardiovascular diseases, some types
of neoplasms, and neurodegenerative diseases in general, and
particularly cognitive impairment or dementia. The dietary factor
is perhaps the most complex, and much research is aimed at
elucidating which foods are associated with this beneficial effect
and why. To this respect, one of the most implicated actors in
this positive effect has shown to be polyunsaturated fatty acids,
especially those belonging to the omega-3 group. Fatty acids are
biomolecules consisting of a linear hydrocarbon chain of variable
length, with a carboxyl group (-COOH) at one end and a methyl
group (-CH3) at the other. The carbon atoms in the chain are joined
by single or double covalent bonds. The absence of double bonds
defines the acid as saturated, while the presence of one double bond
in the chain defines it as monounsaturated acid, and the presence
of multiple double bonds as polyunsaturated. Polyunsaturated fatty
acids are known by their acronym PUFAs (Poly Unsaturated Fatty
Acids). Omega-3 fatty acids (ω-3), together with omega-6 fatty
acids (ω-6), make up the group of so-called essential fatty acids,
which owe their name to the fact that they are essential for the
body since the body is not capable of producing them on its own
and must acquire them from foods that contain them. Whether a
fatty acid is referred to as omega-3 or omega-6 is established by
the location of the first double bond from the methyl-terminal end.
In omega-3s, the double bond is at carbon 3 [C3-C4] and can also be
identified as n-3. In omega-6, the double bond is at carbon 6 (C6-C7)
and is also known as n-6.
Excessive amounts of omega-6 polyunsaturated fatty acids
(PUFA), marked by an increased dietary high omega-6/omega-3
ratio, as is increasingly common in current Western diets, are currently speculated to promote the pathogenesis of many diseases,
including cardiovascular, cancer, inflammatory and autoimmune
diseases [1]. In contrast, omega-3 fatty acids have been shown
to play an important role in altering blood lipid profiles and
membrane lipid composition and affecting eicosanoid biosynthesis,
cell signaling cascades and gene expression, which positively
influences health status. This effect seems to have been proven
in cardiovascular diseases [atrial fibrillation, atherosclerosis,
thrombosis, inflammation, and heart disease, among others],
diabetes, cancer, depression, or autoimmune diseases (e.g.,
rheumatoid arthritis). Its beneficial influence on brain function in
the diet of pregnant and lactating women has also been studied
[2,3]. The first evidence of this beneficial effect was provided
by epidemiological studies which revealed that the traditional
Greenlandic diet, rich in marine mammals and fish, reduced the
incidence of cardiovascular disease in both the Inuit population and
in the Danish people who immigrated to these latitudes, belonging
to a different ethnic group [4]. There is currently a tendency in
today’s diets to over-consume omega-6 in relation to omega-3 due
to the high consumption of vegetable oils by Western society, which
means that this ratio can be as high as 20:1, very different from the
current recommendations which advise that the omega-6/omega-3
acid ratio should be approximately 4:1, as was the case until the
beginning of the 20th century. We could hypothesize that diets rich
in omega-3 polyunsaturated fatty acids would be beneficial for the
functioning of neural structures, since one out of three fatty acids
in the central nervous system are long-chain polyunsaturated fatty
acids, and it could be thought that an inadequate balance between
these (ω-6/ω-3) would lead to neuropsychological alterations [5].
