Abstract
Background and Objective: Blighia unijugata is usually used for its many
therapeutic indications in Côte d’Ivoire. This study evaluated the effects of oral
administration of an active butanol fraction of Blighia unijugata for 28 days on some
biochemical blood parameters of male rats.
Materials and Methods: The active butanolic fraction of Blighia unijugata (BFBu)
was prepared by successive liquid-liquid extractions of the total ethanolic extract of
this plant with water, hexane, chloroform, ethyl acetate and n-butanol. Male albino
rats (Rattus norvegicus) of Wistar strain were assigned into four groups of six animals
each including a control group receiving distilled water and three test groups receiving
BFBu at 50; 500 and 1000 mg/kg bw for 28 days respectively. The substances were
administered orally, once a day. Blood samples were taken on days 7; 14; 21 and 28
of treatment by puncture at the level of the orbital sinus of the eye of rats previously
anesthetized with ether for the determination of biochemical parameters using
an automatic analyzer (KENZA MAX Biochemis Try, France). Biochemical analysis
included creatinine, urea, total protein, transaminases (AST, ALT), total cholesterol,
HDL-cholesterol, triglycerides, bilirubins (direct and indirect), glucose, electrolytes
(sodium, potassium, chlorine, calcium, magnesium) and atherogenicity index.
Results: At repeated doses of 50, 500 and 1000 mg/kg bw for 28 days, the
extract contributed to a significant increase in certain biochemical parameters such
as AST, ALT, urea, creatinine, and total proteins. On the other hand, this same extract
evaluated under the previous conditions, caused a decrease in the levels of glucose,
total cholesterol, HDL-cholesterol, LDL cholesterol, bilirubins (direct and indirect) and
had no noticeable effect on the variation in the concentration of certain electrolytes.
Conclusion: At high doses, the active butanol fraction of Blighia unijugata caused
mid-term hepatic and renal toxicity. However, at a dose of 50 mg/kg bw, this fraction
is non-toxic. This fraction has hypoglycemic effects and does not predispose rats to the
risk of atherosclerosis and coronary heart disease.
Keywords: Blighia Unijugata; Butanol Fraction ; Biochemical Parameters; Male Rat
Introduction
Blighia unijugata (Sapindaceae), a species widespread in tropical and equatorial regions of Africa has been widely used for its multiple therapeutic properties [1]. In Côte d’Ivoire, Nigeria and Congo, this plant is abundantly consumed as vegetables and also used in the treatment of fever, nausea and vomiting, leprosy, eye pain, cough, migraines, rheumatism, kidney pain and joint stiffness, dizziness and especially high blood pressure [2,3]. However, despite the increased use of this medicinal plant, data-t-on its efficacy and safety are not yet available, thus exposing populations to all kinds of dangers. However, scientific studies and the rational evaluation of plants commonly used in traditional medicine could guarantee their best use, reduce the risk of accidents and allow the establishment of specific treatments for its poisonings [4]. Blighia unijugata has been the subject of several scientific publications due to its multiple uses in traditional medicine [5,6]. However, to our knowledge, very few scientific studies have been undertaken to establish the risks of toxicity of this plant on biochemical blood parameters. Previous work in our laboratory has shown that the butanolic fraction of Blighia unijugata (BFBu) has a significant hypotensive effect in rabbit of the species Oryctolagus cuniculus (Leporidae) compared to the other four fractions from the total ethanolic extract of this plant [7]. This study was therefore carried out to evaluate the effects of this active fraction on serum biochemical parameters of male Wistar rats.
Materials and Methods
Materials
Plant Material: The plant material consisted of powdered
leaves of Blighia unijugata. The fresh leaves were collected in
Abidjan (Cocody) in June 2009. This species was identified
by us using available herbaria and a book written by [8]. The
confirmation was made by the National Floristic Center (NFC) of
Félix Houphouët-Boigny University (Abidjan, Ivory Coast) where a
herbarium specimen has been kept under voucher no165.
Animals: The experiments were carried out on 24 albino rats
(Ratus norvegicus), healthy male, of Wistar strain, aged three
months. Their average body weight was 148.00±7.07 g. These
animals came from the animal facility of the Institut Pasteur de Côte
d’Ivoire located in Adiopodoumé (Abidjan) and were reproduced
in the animal facility of the Laboratory of the Ecology Research
Center of Nangui Abrogoua University (Côte d ‘Ivoire) at an ambient
temperature of 25±3 °C. The photoperiod was 12 hours of light and
12 hours of darkness. Water and pellet food (Ivograin®) were fed
ad libitum to the rats before the experiment began. These animals
were treated according to good laboratory practices [9].
Technical Material : The extraction equipment for the total
ethanolic extract and fractions of Blighia unijugata consisted of
an electric grinder (Culatti, France), an electronic scale (Mettler
Toledo, PB 153-L, Switzerland), a magnetic stirrer (Stuart SB 162,
UK), Buchner funnel, cotton wool and Wattman no1 filter paper,
Erlenmeyer flask, 500 mL conical flask, rotary evaporator (Büchi
R110, Germany) and an oven (Retsch, Germany). The subacute
toxicity study was performed using a gastric tube suitable for
gavage of rats, pastor pipettes, and dry tubes. The analysis of the
biochemical parameters was carried out using an automatic device
(KENZA MAX Biochemis Try, France).
Solvents : The total ethanolic extract, hexanic, chloroform, ethyl
acetate, butanolic and aqueous fractions of Blighia unijugata were
obtained by extractions with different solvents (distilled water,
96% ethanol, hexane, chloroform, ethyl acetate and n-butanol).
Methods
Preparation of the Total Ethanolic Extract of Blighia
Unijugata : The total ethanolic extract of Blighia unijugata leaves
was prepared according to the method of some authors [10]. The
fresh leaves were thoroughly washed with tap water and then
dried at room temperature. They were sprayed using an electric
grinder (Culatti, France). Cold maceration was carried out with
100 g of powder in 2 liters of 96% ethanol, for 48 h, with magnetic
stirring. The resulting solution was first filtered through Buchner
and then through Wattman No1 filter paper. This operation
was repeated twice on the same powder residue. The filtrates
obtained were added and concentrated under reduced pressure
at 45 °C using a rotary evaporator (Büchi R110, Germany). The
concentrated product, obtained after evaporation, was collected in
a container and dried in an oven (Retsch, Germany) at 45 °C for
48 h according to the method described by [11]. The 13.3 g paste
obtained corresponds to the total ethanolic extract (TEE) of Blighia
unijugata. It was stored at -5 °C in a hermetically sealed jar.
