Abstract
Now-a-days textiles are used in different sectors and various purposes beyond imagination. Among this an important and emerging part of the textile industry is medical, hygiene and health sector. Chitosan and its derivatives, trimethyl chitosan chloride (TMCC) and carboxymethyl-trimethyl chitosan chloride (CMTMCC) were prepared. Chitosan, TMCC and CMTMCC were applied on cotton fabric by pad-drycure method. Different properties of chitosan derivatives and modified fabric were characterized. The yield percentage of chitosan, TMCC and CMTMCC was 66.5, 102 and 93% respectively. The profiles of FTIR spectroscopy of the chitosan, TMCC and CMTMCC synthesized from chitosan were almost similar except the different characteristic peak which shows evidence of the conversion of chitosan to TMCC and CMTMCC. CMTMCC modified fabric exhibited best antimicrobial activity. TMCC and CMTMCC treated fabric showed more wicking height (cm) comparatively than chitosan treated fabric. Moisture absorption and moisture liberation, swelling and water vapor permeability of treated fabric were investigated. Thermal stability of treated fabric was also studied. All these characteristics support the TMCC and CMTMCC treated fabric could be the valuable materials for medical textile sector.
Keywords: Chitosan; Trimethyl Chitosan Chloride; Carboxymethyl Trimethyl Chitosan Chloride; Cotton Fabric; Antimicrobial Activity
Introduction
The consumers are now increasingly aware of the hygienic
lifestyle and there is necessity and expectation for high quality
healthcare textiles. That’s why it is one of the most important,
continuously expanding and growing fields in technical
textiles. Healthcare textiles represent structurally designed
and accomplished for a new health and hygiene-related textiles
products for the well-being of mankind which applications is
diverse, ranging from hospitals, hotels to personal care products.
Because of their importance, a number of chemicals have been
employed to impart antimicrobial activity to textile materials. These
chemicals include inorganic salts, organometallics, phenols and
thiophenols, onium salts, antibiotics, heterocyclic compounds with
anionic groups, nitro compounds, urea and related compounds,
formaldehyde derivatives, amines and synthetic dyes [1]. However,
with the public’s enhanced awareness of eco-safety, there has been
considerable debate about their use, because majority of such
agents are toxic to humans and are not environmentally friendly.
In addition, another big concern is that some of these agents are
being increasingly resisted by microbial pathogens. Therefore, the
role of textile finishers has now become increasingly demanding
and has strengthened the interest in alternative ecofriendly and
biodegradable finishing agents [2-5].
Chitosan is the deacetylated derivative of chitin that is the
second most abundant polysaccharide found on earth next to
cellulose. When chitin is deacetylated over about 60% it becomes
soluble in dilute aqueous acids and is referred to as chitosan. Chitin is the main component in the shells of crustaceans, such as shrimp,
crab [6-9]. Huge amounts of crab and shrimp (prawn) shells have
been abandoned as wastes by worldwide seafood companies. This
has led to considerable scientific and technological interest in chitin
and chitosan as an attempt to utilize these renewable wastes. The
applications of chitosan include uses in a variety of areas, such
as pharmaceutical and medical applications, paper production,
textile dyeing and finishing, fiber formation, wastewater treatment,
biotechnology, cosmetics, food processing, and agriculture [10,11].
There is a greater demand for antimicrobial finishes on textile
goods. Chitosan, a natural biopolymer, has many chemical attributes,
especially its cationic nature, to make it an interesting candidate for
these applications. However, the major problems of chitosan are its
loss of the antimicrobial activity under alkaline conditions due to its
loss of the cationic nature. But chitosan derivatives will overcome
that problem due to retain antimicrobial activity in wide range of
pH that could have a strong economic, social, and environmental
impact, especially in our country.