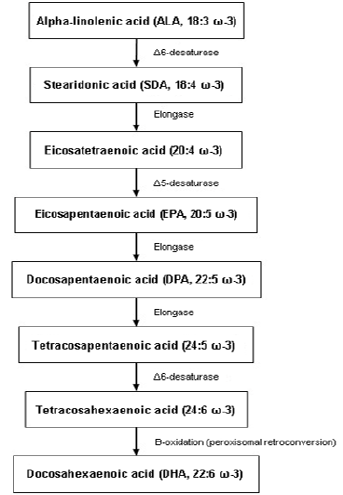
Omega-3 PUFAs originate mainly from the marine environment
or the vegetal kingdom and include α-linolenic acid (ALA; 18:3 ω-3),
stearidonic acid (SDA; 18: 4 ω-3), eicosapentaenoic acid (EPA; 20:5
ω-3), docosapentaenoic acid (DPA; 22:5 ω-3) and docosahexaenoic
acid (DHA; 22:6 ω-3). Some plant seeds, such as flax, chia and
canola seeds, are good sources of ALA, which serves as precursor
for the synthesis of other long-chain PUFAs (see Figure 1) in the
human body such as DHA or EPA. However, the production of longchain
ω-3 PUFA from ALA is very limited, with conversion rates of
around 5% through this metabolism. Therefore, the best sources
of PUFA ω-3 are those of marine origin. Long-chain ω-3s, such as
EPA and DHA, are found in the lipids of fatty fish; in the fat tissue
of marine mammals; in algae and marine fungi; as well as small
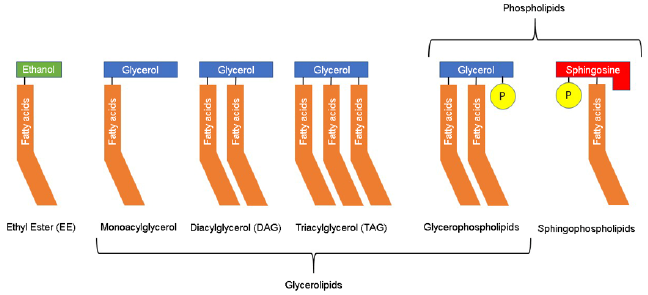
crustaceans that are part of krill. The bioavailability of ω-3 PUFAs is
also influenced by the organic structure of the ingested form from
the diet (see Figure 2), whether ethyl ester (EE), triacylglycerol
(TAG) or phospholipids (PL). The most common form of lipids in
nature is TAG, which has the best bioavailability compared with
EE. PLs are rare in nature and data on their bioavailability are
limited and inconclusive [4]. For these reasons, most supplements
are made from fish oils or other marine organisms such as krill,
as the bioavailability of these products is higher than with vegetal
origin products, in addition to the higher proportion of long-chain
omega-3 PUFA (DHA or EPA) of higher biological value [6].
The ω-3 PUFAs appear to exert a very important action on neuron membranes, especially in the synaptic regions of neurons (and to a greater extent, in areas of grey matter), where they accumulate in greater proportion and are essential components of the phospholipid membrane, so their importance is vital for the stability of the dynamic structure and functional activity of neurons, as they can alter the fluidity of the lipid membrane (displacing cholesterol from it) and promote synaptic plasticity, which is essential for learning, memory and other cognitive processes. They also act as sources of communication for second messengers between neurons, enhance the coupling of G-proteins involved in many signal transduction pathways and are involved in direct lipid-related transcription functions. DHA, one of the most important and final products of their metabolism (see Figure 1), constitutes more than 90% of the ω-3 and 10% to 20% of the total lipids in the brain. It is mainly incorporated into phosphatidylethanolamine, phosphatidylserine and, in smaller amounts, into phosphatidylcholine in synaptic terminals, mitochondria and endoplasmic reticulum. In fact, DHA is able to modulate cellular properties and physiological processes such as membrane fluidity, neurotransmitter release, gene expression, myelination, neuroinflammation and neuronal growth [6].
The purpose of this review is to examine the current evidence on the relationship between dietary omega-3 supplementation and improved cognition or prevention of cognition-related diseases such as Alzheimer’s disease (AD). To this end, we will discuss the evidence both for and against, in preclinical experiments as well as in clinical studies in humans, including normal ageing, subjective memory or cognitive complaints, mild cognitive impairment, and even AD.
Preclinical Data
The potential effect of omega-3 fatty acids on cognition has been
studied in multiple experimental preclinical studies in animals,
both in models of normal ageing and in models of AD. The most
common model of normal animal ageing is the canine model. Dogs
are capable of developing age-related cognitive decline similar to
that found in humans and their diet is similar to ours [7]. They
can therefore be used to study the potential effects of nutritional
supplements in controlled settings. Most studies use nutritional
supplements that combine different types of omega-3s, as well as
amino acids and antioxidants. The results obtained in these studies
support the hypothesis that these supplements would have a
beneficial effect on cognition and learning in older animals [7-9],
although there are some studies that do not find these benefits [10].