Preparation of Fractions of the Total Ethanolic Extract of
Blighia Unijugata : Different fractions of the total ethanolic extract
(TEE) were obtained by successive liquid-liquid extractions, with
four solvents of increasing polarities (hexan, chloroform, éthyl
acétate and n-butanol) according to the methods of [12,13]. Ten
grams of the TEE was dissolved in 200 mL of hot distilled water
(45 °C). The whole was homogenized by magnetic stirring for
15 min and then it was filtered. Aqueous filtrate obtained was
exhausted for 10 min at 27±2 °C with 200 mL of hexane, thus giving
two phases after decantation (hexan phase and residual aqueous
phase). Residual aqueous phase was again treated for 10 min at 27±2 °C with 200 mL of chloroform to in turn give two phases
(chloroform phase and residual aqueous phase). Same operation
was successively treating the residual aqueous phase with ethyl
acetate to also give an ethyl acetate phase and residual aqueous
phase, then with n-butanol to finally obtain butanol phase and
residual aqueous phase after settling. Each organic phases and the
residual aqueous phase obtained was recovered and concentrated
under reduced pressure throught a rotary evaporator (Büchi R110,
Germany). Various concentrated products obtained were collected
in containers and dried in an oven (Retsch, Germany) at 45°C for
24 h according to the method of some authors [14]. Thus, hexan
(0.6 g), chloroform (1.1 g), ethyl acetate (1.6 g), butanol (3.18 g)
and aqueous (3.12 g) fractions were obtained. These end products
were tested on rabbit blood pressure in the laboratory. Of these five
fractions, butanol fraction coded BFBu had a greater hypotensive
effect compared to the other four fractions. BFBu was therefore
chosen for this study. The extractions were repeated several times
in order to obtain a sufficient amount of extract to perform the tests.
BFBu was stored at -5 °C in a tightly closed jar to prevent spoilage.
Methods of the Study of Subacute Toxicity
Preliminary Tests : The results of the acute oral toxicity study
were used as the basis for the selection of the doses of BFBu that
were administered to the rats. The initial dose level, selected on the
basis of this orientation study, is well below the Maximum Tolerated
Dose (MTD). At the end of these tests, the doses of 50; 500 and 1000
mg/kg bw were chosen to be the doses to use during this study.
Distribution and Treatment of Rats : Animals were divided
into 4 homogeneous batches of 6 rats. This homogeneity of the
different batches is a function of the body weight of the rats. The
tests were carried out on a control batch and 3 batches of treated
animals. According to the method described by [15], each rat from
the control group received by gavage distilled water at 10 mL/
kg bw. Butanolic fraction (BFBu), diluted in distilled water was
administered, by gavage, to each of the rats of the 3 test groups,
respectively at doses of 50; 500 and 1000 mg/kg bw. Volume of
FBu administered to the rats of each batch was less than 2 mL. Rats
were given distilled water or BFBu daily by gavage every morning
between 7 and 8 hours AM. GMT for 28 days.
Blood Sample : Days 7, 14, 21 and 28 of treatment, blood
samples were taken on an empty stomach from the rats previously
anesthetized with ether for 2 to 3 minutes, by puncture at the level
of the orbital sinus of the eye, at the level of the eye using pastor
pipettes according to the technique described by [16-18]. Thus, 2
to 4 mL of blood from each animal of the 4 batches were collected
in dry tubes.
Determination of Biochemical Parameters : Automatic
device (KENZA MAX Biochemis Try, France) was used for
determination of the biochemical parameters. Blood collected in
dry tubes was centrifuged at 3000 rpm for 10 min and the resulting
serum was stored at -20 °C until assaying for biochemical blood
parameters. Creatinine, aspartate aminotransferase (AST) and
alanine aminotransferase (ALT) were assayed by cinetic method
[19]. Urea was determined by enzymatic method [19,20]. HDL (High
Density Lipoprotein) Cholesterol Assay Method in VITROS HDL
Plate is based on a non-HDL cholesterol precipitation technique
followed by enzymatic detection [21]. Measurements of serum
concentrations of total proteins, total cholesterol, triglycerides,
bilirubins (direct and indirect) were carried out by the colorimetric
method [19-23]. Glucose present in the serum was determined
according to the glucose oxidase colorimetric method with the
“Glucose Trinder GPO-POD” kit [20,24]. Finally, the electrolytes
(magnesium, calcium, sodium, potassium and chlorine) were also
determined by the colorimetric method [25-28].
Evaluation of the Atherogenicity Index
Atherogenicity index of rats was determined according to the
formula of [29].
Atherogenicity index = total cholesterol / HDL − cholesterol
Statistical Analysis
Statistical analysis of data and graphical representations were performed using Graph Pad Prism 5.01 software (San Diego, USA). The results obtained were expressed as the mean followed by the standard error of the mean (M±ESM). Difference between the mean values of the biochemical parameters was determined by one-way analysis of variance (ANOVA 1) and supplemented by Tukey’s test for the comparison of the means of the parameters of the different test groups compared to the control group. The significance level was set at p<0.05.
