Preface
Otosclerosis is a primary osteodystrophy of the otic capsule or a disorder of bone remodelling incriminating the stapes footplate or bony labyrinth of the inner ear. Otosclerosis literally implies “abnormal hardening of body tissue of the ear”. The condition is confined to the middle ear and is associated with anomalous remodelling of bone. Normally dense endochondral layer of bony otic capsule situated within the labyrinth is substituted by irregular, spongy bone. Consequently, fixation of the stapes ensues. Otosclerosis was initially scripted by Antonio Maria Valsalva in 1735 and is additionally designated as otospongiosis. Otosclerosis alters functioning of the middle ear and inner ear and is a predominant contributor to deafness in adults. Otosclerosis may engender significant disability and morbidity due to hearing loss. Antecedent disease detection with cogent therapy is associated with superior outcomes.
Disease Characteristics
Typically, otosclerosis induces lucent alterations instead
of sclerotic bony modifications. Temporal bones are frequently
incriminated [1,2]. Otosclerosis appears as an antecedent, adult
onset disease. Generally, otosclerosis commences within second
decade or third decade although hearing loss commences following
the fourth decade. Disease occurrence within children is exceptional.
However, the progressive condition demonstrates gradually
worsening clinical symptoms and it may be challenging to ascertain
precise disease onset [1,2]. A female predominance is observed
with a female to male proportion of ~2:1. Otosclerosis worsens
in pregnancy [1,2]. Ethnic predilection demonstrates frequent
disease emergence within the Caucasian population. Otosclerosis
is exceptionally discerned in Asians or Black population. Familial
disease incidence appears in an estimated 50% instances. Around
85% instances are bilateral [1,2]. Otosclerosis is associated with
distinctive phases such as
• Early or active otospongiosis and
• Late or inactive otosclerosis.
Of obscure and multifactorial pathogenesis, otosclerosis may
arise due to genetic, viral, inflammatory or autoimmune components
[1,2]. Preliminary lesions are predominantly comprised of an
admixture of histiocytes, osteoblasts and osteocytes wherein
osteocytes are an active cellular component. Bony circumscription
of pre-existing vascular articulations is resorbed with meliorated
microcirculation. Eventually, osteoblasts configure irregular foci
of nascent, spongy bone, designated as “blue mantles of Manasse”
[1,2].
Disease Pathogenesis
Of obscure aetiology, otosclerosis is posited to denominate a
multifactorial emergence such as
• Anatomical factors wherein fissula ante fenestram is
incriminated along with persisting remnants of embryonic
cartilage [3,4].
• Genetic factors of otosclerosis are associated with several
loci situated upon chromosomes 6p, 9p, 1q, 3q, 6q, 7q, 15q,
16q and a contemporary locus upon chromosome 7q22.1.
Additionally, diverse genes as type I collagen (COL1A1 gene),
transforming growth factor-β1 (TGF-β1) with BMP2 and BMP4
genes, angiotensin II with AGT M235T and ACE I/D genes may
induce otosclerosis [3,4]. Sex hormones, autoimmune reaction,
human leucocyte antigen (HLA), inflammatory or regulatory
cytokines, parathyroid hormone, parathyroid hormonerelated
peptide receptors and oxidative stress may initiate
otosclerosis [3,4].
• Hereditary factors wherein an estimated 50% subjects with
otosclerosis demonstrate a family history. Individuals with
hereditary otosclerosis are associated with an antecedent
disease onset. Majority of instances depict an autosomal
dominant mode of inheritance accompanied by reduced
penetrance in nearly ~40% subjects and variable expression
of disease [3,4].
• Viral infection is implicated in the emergence of otosclerosis.
Ribonucleic acid (RNA) of measles virus may be discerned
within footplate of stapes upon ultrastructural examination
and immunohistochemistry. Vaccination for measles virus
may appear as a safeguard for occurrence of otosclerosis
[3,4]. Additionally, factors such as menopause, trauma,
bone dyscrasia or major surgery may initiate or aggravate
otosclerosis [3,4].
Clinical Elucidation
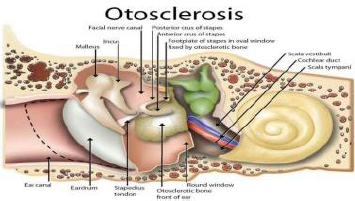
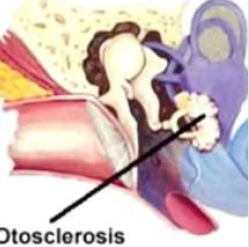
Otosclerosis is commonly confined to the temporal bone and
engenders fixation of footplate of stapes within the oval window.