AD models are usually conducted in rodents. These often consist
of transgenic strains with mutations that predispose to developing
the disease and have been used extensively in AD treatment
research. Most studies of omega-3 supplementation in these animal
models have shown benefits on delayed cognitive impairment [11],
cognitive decline, behavioral symptoms [12], and have even shown
to reduce beta-amyloid deposits [12]. An interesting study used
transgenic mice for amyloid precursor protein (APP)-animal model
for AD-, compared with others that carry the same pathogenic
mutation together as well as another mutation that induces the
passage of endogenous omega-6 to omega-3, obtaining that the
latter showed a lower progression of cognitive and behavioral
symptoms compared to the former, displaying the potential
protective effect of omega-3 versus omega-6 [13]. This beneficial
effect of omega-3s is also observed in other studies in a murine
model of epilepsy [14]. In contrast, other studies have not found
this beneficial effect in transgenic mice, although they do find it in
normal mice [15].
Clinical Evidence
Normal Ageing
We have found and reviewed 13 randomized trials examining
the effects of omega-3 dietary supplements in healthy elderly
subjects, looking at their cognitive performance. Most used DHA
supplements, sometimes combined with EPA, with a daily dose
ranging from 350 mg to 3,000 mg of DHA obtained from fish oil in
most cases, and in one case from krill [16]. Cognitive performance
was measured using a cognitive assessment protocol that typically
includes tests of memory, attention, working memory, verbal fluency, and processing speed [17]. The results of most of them
showed significant differences when assessing the cognitive effects
of omega-3s [16,18-23]. One of the studies used the measurement
of P300 evoked potentials as the primary endpoint, resulting in an
improvement in the omega-3 treated group [24].
In other studies, on the contrary, no significant differences were
found in the parameters evaluated, although most of them used
lower daily doses (200-300 mg DHA), compared to the previous
studies [25-27], or otherwise the sample size was small [28], which
could explain this difference in results. The exception is the study
by Danthiir et al. which used high doses (1,720 mg DHA) and only
obtained a slight tendency towards improvement in some of the
evaluated endpoints [29]. In addition to the above, mention could be
made of the Spanish WAHA study, which did not examine the direct
effect of omega-3 supplements but studied the effect of a dietary
supplement with walnuts in a population-based cohort. Although
there were no significant results in cognitive variables after two
years of intervention, improvements in functional networks
mediated by functional magnetic resonance imaging (fMRI) during
working memory tasks were shown [30].
Subjective Memory Complaints
Studies related to subjective cognitive, or memory complaints
are scarce, perhaps because the concept is more difficult to
categorize or define than normal ageing or the “classic” mild
cognitive impairment or dementia. By subjective memory
complaints (SMC) we mean individuals who present a subjective
perception of poor cognitive performance in general [and memory
in particular] but which present a neuropsychological examination
within the normal range. It has been established that this altered
perception of one’s own cognition could be caused by the onset of
a very incipient cognitive impairment which eventually cannot be
detected in neuropsychological tests, or by a poor estimation of
one’s own abilities (meta-cognition) due to executive dysfunction
[31].
The main study in this subgroup of subjects is the MAPT
study which randomized French elderly people to multi-domain
intervention groups [cognitive stimulation, physical activity, and
nutrition], to omega-3 supplementation or both interventions
versus placebo. This study found no improvement in patients
who underwent intervention [32]. Anyway, a subgroup analysis
subsequently found that omega-3 supplementation would be
partially beneficial in those subjects who had low baseline omega-3
levels [33]. Another ambitious trial in this population subset is the
PONDER study, for which no results have yet been obtained [34].
Otherwise, some studies have found objective improvements on
cognitive performance or in regional blood flow measured by fMRI
in the posterior cingulum [35-37].