Results
Effects of Bfbu on Biochemical Parameters
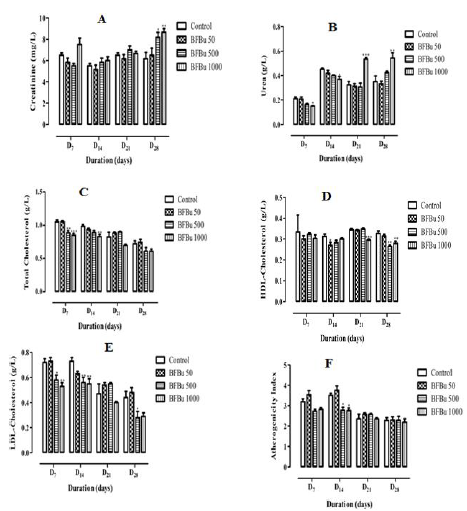
Effect of Bfbu on Creatinine Level : The mean creatininemie of the treated batch with BFBu at 50 mg/kg bw is lower than that of the control from D7 to D21 with the exception of D28 where it is higher than that of the control. D7, the creatinine level of the treated batch with BFBu at 500 mg/kg bw is lower than that of the control. However, from D14, this rate increases until the end of this study with a significantly (p<0.05) high rate with a value of 8.17±0.48 mg/L on D28. Only the creatinine level of the batch treated with BFBu at 1000 mg/kg bw increased throughout this study. This increase of 8.67±0.33 mg/L is significant (p<0.01) on the 28th day of treatment compared to the creatinine level of the control batch which is 6.17±0.60 mg/L (Figure 1A).
Effect of Bfbu on the Urea Rate: In the treated batches with
BFBu at 50 and 500 mg/kg bw, the urea levels decreased compared
to the control during this study, with the exception of D28 where
there was an increase in this level. However, this decrease remains
insignificant throughout this study. Moreover, from D7 to D14, in
the treated batch with BFBu at 1000 mg/kg bw, a significant drop
(p<0.05) in the urea level was noted compared to the control. Rate
decreases and passes respectively from 0.21±0.02 g/L to 0.15±0.0
g/L and from 0.45±0.01 g/L to 0.37±0.02 g/L. However, from the
21st day of treatment, the amount of urea increased significantly
(p<0.01-0.001) in this batch compared to the control batch. The
urea rate increases and goes from 0.33±0.03 g/L to 0.54±0.01 g/L
on D21 and from 0.35±0.04 g/L at 0.54±0.04 g/L on D28 (Figure 1B).
Effect of Bfbu on Total Cholesterol Level: Total cholesterol
level of the treated batch with BFBu at 50 mg/kg body weight is
lower than that of the control on D7 and D14. However, from D21, this
rate undergoes a non-significant increase compared to the control.
Moreover, the treated batches with BFBu at 500 and 1000 mg/kg bw, the total cholesterol level decreased during this study with the
exception of the treated batch with BFBu at 500 mg/kg bw where
this rate increased on D21. This decrease in mean cholesterolemia
is significant (p<0.01-0.001) on D7 and D14 respectively compared
to the mean value of the cholesterol level in the control batch,
but it normalizes from D21. The cholesterol level decreases from
1.05±0.03 g/L to 0.85±0.03 g/L on D7 and from 0.99±0.03 g/L to
0.83±0, 03 g/L on D14 (Figure 1C).
Effect of Bfbu on Hdl-Cholesterol Levels: The mean HDL
cholesterol values of the batch of rats treated with BFBu at 50 mg/
kg bw remained lower than that of the control during this study
with a significant decrease (p<0.05) from a value of 0.27±0.01 g/L
on D14. As for the HDL cholesterol level in the batch of rats treated
with BFBu at 500 mg/kg bw, a decrease was noted during this
work with the exception of D21 where its mean value is similar to
that of the control. The mean value drops significantly (p<0.01)
from 0.33±0.01 g/L to 0.27±0.01 g/L on D28 at this dose. Finally, in
the batch treated with BFBu at 1000 mg/kg bw, the level of HDL
cholesterol decreased throughout this study with a significant drop
(p<0.001-0.01) in this value which went from 0.35±0.01 g/L at
0.30±0.00 g/L and from 0.33±0.01 g/L to 0.28±0.01 g/L respectively
on D21 and D28 after administration of BFBu (Figure 1D).
Effect of BFBu on LDL-Cholesterol Levels: In the treated
batch with BFBu at 50 mg/kg bw, the LDL-cholesterol level did not
undergo any significant variation from D7 to D28 compared to that of
the control batch In the treated batches with BFBu at 500 and 1000
mg/kg bw, the level of LDL-cholesterol decreased during this study.
This drop is significant (p<0.05-0.01) on D7 and D14 in these two
batches. From D21, BFBu does not cause any significant variation
in the LDL cholesterol level with the exception of the treated batch
with FBu at 500 mg/kg of bw where a significant drop (p<0.05) in
this level was recorded on D28 with a value of 0.28±0.05 g/L (Figure
1E).
Evaluation of the Atherogenicity Index of BFBu: At a dose
of 50 mg/kg bw, BFBu did not cause any significant increase in the
atherogenicity index compared to that of the control group during
this study. In the treated batch with BFBu at 500 mg/kg bw, there
was a non-significant decrease on D7 followed by a significant
decrease (p<0.05) in this index on D14 with a value of 2.77±0.16.
From D21 to D28, the atherogenicity index of this batch remains
similar to that of the control batch. Finally, in the treated batch with
BFBu at 1000 mg/kg bw, the atherogenicity index remained low
throughout this study compared to the mean value of the control
batch. This drop is significant (p<0.05) on D14 where the mean value
is 2.76±0.12 (Figure 1F).
Effect of BFBu on Triglyceride Levels: Repeated
administration of BFBu at 50 mg/kg bw causes a non-significant
decrease in the triglyceride level on D7 followed by an increase in
this level from D14 until the end of this study. In the presence of BFBu
at 500 mg/kg bw, the triglyceride level increases during this study
with a significant increase (p<0.01-0.05) respectively on the 7th and
14th day of treatment. The respective average values recorded are
0.66±0.05 g/L and 0.50±0.01 g/L. In the treated batch with BFBu at
1000 mg/kg bw, a significant increase (p<0.01-0.001) in the level
of triglycerides was noted respectively on D7 and D14 where the
average levels are 0.64±0.01 g/L and 0.56±0.02 g/L. This increase
is followed by a non-significant drop in triglyceridemia compared
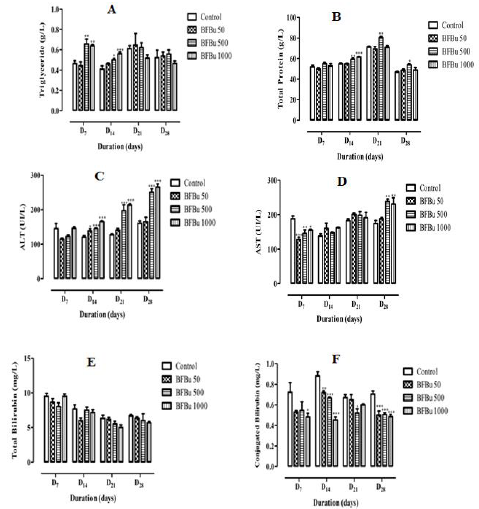
to the control batch on D21 and D28 (Figure 2A).