In view of these ecological, environmental concerns and
to overcome the drawback of chitosan, we are going to explore
chitosan derivatives which will in turn help to develop next
generation healthcare textiles. This will be followed by a focus
on some recent developmental works pertaining to antimicrobial
finishing of textiles using various “green chemistry” approaches in
order to provide safe and novel antimicrobial textiles for aesthetic,
hygienic, and healthcare applications in the near future. The specific
objectives of the present project are: preparation of sustainable
chitosan derivatives from prawn shell waste, modification of cotton
fabric with chitosan derivative and antimicrobial assessment
and different characterization of modified cotton fabric as for
healthcare textiles. The prepared chitosan and its derivatives will
be applied on cotton fabric and the modification will be confirmed
using FTIR, TGA and DTA techniques. As quality healthcare textile,
antimicrobial test, water vapor transmission rate, swelling test,
and tensile strength of finished and unfinished cotton fabric will
be investigated.
Experimental
Materials
Shrimp shell was collected from Mongla (near Sundarban forest), Khulna, Bangladesh that are waste of shrimp processing area. Cotton fabrics were collected from Keya spinning mills Ltd., Bangladesh. Escherichia coli and Staphylococcus aureus bacteria are collected from “Biochemistry and Molecular Biology” department of Rajshahi University. All chemicals that were used in the present investigation were of reagent grade.
Methods
Processing of Prawn Shell Waste: Prawn shell was collected,
washed, dried, and ground to 40-60 mesh using a hammer mill.
The ground prawn shell was then ready for a series of chemical
treatment for extraction of chitin. Through a set of chemical
treatments demineralization, de-protenization and decolouration
of chitin was obtained. Later deacetylation of chitin gave chitosan
[12,13].
Preparation of TMC: TMC was synthesized through
nucleophilic substitution with CH3I as a reagent, sodium iodide
as catalyst and sodium hydroxide (NaOH) as base with N-methyl
pyrrolidone (NMP) as solvent [14].
Preparation of CMTMCC: CMTMCC was synthesized by
reductive carboxymethylation of TMCC that was accomplished by a
chemical reaction between TMCC and monochloroacetic acid in the
presence of sodium hydroxide. An amount of 0.1 g of dried TMCC
was suspended in 10 mL of NMP or isopropanol as solvent and left
to stir overnight at 45°C. After 20 min, under continuous stirring,
0.5 mL of 50% NaOH was slowly added to the reaction mixture
that was stirred for another 45 min. Subsequently, 0.12 g of monochloroacetate
(molar ratio 1:3 to TMC) was added for about 25 min
and reaction was left for 3 h at 60 °C under a nitrogen atmosphere
in order to avoid TMC degradation. Afterwards, pH was adjusted
to neutral with 1M HCl followed by filtration, washing with 70%
ethanol and subsequently with anhydrous ethanol. CMTMC
precipitate was dried under vacuum, dissolved in distilled water
and dialyzed for 3 days against water, changing dialysis medium
twice per day. The dialyzed CMTMCC was lyophilized and later
analyzed [15].
Results and Discussion
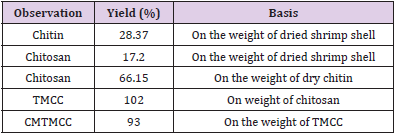
Yield Percentage of Chitin, Chitosan and its Derivatives
The percentage yield was calculated from the ratio of the dried raw sample and the weight of obtained product. This obtained percentage yield is shown in Table 1. The yield percentage of chitosan, TMCC and CMTMCC was 66.5, 102 and 93 % respectively.
FTIR Analysis
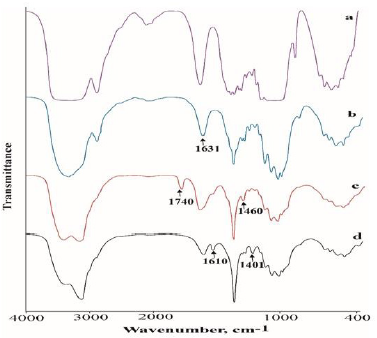
In the FTIR spectrum of the prepared chitosan shown in Figure 1a, there were two absorption peaks at 1631 cm-1 and 2919 cm-1, which correspond to the N−H bending of the primary amine, and the presence of hydroxyl groups respectively. Obvious changes of the FTIR spectra are observed after quaternization of chitosan with methyliodide. In the FTIR spectrum of TMCC, a characteristic peak at 1460 cm-1 indicates the C-H bending of trimethyl ammonium group thus shows evidence of the quaternary ammonium salt group. It should be also noted that the N−H bending (1630 cm-1) of the primary amine disappeared due to the change of the primary amine to the secondary aliphatic amine [16]. A new peak at 1740 cm-1 was also attributed to the quaternary ammonium in TMCC which is shown in Figure 1b. In addition, the spectrum shows a broad band at around 3400 cm 1, probably due to the increased number of hydroxyl groups. The FTIR spectrum of the CMTMCC is shown in Figure 1c. The characteristic peaks of amine (N-H) vibration appeared at 1594 cm-1 for chitosan. Two new absorption peaks at 1401 cm-1 and 1610 cm-1 were attributed to the methyl group of ammonium and carbonyl group in CMTMCC.