The study by Yurko-Mauro et al. in patients with memory
complaints who met criteria for “age-related cognitive decline”
has been considered as positive. This is a randomized, doubleblind,
placebo-controlled clinical trial in which 900 mg per day of
DHA (n=242) or placebo (n=243) was administered to individuals
with memory complaints of average age 70 years for a period of
6 months, showing statistically significant differences in favor of
DHA administration in validated cognitive tests frequently used
for the assessment of memory and learning ability. The authors
concluded that DHA supplementation at a dose of 900 mg/day
improved memory and learning capacity in individuals with SMC
[35]. In addition, there is a meta-analysis evaluating the results of
15 clinical studies, most of them observational, which concluded
that DHA/EPA supplementation has a beneficial effect on memory
in adult individuals. The review concludes that episodic memory
tests of adults with SMC were significantly improved (p<0.004)
with DHA/EPA supplementation. Furthermore, and regardless of
cognitive status at baseline, DHA/EPA supplementation [at doses >1
g per day] was able to improve episodic memory (p<0.04). Changes
in semantic and working memory from baseline were significant
with DHA, but no differences between groups were detected [38].
Mild Cognitive Impairment
Mild cognitive impairment [(MCI) is probably the main risk
factor for developing dementia, and specifically amnestic MCI (the
most prevalent entity) is the most important risk factor for the
development of AD. For this reason, preventive treatments have
become a relevant source of study in patients with MCI, as they could
have a potential role in the prevention of AD. Currently, there is no
drug approved for use in MCI, but some strategies such as cognitive
stimulation, physical exercise, and dietary recommendations,
including omega-3 PUFAs, are under study. Randomized clinical
trials that have studied the effect of omega-3 PUFA in patients with
MCI have been reviewed. Most studies showed improvements in
scores on working memory tests, as well as episodic memory [39-
41], although there are others that evaluated test scores (FSIQ -Full
Scale Intelligence Quotient- and WAIS -Wechsler Adult Intelligence
Scale), or even depressive symptom scales (GDS -Geriatric
Depression Scale) [42,43], which concluded with positive results in
favor of omega-3 supplementation. All the mentioned studies above
followed patients for 6 months to one year, while studies with
shorter follow-up did not seem to obtain statistically significant
differences [44].
Additionally, there have been several published meta-analyses
and systematic reviews on the effect of omega-3s in patients with
MCI. The overall conclusion of all of them points to the beneficial
effect of omega-3 PUFA in MCI patients [45,46]. The most recent
meta-analysis of 25 studies (n=787) indicated that omega-3s appear to have no effect on overall cognitive function (Hedge’s g=
0.02; 95% confidence interval= -0.12 to 0.154), although it may have
a beneficial effect on memory (Hedge’s g=0.31; p=0.003; z=2.945)
[46]. Another meta-analysis by Zhang et al. analysed all studies in
which the MMSE (Mini Mental State Examination) was used for the
assessment of these patients treated with DHA/EPA supplements
and concluded that the treatment seems to statistically decrease
the rate of cognitive decline in terms of MMSE score (WMD=0.15
(0.05-0.25); p=0.003), so the hypothesis that omega-3 could help to
prevent global cognitive decline -in addition to memory- in elderly
people with MCI seems to be supported [47].
Neuroimaging research has looked at the effect that omega-3
PUFA may have on the brain in patients with MCI. One of these
studies used fMRI to study regional blood flow. It appeared that
treatment with omega-3 for 26 weeks is able to increase blood flow
in posterior cortical areas, typically affected in MCI [48]. Another
study analysed the evolution of brain volume measured by MRI
in patients with MCI with results showing that, after treatment
with DHA supplementation for one year, the hippocampal volume
measured by brain MRI was larger in treated patients than in those
receiving placebo [49].