Effect of BFBu on Total Protein Level: Total protein level
of the treated batch with BFBu at 50 mg/kg bw remained almost
lower than that of the control during this study with the exception
of D28 where this rate increased from 46.80±0.95 g/L at 48.70±1.05
g/L. Variations caused by BFBu at this dose are not significant
(p≥0.05) compared to the control batch. In the treated batches with
BFBu at 500 and 1000 mg/kg bw, an increase in this rate was noted
throughout this work. This increase is significant (p<0.05) in the
treated batch with BFBu at 500 mg/kg bw from D14. The increase
in total protein level caused by BFBu at 1000 mg/kg bw is only
significant (p<0.001) on the 14th day of treatment where its value
is 61.50±0.43 g/L (Figure 2B).
Effect of BFBu on Alanine Aminotransferase Level: Alanine
aminotransferase (ALT) level of the treated batches with BFBu at
50 and 500 mg/kg bw is lower than that of the control batch on
D7. However, from D14 until the end of this study, the level of ALT
increased in these batches compared to that of the control batch.
The increase in this level in the treated batch with BFBu at 500
mg/kg bw is significant (p<0.001) from this period, whereas the
increase in this rate is only exceptionally so on the 14th day in the
lot treated with BFBu at 50 mg/kg bw where its value is 138±6.63
IU/L. In the treated batch with BFBu at 1000 mg/kg bw, the level
of ALT, which remained almost the same to the control on D7,
underwent a significant increase (p<0.001) from D14 to D28 with
values of 165±2.63 IU/L (J14), 213±3.62 IU/L (J21) and 265±9.56
IU/L (J28) (Figure 2C).
Effect of BFBu on Aspartate Aminotransferase Level: On D7,
all the doses of BFBu tested caused a significant decrease (p<0.05-
0.001) in the level of aspartate aminotransferase (AST) compared
to the control. The mean AST value of the control batch is 188±7.92
IU/L. The AST levels recorded are 128±6.94 IU/L, 145±11.00 IU/L
and 155±2.79 IU/L respectively for doses of BFBu of 50, 500 and
1000 mg/kg bw. However, from D14, an increase in the AST rate was
recorded in all the batches treated with BFBu. This increase was
not significant in all of the batch test from D14 to D21. On D28, in the
treated batches with BFBu at 500 and 1000 mg/kg bw, a significant increase (p<0.01) in its mean value was recorded compared to that
of the control batch (Figure 2D).
Effect of BFBu on Total Bilirubin Level: All tested doses of
BFBu, the mean values of the total bilirubin level underwent a nonsignificant
decrease (p≥0.05) compared to that of the control lot
during this study with the exception of the lot treated with BFBu at
1000 mg/kg bw on D7 where this rate, a value of 9.50±0.34 mg/L,
remained almost identical to that of the control (Figure 2E).
Effect of BFBu on Conjugated Bilirubin Level: With regard to
the level of conjugated bilirubin, the mean values in the presence of
all the doses of BFBu tested are lower than that of the control during
this study. At 50 mg/kg bw, a significant drop (p<0.01-0.001) in the
conjugated bilirubin level was recorded on D14 and D28, respectively,
but this decrease was not significant on D7 and D21. At 500 mg/kg
bw, the conjugated bilirubin level underwent a significant decrease
(p<0.05-0.001) from the 14th day of treatment with the exception of
D7 where this decrease is not significant with a value of 0.55±0.08
mg/L. Finally, at 1000 mg/kg bw, the level of conjugated bilirubin
decreases significantly (p <0.05 - 0.001) during the treatment
except on D21 when a non-significant drop in its value compared to
the control was recorded. (Figure 2F).
Figure 1: Effect of Bfbu on Some Biochemical Blood Parameters and Atherogenicity Index.
Note: * p < 0.05; ** p < 0.01; *** p < 0.001; n = 6 : Differences were significant when values of treated groups with BFBu were
compared to that of control group, at the same corresponding day.
Figure 2: Effect of Bfbu on Some Biochemical Blood Parameters.
Note: * p <0.05; ** p <0.01; *** p <0.001; n = 6: Differences were significant when values of treated groups with BFBu were
compared to that of control group, at the same corresponding day.
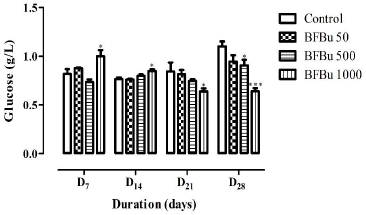
Effect of BFBu on Glucose Level: No significant variation in blood glucose was recorded in the treated batches with BFBu at 50 and 500 mg/kg bw compared to the control during this study. On the 7th and 14th day of treatment, the mean values of the blood glucose level underwent a significant increase (p<0.05) in the treated batch with BFBu at 1000 mg/kg bw compared to the control batch. The average blood sugar values are 1.00±0.06 g/L (D7) and 0.85±0.02 g/L (D14). However, from the 21st to the 28th day of treatment, a significant decrease (p<0.05-0.001) in the blood glucose of the rats was observed in the treated batch with FBu at 1000 mg/kg bw compared to the mean value of the glycemia of the rats of the control group (Figure 3).
Figure 3: Effect of Bfbu on Glucose Levels.
Note: *p<0.05; ** p<0.01; ***p<0.001; n = 6: Differences were significant when values of treated groups with BFBu were
compared to that of control group, at the same corresponding day.