Therefore, inability to transmit sound waves manifests as conductive
hearing loss [5,6]. Generally, otosclerosis engenders conductive
deafness and appears in association with a normal tympanic
membrane. Contingent to focal incrimination of the bony labyrinth,
otosclerosis can be asymptomatic or represent with neurosensory
decimation [5,6]. Frequent clinical representation of otosclerosis
is hearing loss accompanied by tinnitus and vertigo. Gradually
progressive bilateral hearing loss is a common symptom which
typically commences in one ear and progresses to contralateral
ear [5,6]. Inability to adequately discern low-frequency sounds
as a whisper can emerge as an initial clinical symptom. Hearing
may be meliorated in a noisy environment, a feature denominated
as “paracusis willisii” which is indicative of conductive deafness.
Incriminated subjects adopt a low-volume, monotonous tone of
voice.
Extensive otosclerosis is associated with worsening tinnitus.
Mild dizziness appears and deteriorates with disease progression,
simulating Meniere’s disease [5,6]. Otoscopy may be normal or
depict minimal modifications. However, around 10% instances of
active otosclerosis or cochlear otosclerosis are accompanied by
enhanced vascularity of the promontory which can be discerned
through the tympanic membrane and is designated as “Schwartze
sign” [5,6]. Of indolent clinical course, otosclerosis manifests with
frequently bilateral, conductive hearing loss although sensorineural
or mixed deafness may ensue. Hearing loss can be exacerbated in
pregnant subjects [5,6]. Otosclerosis is comprised of distinctive
subtypes denominated as
• Fenestral or stapedial variant which constitutes of a majority
(~80%) of instances and incriminates the oval window along
with stapes footplate. Frequently, conductive hearing loss is
associated with thickening and fixation of stapes [5,6].
• Retrofenestral or cochlear subtype which is composed of
nearly 20% instances. Incriminated cochlea is associated with
demineralization of cochlear capsule. Sensorineural hearing
loss emerges, possibly due to uncertain mechanisms [5,6].
Retrofenestral otosclerosis is usually accompanied by fenestral
variant and the conditions are contemplated to represent a
disease continuum [5,6].
Histological Elucidation
Upon gross examination, foci of otosclerosis appear as
chalky white, greyish or yellowish. Active centric zone and rapid
disease progression is associated with reddish lesions due to
enhanced vascularity. Upon macroscopic examination of dissected
tissue, specimens of un-altered head and crura of stapes are
obtained [5,6]. Microscopically, foci of initial bone resorption
and circumscription of vascular articulations by cellular, fibrovascular
tissue are observed. Subsequently, deposition of immature
bone, perpetual bone resorption and remodelling is exemplified.
Gradually, deposition of bone, enhanced collagen and decimated
ground substance ensue with consequent emergence of densely
sclerotic bone demonstrating prominent cement lines [5,6]. Dense,
endochondral layer of otic capsule appears to be constituted of
spongy bone. Immature, active lesions of otosclerosis are imbued
with abundant bone marrow, vascular spaces and numerous
osteoblasts admixed with osteoclasts. Significant quantities of
cement substance are deposited within the lesion [5,6]. Mature
foci of otosclerosis are mildly vascular and composed of abundant
bone and fibrillar substance with minimal cementum. Localized
foci of bone remodelling and bone resorption of the otic capsule are
followed by subsequent bone deposition.
Otoclerosis predominantly arises due to replacement of normal
bone with sclerotic or spongiotic bone [5,6]. Osteolytic osteocytes
are configured upon growing perimeter of the lesion. Sheets of
connective tissue displace the bone. Delayed stage of otosclerosis
depicts dense, sclerotic bone associated with zones of previous bone resorption. Consequently, disorganized bone is articulated,
osteocytes are quantifiably enhanced and expansive bone marrow
spaces are incorporated with vascular articulations and connective
tissue [5,6]. Bone marrow spaces are eventually substituted by
dense, sclerotic bone imbued with narrow vascular articulations
and minimal Haversian canal system [5,6]. Preliminary and delayed
stage of otosclerosis occurring within a singular temporal bone
engenders pleomorphism. Antecedent lesions may adjoin fissula
ante fenestram and expand through abutting vascular channels
[5,6]. Majority of lesions are confined to anterior oval window
and appear associated with calcification of annular ligament or
incrimination of stapes, thereby engendering conductive hearing
loss [5,6] (Figures 1-8).