Alzheimer´s Disease
There have been numerous studies on omega-3 PUFA in AD
with overall slightly favorable results. In this context, multiple
randomized, double-blind, placebo-controlled trials have been
conducted, some of which have had great scientific impact. So far,
no treatment has been found that is able to significantly improve
AD, so most studies are trying to elucidate whether treatments are
able to reduce the degree of disease progression. One of the first
studies that attempted to address the effect of omega-3s on the
progression of AD was the Omega AD study. In this study, patients
were treated with high-dose DHA and EPA (1.7 g DHA + 0.6 g EPA)
versus placebo, with omega-3 supplementation in both groups
continuing for a further period from 6 months to 1 year. The main
publication derived from the research showed a tendency for the
treatment group to have less disease progression at the cognitive
level [measured by MMSE and ADAS-Cog -Alzheimer´s Disease
Assessment Scale-Cognitive], although significant differences were
only obtained in the mildest AD subgroup [50]. In this trial, a better
outcome in patients with higher plasma levels of omega-3 was found
[51] and also in those with higher homocysteine levels, which the
authors relate to a hypothetical synergistic effect with B vitamins
[52]. The same study attempted to assess the efficacy on behavioral
symptoms without improvement, with the exception of depressive
symptoms, a finding that has been observed in other settings of
cognitive impairment [53]. This beneficial effect of omega-3s has
been replicated in other clinical trials using similar doses of DHA
for 6-12 months with improvements in cognitive scales such as the
ADAS-Cog [54–56].
On the other hand, some studies have found no significant
differences in terms of cognitive assessment [44]. The most
relevant is that of Quinn et al., concluding that treatment with
DHA (2 g/day) for 18 months did not produce a relevant cognitive
effect as measured by MMSE and subscales of the ADAS-Cog [57].
Studies related to some complex nutrient formulations with high
doses of omega-3 PUFA in their composition [1200 mg DHA + 300
mg EPA per 125 ml] are worth mentioning. Some studies with
these products have shown beneficial effects of daily treatment.
The primary endpoint of the trial was the change in memory as
measured by the NTB [Neuropsychological Test Battery] Z-score at
24 weeks of treatment. The study showed statistically significant
differences in NTB score in favor of the treated arm [p=0.023;
Cohen’s d=0.21; 95% confidence interval (-0.06 - 0.9)) [58,59].
Conclusion
Although evidence is not conclusive, several investigations support that supplementation with omega-3 PUFA, especially DHA, may have beneficial effects on cognition in healthy older adults with SMC and even in patients with MCI or AD. These effects should generally appear at high daily doses [800-900 mg and above] and over long periods of time (6 months or longer). This protective effect has not been shown in all studies, so it is possible that the results may be influenced by other variables; in this sense, it is important to mention that ensuring adequate long-term adherence to treatment [at least 6-12 months] may be important to establish a possible objective benefit. Anyway, more soundly designed interventional clinical trials are needed to ascertain relevant issues concerning dose and supplementation duration. It has been found that plasma omega-3 values or homocysteine levels may be related with these discrepancies among different studies, with subjects with lower plasma omega-3 and homocysteine levels benefiting more from this effect. In any case, these supplements appear to have no relevant adverse effects and may have other added benefits such as reduced cardiovascular risk. Moreover, their indication is not at odds with other recommendations such as cognitive stimulation and physical exercise, so that, altogether, these measures can be medically recommended.
Conflicts of Interests
González, Javier is an employee of ITF Research Pharma, S.L.U., Alcobendas, Madrid, Spain.
References
- Simopoulos A (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56(8): 365-379.
- Shahidi F, Miraliakbari H (2004) Omega-3 (n-3) Fatty Acids in Health and Disease: Part 1-cardiovascular disease and Cancer. J Med Food 7(4): 387-401.
- Shahidi F, Miraliakbari H (2005) Omega-3 Fatty Acids in Health and Disease: Part 2—Health Effects of Omega-3 Fatty Acids in Autoimmune Diseases, Mental Health, and Gene Expression. J Med Food 8(2): 133-148.
- Shahidi F, Ambigaipalan P (2018) Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev Food Sci Technol, pp. 345-381.