Effects of BFBu on Electrolytes
Effect of BFBu on Sodium Level: Repeated administration
of BFBu at doses of 50, 500 and 1000 mg/kg bw did not impacted
with sodium levels during this study with the exception of the batch
treated with BFBu at 1000 mg/kg bw where an increase significant
(p<0.05) was recorded on the 28th day of treatment compared to
the control batch. The sodium level is 143±1.31 meq/L at this dose
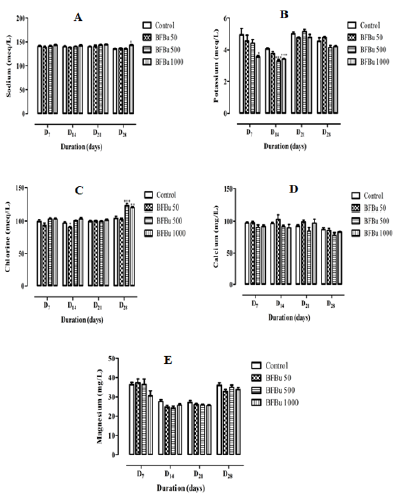
(Figure 4A).
Effect of BFBu on Potassium Level: At 50 mg/kg bw, BFBu
induces a drop in the potassium level with the exception of D28
where this rate increased compared to the control. These variations
are not significant at this dose. At a dose of 500 mg/kg bw, the
potassium level is lower than that of the control during this study.
The drop in this rate is significant (p<0.001) on the 14th day of
treatment in this batch where a value of 3.30±0.10 meq/L was
recorded. However, on D21, the potassium level is higher than that
of the control. As for the treated batch with BFBu at 1000 mg/kg
bw, the potassium level remained lower than that of the control
during this study with significant decreases (p<0.05-0.001) noted
respectively on D7 and D14 but not significant of D21 to D28 compared
to the control batch (Figure 4B).
Effect of BFBu on Chlorine Level: Chlorine level of the
treated batch with BFBu at 50 mg/kg bw decreased significantly
(p<0.05) on D14 compared to the control batch. As for the treated
batches with BFBu at 500 and 1000 mg/kg bw, the chlorine level
increased during this study with exceptionally significant increases
(p<0.01-0.001) in this rate on D28 compared to the control batch.
The respective values recorded at these doses are 122±3.53 meq/L
and 119±1.50 meq/L. However, on D21, the chlorine level is almost
identical in all the batches treated with BFBu (Figure 4C).
Effect of BFBu on Calcium Levels: The calcium level of the
treated batches with BFBu at 50, 500 and 1000 mg/kg bw did
not undergo any significant variation (p≥0.05) during this study
compared to the control (Figure 4D).
Effect of BFBu on the Magnesium Level: On D7, the
magnesium level of the treated batches with BFBu at 50 and 500
mg/kg bw is higher than that of the control. However, from the 14th
day of treatment, this rate decreased in these batches compared
to the control. Only the magnesium level of the treated batch with
FBu at 1000 mg/kg bw continuously decreased during this work.
All the variations caused by BFBu at the doses tested remained
insignificant (p≥0.05) compared to the control during this study
(Figure 4E).
Figure 4: Effect of Bfbu on Electrolytes.
Note: p> 0.05; n = 6: non-significant difference compared to the control batch
* p <0.05; ** p <0.01; *** p <0.001; n = 6: Differences were significant when values of treated groups with BFBu were compared
to that of control group, at the same corresponding day.
Discussion
The various biochemical parameters examined in this study are useful indicators for assessing the toxicity of plant extracts in animals [30]. Analysis of blood parameters is relevant in risk assessment since changes in the hematologic system have a very high predictive value for toxicity in humans [31]. The significant reduction in the level of transaminases (AST and ALT) observed at the start of the experiments indicates that the absorption of BFBu does not cause cytolysis but rather a protective effect in the liver. BFBu does not harm the liver within the first week of administration. However, at the end of this study, the increase in these parameters following the administration of BFBu could be due to the appearance of necrosis in one or more organs, namely the liver, heart and kidneys, thus causing an increase in the level of these enzymes in the blood. Indeed, according to [32], damaged tissue is generally associated with the release of specific enzymes from the tissue, or the affected organ, into the bloodstream. The consequence is an increase in the activity of such enzymes in bodily secretions. ALT is a cytoplasmic enzyme found in very high concentrations in the liver, and an increase in this specific enzyme suggests liver cell damage while AST is less specific than ALT as an indicator of liver function. The increase in the level of transaminases (ALT and AST) has also been observed in liver disorder [33-36]. These results are in agreement with those of [37,38] who reported that the ethanolic and methanolic extracts of Alchornia cordifolia leaves cause an increase in the activity of transaminases. These authors therefore suggested that these extracts exhibit hepatotoxic effects at doses of 250 to 500 mg/kg bw and 800 to 1600 mg/kg bw, respectively. BFBu would therefore be, at the tested doses of 500 and 1000 mg/ kg bw and in the long term, probably poorly tolerated by the liver. According to [39], the increase in the serum total protein level is an indication of tissue damage while the significant decrease in the total protein content of the liver is a reflection of hepatic nontoxicity. The significant increase in the total protein content in the batches of rats treated with BFBu at 500 and 1000 mg/kg bw could therefore indicate an increase in protein stores and therefore suggest hepatic toxicity, which would again confirm the detrimental effects of BFBu at 500 and 1000 mg/kg bw. Bilirubin, a metabolic breakdown product of heme derived from senescent red blood cells, is also one of the most commonly used tests for liver function. Its concentration could indicate the condition of the liver and the type of liver damage [40,41]. The significant reduction in total and conjugated bilirubin levels could be provided by a deficiency in the secretory function of these proteins. Certain substances present in FBu could therefore imply inhibitions at the level of the hepatic cells. This reduction can also affect the functional activity of the liver. Doses of BFBu of 50 and 500 mg/kg bw did not significantly affect blood sugar. Only the dose of 1000 mg/kg bw increased it on D7 and D14 and decreased on D21 and D28. These results suggest that this fraction has hyperglycaemic activity followed by hypoglycaemic activity at high doses. At the end of this study, the hypoglycaemic activity could be attributed to the existence of the molecules demonstrated in this fraction. Indeed, flavonoids from different plants have shown a promising hypoglycemic effect in diabetic animal models [42-44]. Saponins are triterpene, steroidal or alkaloidic glycosides. Authors have shown a hypoglycemic activity of triterpene glycosides [45,46]. It is therefore possible that the presence of flavonoids and saponins in FBu could be responsible for the hypoglycemic effects of this fraction. Some authors reported a significant decrease (p<0.05) in the fasting blood glucose level of rats made diabetic with alloxane when given the ethanolic extract of Ficus microcarpa, orally for two weeks [47]. Finally, these results are in agreement with the studies conducted respectively on the seed cotyledon and leaves of Chrysophyllum albidum at doses between 250 and 1000 mg/kg bw [48,49] on the one hand, and on the other hand on Bixin (10 mg/kg bw) extracted from the seeds of Bixa orellana [50]. These authors have shown that these extracts significantly decrease the glucose level in normal and diabetic rats induced by alloxane. However, the notable increase in blood sugar observed in this study could be linked mainly to the stress undergone by the animals at the time of sampling as suggested by [51] during work with Momordica charantia in rabbits. However, a real hypoglycemic effect could be attributed to this fraction by carrying out further study on suitable models. The significant (p<0.01) increase in mean serum creatinine was seen in the lots that received the highest doses of FBu (500 and 1000 mg/kg bw). This result suggests that BFBu is toxic to the kidneys. Creatinine is the major catabolic product of muscles and is secreted by the kidneys. Serum creatinine levels are used as an indicator of kidney defects [38,52,53]. The significant drop in the urea level observed at the start of treatment in the batch treated with FBu at 1000 mg/kg bw would be a sign of the good functioning of the kidney and the lack of renal toxicity due to this fraction. However, the increase in this level in this batch would be an indication of azotemia. According to Nduka [54], the high urea level is associated with the increased catabolism of tissue proteins. The excess protein in the blood collapses and urea excretion decreases, increasing its level in the blood. The increase in this level would also be a sign of damage to the kidney, which can no longer extract nitrogenous excretion products from the blood and concentrate them in the urine, thus confirming the renal toxicity caused by BFBu. Other indicators of kidney function such as sodium, potassium and chlorine were significantly affected by BFBu except calcium and magnesium which were not. The urea, sodium and chlorine levels significantly increased in the treated batches with BFBu at the highest doses during this study. These results probably suggest nephrotoxicity of BFBu at high doses, which would support the hypothesis made about the increased creatinine level. However, the lack of a significant effect of BFBu on serum calcium and magnesium concentrations in animals suggests that the secretory capacity of the kidney and normal organ function related to these parameters were not affected. According to the work of [41] on the aqueous extract of Felicia muricata at doses of 50, 100 and 200 mg/kg bw, the calcium and magnesium levels of the rats were not significantly modified. The hypokalaemia caused by repeated administration of BFBu may be due to an alteration in tubular reabsorption of potassium. Similar effects have been reported by [55] with the aqueous extract of Arctotis arctotoides on the decrease in potassium concentration. These authors suggested that this plant would not cause any damage to the cardiovascular system, which could also be the case with BFBu. Changes in the concentration of major lipids such as total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides, as well as the atherogenicity index may provide useful information on lipid metabolism and heart predisposition, atherosclerosis and its associated coronary heart disease [29,56-58]. In this study, the decrease in serum cholesterol level could be explained by the deterioration of cholesterol biosynthesis. This has also been suggested by [59] who showed that the aqueous extract of Chrysocoma ciliata at doses of 50, 100 and 200 mg/kg body weight decreases the cholesterol level in rats. Elevated cholesterol concentration is an important risk factor for cardiovascular disease [60]. Therefore, lowering cholesterol at the doses of BFBu tested could be clinically beneficial as this fraction is unlikely to be associated with cardiovascular risk. Triglycerides are the storage forms of fatty acids. The decrease in the level of lipids such as HDL and LDL cholesterols as well as the decrease in the atherogenicity index may be an indication that FBu would not predispose animals to the risk of atherosclerosis and associated coronary heart disease. According to several authors, the total cholesterol/cholesterol- HDL ratio constitutes a revealing index of arterial and especially coronary risk. This ratio would indicate an increased risk if it is greater than 4.4 [29]. However, according to the results obtained during this study, the atherogenicity index of each animal was lower than this value. BFBu could therefore be protective of heart tissue. Furthermore, the decrease in serum lipid parameters investigated in this study suggests that the lipid metabolism of animals was disturbed by the administration of BFBu. This disturbance could be an indication that BFBu would probably not predispose the animal to atherosclerosis and associated coronary heart disease despite the notable elevation (p<0.001) in triglyceride levels during the first 14 days in batches treated with BFBu at 500 and 1000 mg/ kg bw.
Conclusion
This study showed that butanol fraction of Blighia unijugata (BFBu) produced changes in biochemical parameters following repeated administration. BFBu caused disruption of the carbohydrate, lipid and protein balances. At high doses, BFBu caused hepatic and renal toxicity. However, the fraction tested at dose of 50 mg/kg bw, is non-toxic. This fraction has hypoglycemic effects and could be used in the management of diabetes, respectively. BFBu would not predispose rats to the risk of atherosclerosis and associated coronary heart disease.
Conflict of Interest
The authors declare no conflict of interest.
References
- Hyde MA, Wursten B (2002) Flora of Zimbabwe: Species information: Blighia unijugata. CITIS Ed, Harare, pp. 317-318.
- Adjanohoun E, Aké Assi L (1979) Contribution au recensement des plantes médicinales de Côte d’Ivoire. Centre National de Floristique, Abidjan, Côte d’Ivoire, pp. 272-274.
- Burkill HM (2000) The useful plants of West tropical Africa (2nd)., Volume 5, Families S-Z, Addenda Royal Botanic Gardens, Kew, United Kingdom; Richmond, p. 11-13.
- Gies JP (1993) Bases de pharmacologie molé Ed. Marketing, Paris, France, p. 5-6.
- N'dia KF, Bléyéré NM, Kouakou KL, Abo KJC, Yapo AP, et al. (2013a) Acute toxicity in mice and effects of a butanol extract from the leaves of Blighia unijugata (Sapindaceae) on electrocardiogram of Rabbits. Scholars Academic Journal of Pharmacy 2(6): 429-435.
