Abstract
Background: Pontocerebellar hypoplasia (PCH) is a genetically heterogeneous
condition, characterized by malformation of the cerebellum, the ventral portion of
the pons and inferior olivary nucleus, as well as supratentorial atrophic alterations.
Cortical migration defects such as lissencephaly may be associated. To date, at least
thirteen subtypes of PCH with distinct genotypes and phenotypes have been described.
Case Report: We report a case of classical PCH, without cortical migration defect,
presented with severe global developmental delay, spasticity and dystonia. Whole
exome sequencing detected a missense heterozygous mutation in exon 8 of PAFAH1B1
gene. Parental analysis confirmed that it was a novel de novo mutational event,
supporting its association with PCH.
Conclusion: Pathogenic variants in PAFAH1B1, which have been widely associated
with laminar heterotopia and lissencephaly, had never been linked to isolated PCH.
However, we present a case report in which this association was found. Does this case
report bring a new phenotype for an “old” gene, or an “old” phenotype with a “new”
gene?
Keywords: Pontocerebellar Hypoplasia; PAFAH1B1; LIS1; Whole Exome Sequencing; Brain Malformation
Abbreviations: PCH: Pontocerebellar Hypoplasia; PAFAH1B1: Platelet-Activating Factor Acetylhydrolase IB Subunit Alpha Gene; LIS1: Lissencephaly 1 Gene; DCX: X-Linked Doublecortin Gene; ARX: Aristaless-Related Homeobox X-Linked Gene; tRNA: Transfer Ribonucleic Acid; TSEN2: tRNA- Splicing Endonuclease Subunit 2; SEPSECS: O-Phosphoserine t-RNA Selenocysteine tRNA Synthase; CLAM: Cerebellar Atrophy With Progressive Microcephaly; RARS2: Arginyl-tRNA Synthetase 2; VRK1: Vaccinia Related Kinase 1; CASK: X-Linked Calcium/Calmodulin-Dependent Serine Protein Kinase; WD40: Water Displacement 40th Formula
Introduction
The advance of gene sequencing techniques has made it
possible to determine the genetic origin of an increasing number
of central nervous system malformations which previously did
not have a defined etiology. Pontocerebellar hypoplasia (PCH) is a
robust example of great variability of phenotypes associated with a
specific group of malformations, characterized by atrophic changes
of the cerebellar vermis and hemispheres, the ventral portion of the
pons and inferior olivary nucleus, often associated with defects in cortical development and derived from mutations in a wide range
of genes. To date, at least thirteen subtypes of pontocerebellar
hypoplasia with distinct genotypes and phenotypes have been
described, but none of them were caused by variants on plateletactivating
factor acetylhydrolase IB subunit alpha gene (PAFAH1B1),
related to lissencephaly [1]. Lissencephaly is a spectrum of cortical
development malformations, characterized by neuronal migration
defects, which comprises agyria, pachygyria and subcortical band
heterotopia [2,3]. PAFAH1B1, also known as Lissencephaly 1 gene
(LIS1), was the first gene identified as being related to lissencephaly,
followed by X-linked doublecortin gene (DCX) [4].
Classical lissencephaly (or type 1 - lissencephaly), characterized
by the presence of a thick cortex (composed of four abnormal
layers) and the absence of other associated brain abnormalities
(e.g severe congenital microcephaly, agenesis of the corpus
callosum, or cerebellar hypoplasia) [4], is caused by mutations
in some specific genes: PAFAH1B1, DCX (in males; in females,
DCX mutations are associated with subcortical band heterotopia)
and Aristaless-related homeobox, X-linked gene (ARX), in this
case, characterized by a three-layered cortex [4]. There are other
phenotypes of lissencephaly, associated with microcephaly (called
“microlissencephaly”), agenesis of the corpus callosum or even
cerebellar hypoplasia. However, until the present moment, there
has been no record of mutations in PAFAH1B1 presented with
pontocerebellar hypoplasia without cortical malformations, as we
describe in this report.
Case Report
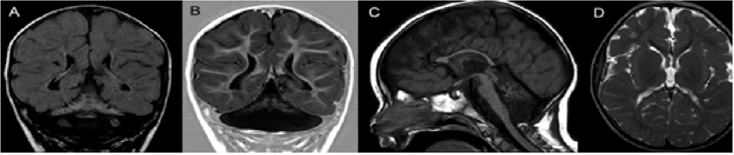
The proband is an 8-year-old male, first-child of nonconsanguineous parents, who was born after an uneventful pregnancy, labor and perinatal period (Birth weight: 3,960 g; occipitofrontal circumference: 33 cm). He presented micropenis and surgically corrected bilateral cryptorchidism. He has presented severe global developmental delay, a failure to thrive and deceleration of occipitofrontal circumference growth. Currently he has profound intellectual deficiency, inconstant eye contact, bilateral strabismus, inability to maintain his head up and presents spasticity and dystonia. His occipitofrontal circumference is 47.5 cm (z-score<-3) and fundoscopy is normal. Brain MRI (Figure 1) at 18 months of age disclosed a classic PCH with reduced white matter but normal cortical gyration pattern.
Note: Brain MRI at 18 months of age. Coronal FLAIR (A) and T1 inversion--recovery (B) images disclose a butterfly-type cerebellum, characterized by severe proportional hypoplastic vermis and cerebellar hemispheres. FLAIR image also shows diffuse cerebellar hyperintensity. A sagittal T1-weighted image (C) demonstrates thinning of the corpus callosum, attenuation of the pons, which is almost flat, and a small hypoplastic cerebellar vermis. Axial T2-weighted image (D) shows bilateral reduction of cerebral white matter with unremarkable cortical gyration pattern.