The profiles of FTIR spectroscopy of the chitosan, TMCC and CMTMCC synthesized from chitosan are almost similar but have different characteristic peak which shows evidence of the conversion of chitosan to TMCC and CMTMCC.The FTIR spectra of unmodified, chitosan, TMCC modified and CMTMCC modified cotton fabrics were shown in Figure 2. The obtained spectra of both types of fabrics were mostly similar except the new additional peak in the modified cotton fabrics. In case of chitosan modified cotton fabric a new peak at 1631 cm-1 was appeared due to the N-H bending of primary amine. Similarly, characteristic peaks at 1460 cm-1 (C-H bending of trimethyl ammonium group) and 1610 cm-1 (carbonyl group) were attributed to TMCC and CMTMCC modified cotton fabric.
Figure 2: The FTIR spectra of, (a) Unmodified; (b) Chitosan modified; (c) TMCC modified; (d) CMTMCC modified cotton fabric.
Surface Morphology
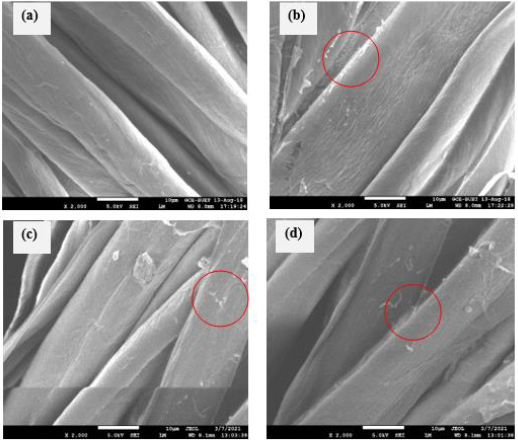
Surface morphology of the unmodified and modified cotton fabric were evaluated using three dimensional images getting from scanning electron microscope Figure 3. Unmodified cotton fabric exhibits regular surface as well as no adherence. Besides modified fabrics were overlaid with mentioned modifier which reveals little bit irregular, or microstructure roughness helps to increase surface area as well as antimicrobial functionality [17]. Conglomerated granule was evidently visible on the fiber surface which proofs the attachment of applied modifier on the fabric.
Figure 3: The SEM images of, (a) Unmodified; (b) Chitosan modified; (c) TMCC modified; (d) CMTMCC modified cotton fabric.