- N'dia KF, Kouakou KL, Bléyéré NM, Yapo AP, Ehilé EE (2013b) Hypotensive effects of a butanol active fraction from leaves of Blighia unijugata (Sapindaceae) on arterial blood pressure of rabbit. World Journal of Pharmacy and Pharmaceutical Sciences 2(6): 6693-6705.
- N’dia KF (2015) Effets pharmacologiques et physiologiques d’extraits de Blighia unijugata (Sapindaceae) chez des mammifères (lapins, rats, souris). Thèse unique, Université Nangui Abrogoua, Abidjan (Côte d’Ivoire), pp. 184.
- Aké Assi L (2002) Boissiera 58. Flore de la Côte d’Ivoire : catalogue systématique, biogéographie et écologie II. Mémoires de botanique systématique, pp. 117-124.
- (1998) OCDE (1998) Série sur les principes de bonnes pratiques de laboratoire et vérification du respect de ces principes. ENV/MC/CHEM 98(17): 22-23.
- N’dia KF, Traoré F, Kouakou KL, Ehilé EE (2009) Effets pharmacologiques d’un extrait aqueux de Mirabilis jalapa L (Nyctaginaceae) sur le système cardiovasculaire, la respiration et l’activité mécanique intestinale de mammifè Afrique Science 5(2): 330-348.
- Guédé-Guina F, Vangah MM, Harouna D, Bahi C (1993) Potencies of Misca, a plant source concentrate against fungi. J Ethnopharmacol 14(2): 45-53.
- Kéita AA, Mariko E, Haïdara TK (1998) Etude de l’activité hypoglycémiante des feuilles de Sclerocarya birrea (A. Rich.) (Anacardiaceae). Pharm Méd Trad Afr 10: 16-25.
- Sakandé J, Nacoulma OG, Nikiéma JB, Lompo M, Bassené E, et al. (2004) Etude de l’effet antipyrétique d’extraits des inflorescences mâles du rônier Borassus aethiopum Mart (Arecaceae). Méd Afr N 51(5): 280-282.
- Zirihi GN, Kra AKM, Bahi C, Guédé-Guina F (2003) Plantes médicinales immuno stimulantes : critères de sélection, techniques rapides d’extraction des principes actifs et méthodes d’évaluation de l’activité immunogè Rev Pharm Afr 17: 131-138.
- Watcho P, Nchegang B, Nguelefack TB, Kamanyi A (2010) Evaluation des effets prosexuels des extraits de Bridelia ferruginea chez le rat mâle naï Androl 20(3): 209-215.
- Waynforth BH (1980) Injection Techniques-Experimental and Surgical Techniques in the Rat. Academic Press, London, p. 3-61.
- Jones W, Mohr H (1990) Hemopoietic system-Springer Verlag. Lea & Febliger, Philadelphia 9: 149-154.
- Lima LB, Vasconcelos CFB, Maranhão HML, Leite VR, Ferreira PA, et al. (2009) Acute and subacute toxicity of Schinus terebinthifolius bark extract. J Ethnopharmacol 126(3): 468-473.
- Tietz NW, Prude EL, Sirgard-Anderson O (1994) Tietz textbook of clinical chemistry (2nd)., WB Saunders Company, London, pp. 1354-1374.
- Young DS (1997) Effects of Drugs on Clinical Laboratory Tests. Annals of Clinical Biochemistry 34(6): 579-581.
- Friewald WT, Levy RI, Fredrickson DS (1972) Estimation of the Concentration of Low Density Lipoprotein Cholesterol in Plasma without the use of Preparative Ultracentrifuge. Clinical Chemistry 18(6): 499-502.
- Pearlman FC, Lee RTY (1974) Detection and measurement of total bilirubin in serum, with use of surfactants as solubilizing agents. Clin Chem 20(4): 447-453.
- Zoppi F, Peracino A, Fenili D, Marcovina S, Ramella C (1976) Metodo per la determinazione della bilirubina totale e coniugata. Uso di un tensioattivo cationico come agente solubilizzante. Giom It Chim Cl 1(1): 343-359.
- Sasaki T, Matsy S, Sonae A (1972) Effect of acetic acid concentration on the color reaction in the O-toludine boric acid method for blood glucose estimation. Rinsho Kagaku 1(3): 346-353.
- Gindler ME, Heth DA (1971) Colorimetric determination with bound "Calmagite" of magnesium in human blood serum. Clin Chem 17(7): 662.
- Michaylova V, Illkova P (1971) Photometric determination of micro amounts of calcium with Arsenazo III. Anal Chim Acta 53(1): 194-198.
- Tietz NW (1976) Fundamentals of clinical chemistry. Ed. WB. Saunders Co Philadelphia 2(11): 875-877.
- Tietz NW (1987) Fundamentals of clinical chemistry. Ed. WB. Saunders Co Philadelphia 2: 426-428.
- Panagiotakos B, Pitsavos C, Skoumas J, Chrysohou C, Toutouza M, et al. (2003) Importance of LDL/HDL ratio as a predicator for coronary heart disease events in patients with heterozygous familial hypercholesterolemia: A 15-year follow-up (1987-2002). Curr Med Res Opin 19(2): 89-94.
- Yakubu MT, Akanji MA, Oladiji AT (2007) Haematological evaluation in male albino. Rats following chronic administration of aqueous extract of Fadogia argrestis stem. Pharmacog Mag 3(9): 34-38.
- Olson H, Betton G, Robinson D, Thomas K, Monro A, et al. (2000) Concordance of toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32(1): 56-67.
- Etame Loe G, Dibong SD, Yinyang J, Elimbi M, Ngoule CC, et al. (2018) Étude de la toxicité aigüe et subaigüe de l’extrait au vin de palme des rhizomes de Curcuma longa Journal of Applied Biosciences 132: 13452-13460.
- Varley H (1975) Practical clinical chemistry (4th)., Arnold-Heinemann publishers, India, pp. 350-363.
- Shanker K, Pathak NKR, Trivedi VP, Chansuria JPN, Pandey VB (2002) An evaluation of toxicity of Taxus baccata Linn. (Talispatra) in experimental animals. J Ethnopharmacol 79(1): 69-73.
- Ramaiah SK (2011) Preclinical safety assessment : current gaps, challenges, and approaches in identifying translatable biomarkers of drug-induced liver injury. Clinics in Laboratory Medicine 31(1): 161-172.