- Caballer García J (2011) Los ácidos grasos omega-3 en la prevención de la enfermedad de Alzheimer. Alzheimer Real e Investig en Demenc 49: 12-18.
- Avallone R, Vitale G, Bertolotti M (2019) Omega-3 fatty acids and neurodegenerative diseases: New evidence in clinical trials. Int J Mol Sci 20(17): 4256.
- Araujo JA, Studzinski CM, Head E, Cotman CW, Milgram NW (2005) Assessment of nutritional interventions for modification of age-associated cognitive decline using a canine model of human aging. Age 27(1): 27-37.
- Milgram NW, Araujo JA, Hagen TM, Treadwell BV, Ames BN (2007) Acetyl‐L‐carnitine and α‐lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J 21(13): 3756-3762.
- Pan Y, Kennedy AD, Jönsson TJ, Milgram NW (2018) Cognitive enhancement in old dogs from dietary supplementation with a nutrient blend containing arginine, antioxidants, B vitamins and fish oil. Br J Nutr 119(3): 349-358.
- Christie LA, Opii WO, Head E, Araujo JA, de Rivera C, et al. (2009) Short-term supplementation with acetyl-l-carnitine and lipoic acid alters plasma protein carbonyl levels but does not improve cognition in aged beagles. Exp Gerontol 44(12): 752-759.
- Hosono T, Mouri A, Nishitsuji K, Jung C, Kontani M, et al. (2015) Arachidonic or Docosahexaenoic Acid Diet Prevents Memory Impairment in Tg2576 Mice. J Alzheimer’s Dis 48(1): 149-162.
- Teng E, Taylor K, Bilousova T, Weiland D, Pham T, et al. (2015) Dietary DHA supplementation in an APP / PS1 transgenic rat model of AD reduces behavioral and A β pathology and modulates A β Neurobiol Dis 82(Octubre): 552-560.
- Wu K, Gao X, Shi B, Chen S, Zhou X, et al. (2016) Enriched endogenous n-3 polyunsaturated fatty acids alleviate cognitive and behavioral deficits in a mice model of Alzheimer’s disease. Neuroscience 333: 345-355.
- Abdel-Wahab BA, Al-Qahtani JM, El-Safty SA (2015) Omega-3 polyunsaturated fatty acids in large doses attenuate seizures, cognitive impairment, and hippocampal oxidative DNA damage in young kindled rats. Neurosci Lett 584: 173-177.
- Arendash GW, Jensen MT, Salem N, Hussein N, Cracchiolo J, et al. (2007) A diet high in omega-3 fatty acids does not improve or protect cognitive performance in Alzheimer’s transgenic mice. Neuroscience 149(2): 286-302.
- Konagai C, Yanagimoto K, Hayamizu K, Li H, Tsuji T, et al. (2013) Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin Interv Aging 8: 1247-1257.
- Danthiir V, Burns NR, Nettelbeck T, Wilson C, Wittert G (2011) The older people, omega-3, and cognitive health (EPOCH) trial design and methodology: A randomised, double-blind, controlled trial investigating the effect of long-chain omega-3 fatty acids on cognitive ageing and wellbeing in cognitively healthy older ad. Nutr J 10(1): 117.
- De Souto Barreto P, Rolland Y, Cesari M, Dupuy C, et al. (2018) Effects of multidomain lifestyle intervention, omega-3 supplementation or their combination on physical activity levels in older adults: Secondary analysis of the Multidomain Alzheimer Preventive Trial (MAPT) randomised controlled trial. Age Ageing 47(2): 281-288.
- Külzow N, Witte AV, Kerti L, Grittner U, Schuchardt JP, et al. (2016) Impact of Omega-3 Fatty Acid Supplementation on Memory Functions in Healthy Older Adults. J Alzheimer’s Dis 51: 713-725.
- Nilsson A, Radeborg K, Salo I, Björck I (2012) Effects of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: a randomized controlled cross-over study. Nutr J 11: 1-9.