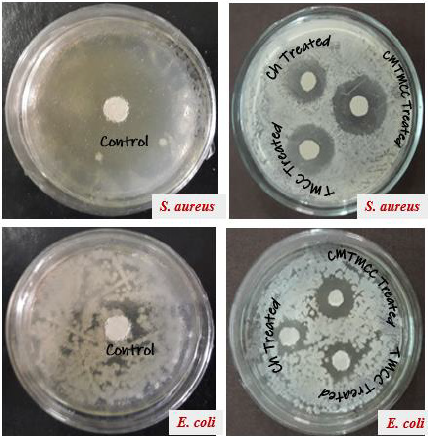
Antimicrobial Activity
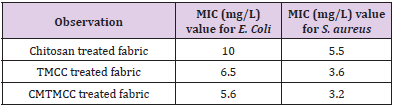
Minimum Inhibition Concentration (MIC) describes the minimum concentration of the modifier in which concentration the multiplication of the bacteria is inhibited. For being different in structure, different modifiers have variation in antimicrobial activity. The Table 2 & Figure 4 show that antimicrobial activity of chitosan, TMCC and CMTMCC treated modified fabric. Chitosan have antimicrobial activity against both E. coli and S. aureus, because of the positive charge on the C-2 of the glucosamine monomer below pH 6. The exact mechanism of the antimicrobial action of chitosan, and their derivatives is still unknown, but different mechanisms have been proposed. Interaction between positively charged chitosan molecules and negatively charged microbial cell membranes leads to the leakage of proteinaceous and other intracellular constituents. Chitosan acted mainly on the outer surface of the bacteria. At a lower concentration the polycationic chitosan does probably bind to the negatively charged bacterial surface to cause agglutination, while at higher concentrations, the larger number of positive charges may have imparted a net positive charge to the bacterial surfaces to keep them in suspension [18,19]. Chitosan interacts with the membrane of the cell to alter cell permeability. But the problem is that it has lower solubility above pH 6 and also its lower attachment capacity on cotton fabric [20]. For TMCC, the mechanism of antimicrobial activity using on cotton fabric is almost similar to chitosan. The slight difference in antimicrobial activity of chitosan and TMCC is due to having positive charge in nitrogen atom and its higher solubility in water than chitosan.
Figure 4: Antimicrobial activity of unmodified and modified cotton fabric against S. aureus and E. coli stain.
The antimicrobial action is believed to occur when the compounds are absorbed onto the bacterial cell surface, increasing the permeability of the lipid cell membrane and causing death through the loss of essential cell materials. In addition, these derivatives of chitosan are generally more active against grampositive and gram-negative bacteria than chitosan. This effect is believed to be due to adsorption of the polymers onto the bacterial cell surface and membrane with subsequent disruption of membrane integrity. Antimicrobial activity generally increases as the content of the quaternary ammonium moiety increases comparatively than chitosan. CMTMCC has better antimicrobial activity than chitosan and TMCC. Here the mechanism is approximately same to them but the difference is that the attachment capacity of CMTMCC (MIC=5.8mg/L for E. coli and 3.3 mg/L for S. aureus) is more than chitosan and TMCC on the cotton fabric.
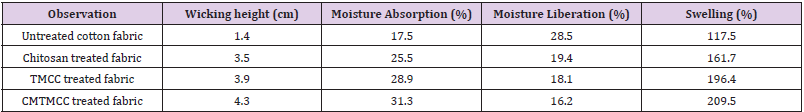
Wicking, Moisture Absorption, Moisture Liberation and Swelling Test Result
Wicking Test: Wicking is the spontaneous flow of a liquid in a porous substrate, driven by capillary forces. Because capillary forces are caused by wetting, wicking is a result of spontaneous wetting in a capillary system. The wicking test results for untreated cotton fabric, chitosan, TMCC and CMTMCC modified cotton are given in Table 3. Untreated cotton fabric shows lower wicking height than the treated cotton fabrics due to the absence of the coating of chitosan, TMCC, and CMTMCC having channel like structure on cotton fabric. Chitosan and its derivatives show higher wicking height (cm). TMCC and CMTMCC have also higher wicking height (cm) comparatively than chitosan treated fabric. The reason for this is TMCC and CMTMCC have channel like structure same as chitosan and also have the presence of positive charge which helps to attract water molecules.
Table 3: Wicking, moisture absorption, moisture liberation and swelling test result of unmodified and modified cotton fabric.
Moisture Absorption: The properties of cotton fiber are
strongly affected by moisture content because it is a hygroscopic
fiber and absorbs or desorbs moisture from the surrounding
atmosphere. In general, the fibers that absorb the greatest amount
of moisture are the ones whose properties change the most. The
main types of properties affected are: dimensional, mechanical
and electrical. The molecular structure of the cotton fiber includes
hydroxyl groups that attract water molecules and hydrogen bond
with the cellulose [21]. Ability to retain a liquid in the fabric is
known as absorption. Absorption consists of several parts, first the
fiber surface is wetted and the liquid is transported into the voids
between the fibers and are absorbed into the fibers and diffuse
[22]. The moisture absorption test results are given in Table 3. The
rate of moisture absorption in untreated fabric sample decreased
as compared to the treated fabric due to the absence of channel like
structure. On the other hand, chitosan, TMCC, and CMTMCC treated
cotton fabrics show more moisture absorption (%) due to the
presence of hydroxyl groups (-OH) and channel like structure. Also
for having the good attachment capacity on cotton fabric CMTMCC
treated cotton fabric showed higher moisture absorption (%).