- Aïssatou DS, Metsagang JTN, Sokeng CD, Njintang NY (2017) Anthihyperlipidemic and hypolipidimic properties of Tacca leontopetaloides (L) Kuntze (Discoreales : Discoreaceae) tuber’s aqueous extracts in the rats. Brizilian Journal of Biological Sciences 4(7): 67-80.
- Akanmu MA, Adeloye AO, Obuotor EM, Adelusola KA, Iwalewa EO, et al. (2010) Acute and Sub-Chronic Toxicity Potential Effects of Alchornea cordifolia (Euphorbiaceae) in Rats. Nigerian Journal of Natural Products and Medicine 14(1): 14-20.
- Ajibade TO, Olayemi FO (2015) Reproductive and toxic effects of methanol extract of Alchornea cordifolia leaf in male rats. Andrologia 47(9): 1034-1040.
- Gatsing D, Aliyu R, Kuiate JR, Garba IH, Jaryum KH, et al. (2005) Toxicological evaluation of the aqueous extract of Allium sativum bulbs on laboratory mice and rats. Cameroun J Exp Biol 1(1): 39-45.
- Shahjahan M, Sabitha KE, Jamu M, Shyamala Dev CS (2004) Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Indian J Med Res 120(3): 194-198.
- Ashafa AOT, Yakubu MT, Grierson DS, Afolayan AJ (2009a) Toxicological evaluation of the aqueous extract of Felicia muricata Thunb. Leaves in Wistar rats. Afr J Biotechnol 8(6): 949-954.
- Zarzuelo A, Jimenez I, Gamez MJ (1996) Effects of luteolin 5-O-beta-rutenoside in streptozotocin-induced diabetic rats. Life Sci 58(25): 2311-2316.
- Nojima H, Kimura I, Chen FJ (1998) Anti-hyperglycemic effects of N-containing sugars from Xanthocercis zambesiaca, Morus bombycis, Aglaonema trubii and Castanosperrmi australe in streptozotocin-diabetic mice. J Nat Prod 61(3): 397-400.
- Kim HY, Moon BH, Lee HJ, Choi DH (2004) Flavonoids glycosides from the leaves of Eucommia ulmoides with gyration inhibitory activity. J Ethnopharmacol 93(2-3): 227-230.
- Reher G, Slijepcevic M, Krans L (1991) Hypoglycemic activity of triterpenes and tannins from Sarcopoterium spinosum and two Sanguisorba Species. Planta Med 57(8): 57-58.
- Kako M, Miura T, Nishiyama Y, Icchimaru M, Mariyasu M, et al. (1997) Hypoglycemic activity of some triterpenoid glycosides. J Nat Prod 60(6): 604-605.
- Kumar KA, Maheswari MU, Sivashanmugam AT (2007) Hypoglycemic effects of Ficus microcarpa leaves (Chinese Baryan) on alloxan-induced diabetic rats. J Biol Sci 7(2): 321-326.
- Olorunnisola DS, Amao IS, Ehigie DO, Ajayi ZAF (2008) Anti-hyperglycemic and hypoglycemic effect of ethanolic extract of Chrysophyllum albidum seed cotyledon in alloxan induced-diabetic rats. Res. J Applied Sci 3(2): 123-127.
- Adebayo AH, Abolaji AO, Opata TK, Adegbenro IK (2010) Effects of ethanolic leaf of Chrysophyllum albidum G. on biochemical and haematological parameters of albino Wistar rats. Afr J Biotechnol 9(14): 2145-2150.
- Keita H, Dos Santos CBR, Ramos MM, Padilha EL, Serafim RB, et al. (2021) Assesment of the hypoglycemic effect of Bixin in alloxan-induced diabetic rats: in vivoand in silico Journal of Biomolecular Structure and Dynamics 39(3): 1017-1028.
- Sakandé J, Ahiboh H, Edjeme A, Yapo AE (2003) Etude de la tolérance biologique d’une plante à activité antiplasmodiale: Momordica charantia L. (Cucurbitaceae). Mali Médical, XVIII (1&2): 1-4.
- Kaplan LA, Szabo LL, Opherin EK (1988) Clinical chemistry: Interpretation and techniques (3rd)., Lea & Febliger, Philadelphia, pp. 112-231.
- Aliyu R, Adebayo AH, Gatsing D, Garba IH (2007) The effects of ethanolic leaf extract of Commiphora africana (Burseraceae) on rat liver and kidney functions. J Pharmacol Toxicol 2(4): 373-379.
- Nduka N (1999) Clinical biochemistry for students of pathology. Amino Press Ltd, Nigeria, pp. 142-143.
- Jimoh FO, Adepapo AA, Sofidiya MO, Masika PJ, Afolayan AJ (2008) Safety evaluation of the extract from the shoots of Arctotis arctotoides in rats and mice. Afr J Biotechnol 7(18): 3173-3177.
- Philip DM (1995) Plasma enzyme in diagnosis. In: Clinical chemistry in diagnosis and treatment (6th)., Arnold Publishers, London, pp. 303-307.
- Mayes PA (1996) Lipid transport and storage (24th).,. In: Harper’s biochemistry. Murray RK., Granner DK., Mayes PA, Rodwell VW (Eds.)., Prentice Hall International, USA, pp. 254-255.
- Oyebanji BO, Oridupa OA, Ajibade TO, Saba AB (2015) Effect of Sub-Chronic Administration of Tetracera potatoria Roots Extract and Betulinic Acid from the Plant on Haematology and Serum Biochemistry of Wistar Rats. Nigerian Veterinary Journal 36(1): 1144-1153.
- Ashafa AOT, Yakubu MT, Grierson DS, Afolayan AJ (2009b) Effects of aqueous extract from the leaves of Chrysocoma ciliata L. on some biochemical parameters of Wistar rats. Afr J Biotechnol 8(8): 1425-1430.
- Abolaji AO, Adebayo AH, Odesanni OS (2007) Effect of ethanolic extract of Parinari polyandra (Rosaceae) on serum lipid profile and some electrolytes in pregnant rabbits. Res J Med Plants 1(4): 121-127.

 Research Article
Research Article