Figure 1: Neuroimaging of Pontocerebellar Hypoplasia.
Genetic Study
Whole exome sequencing was performed in order to identify genetic abnormalities that might be responsible for the clinical and radiological phenotype. No deleterious variants were detected in genes previously associated to PCH, but the patient harbors a missense heterozygous variant p. Arg273Gln (c.818G>A, NM_000430.3; Chr17:2,577,500) in exon 8 of PAFAH1B1, a highlyconserved (PhyloP>2) region and classified by SIFT and Polyphen as deleterious. This variant was neither present in 123,115 individuals from the Genome Aggregation Database (gnomad. broadinstitute.org) nor had been reported before. The variant c.818G>A was confirmed by Sanger sequencing in the index case and his parents were also examined, but it was not present in them.
Discussion
Pontocerebellar hypoplasia (PCH) is inherited as an autosomal
recessive or X-linked trait, and it is characterized by profound
congenital size reduction of the pons and cerebellum. Several genes
have been implicated in PCH, including the autosomal transfer
ribonucleic acid (tRNA) splicing endonuclease subunit 2 (TSEN2),
TSEN15, TSEN34, TSEN54, O-phosphoserine t-RNA selenocysteine
tRNA synthase (SEPSECS), cerebellar atrophy with progressive microcephaly (CLAM), arginyl-tRNA synthetase 2 (RARS2), vaccinia
related kinase 1 (VRK1) and the X-linked Calcium/Calmodulin-
Dependent Serine Protein Kinase (CASK) [5,6]. Up to now, no
dominant inheritance has been associated with PCH. Herein, we
report a case of classical PCH, associated with decreased white
matter volume, although there is no cortical migration defect.
However, molecular testing revealed a novel de novo heterozygous
mutation in PAFAH1B1 (LIS1). This gene has been associated with
laminar heterotopia and lissencephaly, occasionally combined
with PCH [7,8]. In a comprehensive investigation of a large series
of PCH, only 60% of cases have their molecular basis unraveled
[5]. PAFAH1B1 product plays a critical role in neuronal migration
during brain development [7,9].
Haploinsufficiency of PAFAH1B1 leads to neuronal migration
defects of variable degrees of severity of the lissencephaly
spectrum (OMIM # 607432), including Miller-Dieker syndrome
(OMIM#247200). The p. Arg273Gln occurs in the hinge between two
of the seven Water Displacement 40th Formula (WD40) domains,
which are supposed to form a ring propeller-like structure. No
missense mutations in any of the hinge regions of WD40 domains
have been reported so far (The Human Gene Mutation Database).
The variant Arg273*, leading to premature stop codon, has been
reported several times associated with lissencephaly. De novo
mutations in coding regions leading to protein structural change
occur on average once at every generation [10]. Finding this type
of change in a highly conserved 6 gene that is active during central
nervous system formation strongly supports its association with
PCH. As WES becomes more widespread, the number of genes
associated with PCH will probably increase, and PAFAH1B1 might
be one of these newcomers. A “new” phenotype for an “old” gene,
or an “old” phenotype with a “new” gene?
Conflict of Interest Disclosures
This study has no sponsorship or funding of any kind. There are no conflicts of interest to disclose.
Ethics
This report complies with Brazilian law. The family gave written permission to publish this report. Whole exome sequencing has been performed for clinical diagnosis only, so an institution’s ethics committee’s approval is not required for this purpose.
Acknowledgement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES/ PROEX) - Finance Code 001 - according to process number 23038.018285/2019-21/PROEX PPGN/FMUSP.
References
- Van Dijk T, Baas F, Barth PG, Poll-The BT (2018) What's new in pontocerebellar hypoplasia? An update on genes and subtypes. Orphanet J Rare Dis 13(1): 92.
- Di Donato N, Timms AE, Aldinger KA, Mirzaa GM, Bennett JT, et al. (2018) Analysis of 17 genes detects mutations in 81% of 811 patients with lissencephaly. Genet Med 20(11): 1354-1364.
- Saillour Y, Carion N, Quelin C, Leger PL, Boddaert N, et al. (2009) LIS1- Related Isolated Lissencephaly Spectrum of Mutations and Relationships with Malformation Severity. Arch Neurol 66(8): 1007-15.
- Fry AE, Cushion TD, Pilz DT (2014) The genetics of lissencephaly. Am J Med Genet Part C Semin Med Genet 166C(2): 198-210.
- Namavar Y, Barth PG, Kasher PR, Van Ruissen F, Brockmann K, et al. (2011) Clinical, neuroradiological and genetic findings in pontocerebellar hypoplasia. Brain 134(Pt 1): 143-156.
- Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, et al. (2008) Mutations of CASK cause an X- linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet 40(9): 1065-1067.
- Coquelle FM, Caspi M, Cordelières FP, Domplerre JP, Dujardin DL, et al. (2020) LIS1, CLIP- 170's key to the dynein/dynactin pathway. Mol Cell Biol 22(9): 3089-3102.
- Uyanik G, Morris-Rosendahl DJ, Stiegler J, Klapecki J, Gross C, et al. (2007) Location and type of mutation in the LIS1 gene do not predict phenotypic severity. Neurology 69(5): 442-447.
- Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, et al. (2004) Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol 165(5): 709-721.
- Veltman JA, Brunner HG (2012) De novo mutations in human genetic disease. Nat Rev Genet 13: 565-575.

 Case Report
Case Report