Moisture Liberation: The moisture liberation ability is one
of the crucial factors for wounds healing, because wound dressing
manages the moisture on the surface and bed of the wound by
absorbing and releasing exudates. The Moisture liberation test
results of untreated and treated cotton fabrics are given in Table
3. Untreated fabric can easily liberate moisture because of having
low capillaries. On the other hand, chitosan, TMCC, and CMTMCC
treated cotton fabrics have channel like structure and for this it is
unable to moisture liberation.
Swelling Test: Moisture/liquid transport in textile fabrics is one
of the critical factors affecting physiological comfort. In conditions
where wearers sweat a lot (e.g. high-level bodily activity), it is not
only desirable for the fabric next to the skin to absorb liquid rapidly
but also to transport it through the fabric promptly to avoid the
discomfort of the fabric sticking to the skin. The comfort afforded
by textile fabrics can be improved by understanding the liquid
transport mechanism [23]. The swelling capacity of an antibacterial
fabric plays an important role in the antibacterial activity, wound
healing capacity, and for biomedical application due to their high
water/solvent holding capacity. They can further absorb a slight to
moderate amount of the wound exudates by swelling which helps
in fast healing of the wound. The swelling test results are list in
Table 3. Swelling % of untreated fabric is lower than others because
of lacking the cross-linked channel structure to hold water for being
swollen. Then the swelling capacity of CMTMCC is more than others
for having not only the presence of hydrophilic groups in the film
networks - as like as chitosan and TMCC which assist in improving
the swelling characteristic of the fabric but also N atom containing
positive charge and higher attachment capacity to the fabric.
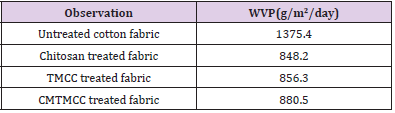
Water Vapor Permeability (WVP) Test
Bolton studied a variety of dressings and determined that a WVTR of about 840 g/m2/day is required to maintain a moist wound surface [24]. Water Vapor permeability occurs mainly for having different vapor pressure on both sides of the cotton fabrics. The WVP test results are given in Table 4. WVP of untreated cotton fabric is high because covering of fabrics with modifiers isn’t occurred on it. For Chitosan, TMCC, and CMTMCC treated fabrics WVP comparatively increases due to the presence of hydroxyl groups. Here after applying Chitosan, TMCC, and CMTMCC on cotton fabric hydrophilic properties are raised and channel like structures are formed which attract water vapor and help to pass away to the outer side of the fabric to saturate the outer side. Among of them CMTMCC have higher WVP because it further has higher attachment capacity on cotton fabric causes more water vapor permeability through cotton fabrics. Fabrics used for wound dressing should have neither higher nor lower WVP value. So for this CMTMCC treated cotton fabrics are more suitable to provide moist wound condition.
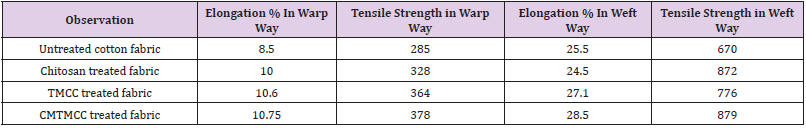
Tensile Strength Test
The tensile strength is one of the most important physical properties of the fabrics. The tensile strength of the fabric contributes greatly to its quality as well as its life. The strength of the fabrics depends on of many different factors that affect it. Molecular structure, temperature, composition, are some of the factors that are responsible for the increase or decrease in the tensile strength of fabric. It is well known that chitosan significantly imparts stiffness in fabric and affect tensile strength of fabric. In this study cotton fabrics are bio-scoured in presence of cellulase enzyme and for this hydrolysis is occurred which causes the loss of tensile strength of cotton fabric. Then to regain the tensile strength the cotton fabrics are treated with chitosan and its derivatives. Tensile strength test results are listed in Table 5. Tensile strength of untreated fabric is low due to hydrolysis during bio-scouring with cellulase. For the chitosan, TMCC, and CMTMCC treated cotton fabrics, tensile strength increases because of binding between fibres and yarns. Chitosan, TMCC, and CMTMCC to bind fibres and yarns and increases tensile strength. Among the samples, CMTMCC treated fabric have higher tensile strength than others.