- Pase MP, Grima N, Cockerell R, Stough C, Scholey A, et al. (2015) The Effects of Long-Chain Omega-3 Fish Oils and Multivitamins on Cognitive and Cardiovascular Function: A Randomized , Controlled Clinical Trial The Effects of Long-Chain Omega-3 Fish Oils and Multivitamins on. J Am Coll Nutr 34(1): 21-31.
- Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, et al. (2014) Long-Chain Omega-3 Fatty Acids Improve Brain Function and Structure in Older Adults. Cereb Cortex 24(November), pp. 3059-3068.
- Baleztena J, Ruiz-Canela M, Sayon-Orea C, Pardo M, Añorbe T, et al. (2018) Association between cognitive function and supplementation with omega-3 PUFAs and other nutrients in 75 years old patients: A randomized multicenter study. PLoS One 13(3): 1-15.
- Tokuda H, Sueyasu T, Kontani M, Kawashima H, Shibata H, et al. (2015) Low Doses of Long-chain Polyunsaturated Fatty Acids Affect Cognitive Function in Elderly Japanese Men: A Randomized Controlled Trial. J Oleo Sci 644(6): 633-644.
- Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, et al. (2010) Effect of 2-y n−3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr 91(6): 1725-1732.
- Chew EY, Clemons TE, Agrón E, Launer LJ, Grodstein F, et al. (2015) Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function. JAMA 314(8): 791.
- Stough C, Downey L, Silber B, Lloyd J, Kure C, et al. (2012) The effects of 90-day supplementation with the Omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiol Aging 33(4): 824.e1-824.e3.
- Jackson PA, Forster JS, Bell JG, Dick JR, Younger I, et al. (2016) DHA Supplementation Alone or in Combination with Other Nutrients Does not Modulate Cerebral Hemodynamics or Cognitive Function in Healthy Older Adults. Nutrients 8(2): 86.
- Danthiir V, Hosking DE, Nettelbeck T, Vincent AD, Wilson C, et al. (2018) An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. Am J Clin Nutr 107(5): 754-762.
- Sala-Vila A, Valls-Pedret C, Rajaram S, Coll-Padrós N, Cofán M, et al. (2020) Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts and Healthy Aging (WAHA) study: a randomized controlled trial. Am J Clin Nutr 111(3): 590-600.
- Ruiz-Sánchez De León JM, Llanero-Luque M, Lozoya-Delgado P, Fernández-Blázquez MA, Pedrero-Pérez EJ (2010) Neuropsychological study of young adults with subjective memory complaints: Involvement of the executive functions and other associated frontal symptoms. Rev Neurol 51(11): 650-660.
- Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, et al. (2017) Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 16(5): 377-389.
- Hooper C, de Souto Barreto P, Coley N, Cantet C, Cesari M, et al. (2017) Cognitive changes with omega-3 polyunsaturated fatty acids in non-demented older adults with low omega-3 index. J Nutr Health Aging 21(9): 988-993.
- Macpherson H, Brownell S, Duckham RL, Meyer B, Mirzaee S, et al. (2019). Multifaceted intervention to enhance cognition in older people at risk of cognitive decline: Study protocol for the Protein Omega-3 and Vitamin D Exercise Research (PONDER) study. BMJ Open 9(5).
- Yurko-Mauro K, Mccarthy D, Rom D, Nelson EB, Ryan AS, et al. (2010) Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer’s Dement 6(6): 456-464.
- Mcnamara RK, Kalt W, Shidler MD, Mcdonald J, Suzanne S, et al. (2019) Cognitive Response to Fish Oil, Blueberry, and Combined Supplementation in Older Adults with Subjective Cognitive Impairment. Neurobiol Aging 64: 147-156.
- Boespflug EL, Mcnamara RK, Eliassen JC, Schidler MD, Krikorian R (2016) Fish Oil Supplementation Increases Event-Related Posterior Cingulate Activation in Older Adults with Subjective Memory Impairment. J Nutr Health Aging 20(2): 161-169.
- Yurko-Mauro K, Alexander DD, Elswyk ME Van (2015) Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLoS One 10(3): 1-18.
- Bo Y, Zhang X, Wang Y, You J, Cui H, et al. (2017) The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients 9(1): 1-11.