Figure 1: Otosclerosis exhibiting sclerotic bone of footplate of stapes and bony labyrinth of inner ear [9].
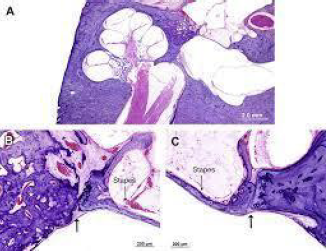
Figure 2: Otosclerosis enunciating spongiotic and sclerotic bones replacing foot plate of stapes [10].
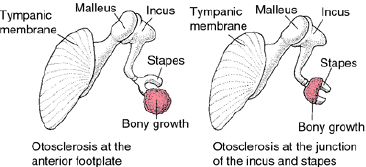
Figure 3: Otosclerosis exemplifying bony growth within footplate of stapes and spongiotic bone at the junction of incus and stapes [11].
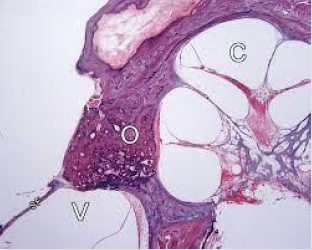
Figure 4: Otosclerosis exemplifying bony sclerosis at footplate of stapes with an admixture of spongiotic bone, osteocytes and minimal Haversian system [12].
Figure 5: Otosclerosis delineating bony sclerosis of footplate of stapes with configured spongiotic bone and associated conductive hearing loss [13].
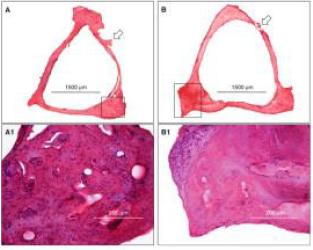
Figure 6: Otosclerosis demonstrating bony sclerosis and spongiotic bone replacing footplate of stapes with intermingled osteocytes and fibro-connective tissue [14].
Figure 7: Otosclerosis displaying spongy and sclerotic bone replacing footplate of stapes intermingled with abundant osteocytes, osteoblasts and fibro-connective tissue [15].
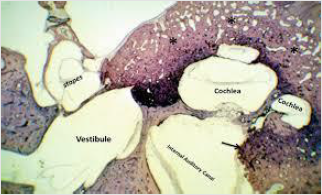
Figure 8: Otosclerosis exhibiting bony sclerosis of the inner ear labyrinth with several osteocytes, decimated Haversian canal and fibroconnective tissue [16].
Differential Diagnosis
Otosclerosis requires a segregation from conditions such as
• Osteogenesis imperfecta where otic capsule demonstrates
lucencies simulating otosclerosis. Osteogenesis imperfecta is
associated with non deformed, fragile bones and blue sclera.
Severe variants of osteogenesis imperfecta are devoid of an
organized bone trabecular pattern. Crowding of osteocytes
within bone occurs due to decimated collagen synthesis.
Enlarged foci of woven bone are delineated. Minimally severe
instances depict crowding of osteocytes associated with
attenuated lamellar bone [2,4].
• Paget’s disease of bone predominantly occurs in elderly
individuals and demonstrates bony expansion. Acute stage is
primarily composed of woven bone admixed with focal, mosaic
configuration of lamellar bone associated with irregular
cement lines. Osteoclasts are preponderant upon the bone
surface. Osteoclasts appearing within osteolytic phase may
depict around ~100 nuclei [2,4]. Chronic stage is comprised of
thickened bony trabeculae and thick bones which are imbued
with finely fibrotic bone marrow [2,4].
• Osteoradionecrosis arises due to vascular injury with
consequent emergence of bony ischemia. Osteoradionecrosis
is associated with distinctive phases denominated as
• Pre-fibrotic phase demonstrating foci of chronic inflammatory
cell exudate
• Phase of organized fibrosis predominantly displaying aberrant
fibroblastic activity along with an inadequately organized
bone matrix. Aforesaid regions appear adjacent to foci of aging
fibroblasts disseminated within a minimally cellular, fibrotic,
densely sclerotic matrix [2,4].