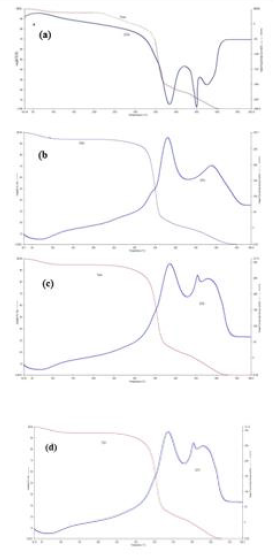
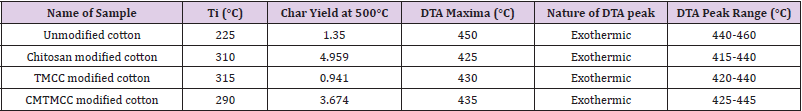
Thermal Analysis
Figure 5 shows TGA and DTA curves of unmodified-, chitosan modified-, TMCC modified- and CMTMCC modified cotton fabrics. It can be seen that the initial decomposition temperature of unmodified cotton fabric is 225OC as shown in Table 6. Also, for DSC curve it is observed that there is a exothermic peak at 450°C which corresponds the burning of the char present in cotton fabric during oxidation. Figure 5b represents TGA and DTA curve for chitosan treated cotton fabric, from this Figure it is obtained two exothermic peaks are seen which correspond to the burning of the incorporated hydrogens and the burning of the char of cotton fabric. The initial decomposition temperature of chitosan cotton fabric is 310OC as shown in Table 6. Now from Figure 5c it is obtained that at 50°C phase transition is occurred, at 80°Can endothermic peak is appeared due to melting behavior and at 340°C another endothermic peak is occurred for the same at 50°C. In this Figure the Initial decomposition temperature was 315°C. And Initial decomposition temperature of CMTMCC modified cotton was 290°C as shown in Table 6.
Figure 5: TGA and DTA curves of, (a) Unmodified cotton; (b) Chitosan modified cotton; (c) TMCC modified cotton; (d) CMTMCC modified cotton.
Conclusion
Different experimental methods were established that chitosan, TMCC and CMTMCC were successfully attached on cotton fabric by pad-dry-cure method. Among the samples, CMTMCC treated fabric have higher tensile strength than others. The rate of moisture absorption in untreated fabric sample decreased as compared to the treated fabric. Antimicrobial activity is the main factor for medical and health care textiles. In this study, chitosan derivatives TMCC and CMTMCC treated cotton fabric exhibited excellent antimicrobial performance. It is expected that this sustainable chitosan derivatives-based healthcare textile retaining durable antimicrobial activities that will be used in hospitals, hotels and personal cares textiles as not only fashion and comfort but also safeguard for human health without disturbing environment. At the same time, the project will help to utilize the by-product or wastage of prawn processing industries.
Acknowledgment
The authors would like to acknowledge the Faculty of Engineering, Rajshahi University, Bangladesh for funding the Project Ref. No.: 1208/5/52/RU/Eng-10/2019-2020.
References
- Gao Y, Cranston R (2008) Recent advances in antimicrobial treatments of textiles. Textile research journal 78(1): 60-72.
- Ibrahim N, Hussain CM (2021) Green Chemistry for Sustainable Textiles. Modern Design and Approaches. Woodhead Publishing, UK.
- Periyasamy AP, Venkatesan H (2017) Eco-materials in Textile Finishing. In: Martínez LMT, Kharissova OV, Kharisov BI (Eds.)., Handbook of Ecomaterials. Springer Nature Switzerland, pp: 1461-1482.