- Lee LK, Shahar S, Chin AV, Yusoff NAM (2013) Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): A 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl) 225(3): 605-612.
- Yurko-Mauro K (2010) Cognitive and Cardiovascular Benefits of Docosahexaenoic Acid in Aging and Cognitive Decline. Curr Alzheimer Res 7(3): 190-196.
- Li M, Li W, Gao Y, Chen Y, Bai D, et al. (2020) Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: a randomized double-blind, placebo-controlled trial. Eur J Nutr (0123456789).
- Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, et al. (2012) Effects of n -3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr 107(11): 1682-1693.
- Phillips MA, Childs CE, Calder PC, Rogers PJ (2015) No Effect of Omega-3 Fatty Acid Supplementation on Cognition and Mood in Individuals with Cognitive Impairment and Probable Alzheimer’ s Disease : A Randomised Controlled Trial. Int J Mol Sci 16(10): 24600-24613.
- Mazereeuw G, Lanctôt KL, Chau SA, Swardfager W, Herrmann N (2012) Effects of omega-3 fatty acids on cognitive performance: A meta-analysis. Neurobiol Aging [Internet] 33(7): 1482.e17-1482.e29.
- Alex A, Abbott KA, McEvoy M, Schofield PW, Garg ML (2020) Long-chain omega-3 polyunsaturated fatty acids and cognitive decline in non-demented adults: a systematic review and meta-analysis. Nutr Rev 78(7): 563-578.
- Zhang XW, Hou WS, Li M, Tang ZY (2016) Omega-3 fatty acids and risk of cognitive decline in the elderly: a meta-analysis of randomized controlled trials. Aging Clin Exp Res 28(1): 165-166.
- Schwarz C, Wirth M, Gerischer L, Grittner U, Witte AV, et al. (2018) Effects of Omega-3 Fatty Acids on Resting Cerebral Perfusion in Patients with Mild Cognitive Impairment: A Randomized Controlled Trial. J Prev Alzheimer´s Dis 5(1): 26-30.
- Zhang YP, Miao R, Li Q, Wu T, Ma F (2017) Effects of DHA Supplementation on Hippocampal Volume and Cognitive Function in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized, Double-Blind, Placebo-Controlled Trial. J Alzheimer’s Dis 55(2): 497-507.
- Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, et al. (2006) ω-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study - A randomized double-blind trial. Arch Neurol 63(10): 1402-1408.
- Eriksdotter M, Vedin I, Falahati F, Freund-Levi Y, Hjorth E, et al. (2015) Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients During Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J Alzheimer’s Dis 48(3): 805-812.
- Jernerén F, Cederholm T, Refsum H, Smith AD, Turner C, et al. (2019) Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. Korczyn A, editor. J Alzheimer’s Dis 69(1): 189-197.
- Freund-Levi Y, Basun H, Cederholm T, Faxén-Irving G, Garlind A, et al. (2008) Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry 23(2): 161-169.
- Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, et al. (2008) The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog Neuro-Psychopharmacology Biol Psychiatry 32(6): 15381-1544.
- Hashimoto M, Kato S, Tanabe Y, Katakura M, Mamun A Al, et al. (2016) Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function and mental health of the oldest elderly in Japanese care facilities and nursing homes. Geriatr Gerontol Int 17(2): 330-337.
- Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, et al. (2013) A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s Disease. J Alzheimer’s Dis 38(1): 111-120.
- Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, et al. (2010) Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA - J Am Med Assoc 304(17): 1903-1911.
- Scheltens P, Twisk JWR, Blesa R, Scarpini E, von Arnim CAF, et al. (2012) Efficacy of Souvenaid in Mild Alzheimer’s Disease: Results from a Randomized, Controlled Trial. J Alzheimer’s Dis 31(1): 225-236.
- Rasmussen J (2019) The LipiDiDiet trial: what does it add to the current evidence for Fortasyn Connect in early Alzheimer’s disease? Clin Interv Aging 14: 14811-1492.

 Review Article
Review Article