• Fibro-atrophic phase is composed of dense hyalinization
and fibrosis along with absence of bone marrow cells. Focal,
reactive stratified squamous epithelium may layer a fistulous
tract. Patchy, secondary infiltration of chronic inflammatory
cells may occur. Lesions may display necrotic or sclerotic bone
along with empty osteocyte lacunae. Fibrosis of bone marrow
is admixed with irregular bony trabeculae devoid of osteocytes
within the lacuna. Foci of bone marrow necrosis may arise
due to emergence of a preliminary stage of oedematous,
fibromyxoid disease [2,4]. Additionally, otosclerosis requires
a segregation from conditions which are associated with
conductive deafness such as serous otitis media, adhesive
otitis media, congenital fixation of the stapes, Meniere disease,
tympanosclerosis, attic fixation of head of the malleus or
ossicular discontinuity [2,4].
Investigative Assay
Upon otoscopy, features indicative of otosclerosis are minimal
to absent. However, severe instances with cochlear incrimination
can engender hyperaemia of the cochlear promontory, a feature
designated as “Schwartze sign” [7,8]. Otosclerosis can be
appropriately investigated with the tuning fork and demonstrates
a negative Rinne’s test. Weber’s test appears lateralized to the ear
and is indicative of a severe conductive hearing loss [7,8]. Pure tone
audiometry demonstrates low frequency loss of air conduction
with normal bone conduction. Pure tone audiometry exhibits a
characteristic decimation of bone conduction, especially at higher
frequencies [7,8]. Mixed hearing loss can be observed wherein
preliminary otosclerosis is associated with normal tympanometry
[7,8]. High resolution computerized tomography (CT) of temporal
bone is optimal in discerning otosclerosis besides recognizing
and segregating associated causes of deafness. An estimated 80%
instances depict fenestral foci which are situated anterior to the oval
window. Also, thickened stapedial footplate and incriminated round
window can be discerned, features which regulate appropriate
therapy [7,8]. Retrosternal focus emerges as a “double halo” sign, a
feature encountered with cochlear otosclerosis. Otosclerosis can be
appropriately graded with CT [7,8].
Pertinent imaging depicts representations such as
• Fenestral otosclerosis which is the commonest variant and
demonstrates site of incrimination just anterior to oval window
while configuring a miniature cleft denominated as fissula ante
fenestram. Besides, bony overgrowth may engender fixation of
the stapes [7,8].
• Retrofenestral otosclerosis incriminates the niche of
round window and usually accompanies fenestral disease.
Nevertheless, isolated round window otosclerosis may be
occasionally discerned. Predominant site of disease emergence
appears as focal or circumferential bone circumscribing
the cochlea. Circumferential bony implication engenders a
“fourth turn” or “double ring” sign [7,8]. Imaging features are
contingent to phase of the disease and appear as
• Otospongiotic phase composed of demineralization and
configuration of spongy bone. Pertinent phase manifests as
decimated attenuation or lucent zone within homogeneously
dense perimeter of otic capsule [7,8].
• Otosclerotic phase is associated with enhanced attenuation
within disease specific region. As it may be challenging to
discern otosclerotic bone from encompassing normal bone,
features such as thickness of otic capsule or aberrant convexity
of contour of otic capsule cortex anterolateral to anterior
margin of the oval window may aid the distinction [7,8]. Severe
instances are associated with comprehensive impaction of oval
window or round window with a dense, bony plate associated
with complete fixation of stapes [7,8]. Thin-slice computerized
tomography (CT) through the temporal bone is a preferred
imaging modality for adequately exemplifying anatomy of
inner ear and emergent, subtle, preliminary modifications of
otosclerosis. Upon computerized tomography, otosclerosis is
categorized as
• Grade I which is singularly comprised of fenestral, spongiotic
or sclerotic lesions which appear as thickened footplate of
stapes along with decalcified, narrowed or enlarged round
window or oval window [7,8].
• Grade 2 is constituted of patchy, localized cochlear disease
along with the presence or absence of fenestral otosclerosis
and is subdivided into~grade 2A which implicates basal
cochlear turn~grade 2B which implicates middle or apical
cochlear turn~grade 2C which implicates basal turn and
middle or apical cochlear turn [7,8].
• Grade 3 is comprised of diffuse, confluent involvement of
cochlea and otic capsule along with the presence or absence
of fenestral involvement [7,8]. Magnetic resonance imaging
(MRI) of retrofenestral otosclerosis exhibits a distinctive
pericochlear and perilabyrinthine soft tissue intensity upon
T1 weighted imaging with contrast enhancement. Besides,
enhanced signal intensity upon T2 weighted imaging may be
observed [7,8].