- Khan MI, Ahmad A, Khan SA, Yusuf M, Shahid M, et al. (2011) Assessment of antimicrobial activity of catechu and its dyed substrate. Journal of Cleaner Production 19(12): 1385-1394.
- Oh SW, Kang MN, Cho CW, Lee MW (1997) Detection of carcinogenic amines from dyestuffs or dyed substrates. Dyes and Pigments 33(2): 119-135.
- Roy JC, Salaün F, Giraud S, Ferri A (2017) Chapter -7. Solubility of Chitin: Solvents, Solution Behaviors and Their Related mechanism. In: Xu Z (Edt.)., Solubility of Polysaccharides. Intech Open.
- Elieh-Ali-Komi D, Hamblin MR (2016) Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. International Journal of Advanced Research 4(3): 411-427.
- Rajendran R, Rajalakshmi V, Radhai R (2014) Fabrication of antimicrobial medical textiles using V. Negundo loaded nanoparticle. International Journal of Pharmacy and Pharmaceutical Sciences 3(8): 1394-1406.
- Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan-A versatile semi-synthetic polymer in biomedical applications. Biomaterials Progress in Polymer Science 36(8): 981-1014.
- Bouhenna M, Salah R, Bakour R, Drouiche N, Abdi N, et al. (2015) Effects of chitin and its derivatives on human cancer cells lines. Environmental Science and Pollution Research 22(20): 15579-15586.
- Ahmed TA, Aljaeid BM (2016) Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug design, development and therapy 10: 483-507.
- Hamdan IA, Alhnon, FJ (2020) Extraction, characterization and bioactivity of chitosan from farms shrimps of Basra province by chemical method. Journal of Physics: Conference Series. 1st International Conference on Pure Science (ISCPS-2020).
- Alam R, Khan MA, Khan RA, Ghosal S, Mondal MIH, et al. (2008) Study on the physic mechanical properties of photo-cured chitosan films with oligomer and acrylate monomer. Journal of Polymers and Environment 16(3): 213-219.
- Sieval AB, Thanou M, Kotze AF, Verhoef JC, Brussee J, et al. (1998) Preparation and NMR characterization of highly substitutedN-trimethyl chitosan chloride. Carbohydrate Polymers 36(2-3): 157-165.
- Patrulea V, Applegate LA, Ostafe V, Jordan O, Borchard G, et al. (2015) Optimized synthesis of O-carboxymethyl-N, N, N-trimethyl chitosan. Carbohydrate polymers 122: 46-52.
- Pavia DL, Lampman GM, Kriz GS, Vyvyan JR (2001) Introduction to Spectroscopy (2nd)., Brooks/Cole Thomson Learning, Australia.
- Naebe M, Li Q, Onur A, Denning R (2016) Investigation of chitosan adsorption onto cotton fabric with atmospheric helium/ oxygen plasma pre-treatment. Cellulose 23: 2129-2142.
- Sudarshan NR, Hoover DG, Knorr D (1992) Antibacterial action of chitosan. Food Biotechnology 6(3): 257-272.
- Papineau AM, Hoover DG, Knorr D, Farkas DF (2009) Antimicrobial effect of water‐soluble chitosans with high hydrostatic pressure. Food Biotechnology 5(1): 47-57.
- Martins AF, Facchi SP, Follmann HDM, Pereira AGB, Rubira AF, et al. (2014) Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. International Journal of Molecular Science 15(11): 20800-20832.
- Montalvo JG, Von Hoven TM (2008) Review of standard test methods for moisture in lint cotton. The Journal of Cotton Science 12: 33-47.
- Petrulyte S, Baltakyte R (2008) Investigation into the wetting phenomenon of terry fabrics. Fibres & Textiles in Eastern Europe 16(4): 62-66.
- Benltoufa S, Fayala F, Nasrallah SB (2012) Determination of yarn and fiber diameters after swelling using a capillary rise method. Journal of The Textile Institute 103(5): 517-522.
- Bolton L, Monte K, Pirone LA (2000) Moisture and healing: Beyond the jargon. Ostomy/wound Management 46(1A): 51S-62S.

 Research Article
Research Article