Therapeutic Options
Medical management of otosclerosis is required to circumvent or arrest disease progression. However, optimal, efficacious medical therapy which alleviates otosclerosis remains lacking. Administration of sodium fluoride in order to decimate disease progression is debatable [7,8]. Bisphosphonates are antiresorptive and induce osteoclastic apoptosis. Contemporary bisphosphonates, commonly employed to treat otosclerosis, appear promising [7,8]. Bilateral hearing aids can be utilized singularly or in combination with diverse therapies [7,8]. Fenestral otosclerosis can be appropriately treated with stapedectomy along with the employment of stapes prosthesis. Optimal and recommended surgical treatment of otosclerosis is stapedotomy or stapedectomy or correction of fixation of foot plate of stapes. Surgical intervention is commonly followed by implantation of prosthesis [7,8]. Therapeutic outcomes of surgical intervention in otosclerosis appear to be superior, regardless of surgical manoeuver adopted [7,8]. Though surgical intervention is beneficial, hearing aids may be required in certain subjects following surgery [7,8]. Untreated otosclerosis may engender significant hearing loss although complete deafness is uncommon [7,8].
Although infrequent, surgical intervention for treating otosclerosis can induce comprehensive sensorineural deafness within the operated ear. Surgical manoeuvers may induce facial nerve injury or tinnitus. An unpleasant taste may arise for a brief duration [7,8]. Prosthetic implantation is associated with granulomatous inflammation and erosion or necrosis of the incus [7,8]. Following surgical intervention, an estimated 90% subjects demonstrate significant amelioration of hearing ability. Hearing remains unaltered or declines in occasional instances [7,8]. Laser techniques and vein grafting is associated with superior outcomes. Additionally, reoccurrence of conductive hearing loss may ensue due to displacement of prosthetic implant. Original position of prosthesis is altered on account of collagen contraction within the neo-membrane configured between prosthesis and bony labyrinth [7,8]. Revision surgery may exhibit ambiguous outcomes and is usually adopted for treating associated clinical symptoms as facial nerve palsy, persistent vertigo or failure of meliorated hearing [7,8]. Nevertheless, revision surgery may be accompanied by inferior outcomes [7,8]. Strict monitoring is required in subjects with a family history of otosclerosis. Hearing loss in pregnant subjects may appear due to otosclerosis [7-16].
References
- Zafar N, Jamal Z, Moien AB Khan. ”Otosclerosis” Stat Pearls International, Treasure Island, Florida.
- Rudic M, Keogh I, R Wagner, E Wilkinson, N Kiros, et al. (2015) The pathophysiology of otosclerosis: Review of current research. Hear Res 330(Pt A): 51-6.
- Viza Puiggrós I, Granell Moreno E, Carlos Calvo Navarro, Mercè Bohé Rovira, César Orús Dotu, et al. (2020) Diagnostic utility of labyrinth capsule bone density in the diagnosis of otosclerosis with high resolution tomography. Acta Otorrinolaringol Esp 71(4): 242-248.
- Bittermann AJ, Wegner I, Bo Jan Noordman, Robert Vincent, Geert J M G van der Heijden, et al. (2014) An introduction of genetics in otosclerosis: a systematic review. Otolaryngol Head Neck Surg 150(1): 34-39.
- Schrauwen I, Weegerink NJ, E Fransen, C Claes, R J E Pennings, et al. (2019) A new locus for otosclerosis, OTSC10, maps to chromosome 1q41-44. Clin Genet 79(5): 495-497.
- Crompton M, Cadge BA, Joanna L Ziff, Andrew J Mowat, Robert Nash, et al. (2019) The Epidemiology of Otosclerosis in a British Cohort. Otol Neurotol 40(1): 22-30.
- Arli C, Gulmez I, Elif Tuğba Saraç, Şemsettin Okuyucu (2020) Assessment of inflammatory markers in otosclerosis patients. Braz J Otorhinolaryngol 86(4): 456-460.
- Morrison AW (1967) Genetic factors in otosclerosis. Ann R Coll Surg Engl 41(2): 202-237.
- Image 1 Courtesy: Medic for you.
- Image 2 Courtesy: UCL discovery.
- Image 3 Courtesy: Medical dictionary.
- Image 4 Courtesy: Springer link.
- Image 5 Courtesy: Health Jade.
- Image 6 Courtesy: Otolaryngology clinics of North America.
- Image 7 Courtesy: Plural publishing.
- Image 8 Courtesy: Research gate.

 Review Article
Review Article