Abstract
Background: The present study was focused on the study of essential oil extraction
from the zest of grapefruit (Citrus paradisi) and the determination of antioxidant and
antitumor activities.
Methods: Grapefruit essential oil was extracted by hydro distillation, grapefruit
essential oil was analyzed for antioxidant potential in the chemical system by the
DPPH, ABTS, FRAP test, and scavenging activity of nitric oxide. The cytotoxicity study
of grapefruit essential oil was assessed using MTT assay against HeLa and MCF-7 tumor
cells.
Results: The extraction yield obtained after optimization was 1.32 %. Antioxidant
activities were studied using chemical systems. The results have been very interesting
since we got 54 %, 83 %, 66 % respectively with DPPH, ABTS, FRAP, and a notable
effect to scavenging NO• radical. In addition, a significant antitumor effect of grapefruit
essential oil was observed in HeLa and MCF-7 tumor cells.
Conclusion: The data serve as the basis for the consumption of Citrus paradisi
essential oil, with significant antioxidant activity, with no harmful effect for the normal
cells, and with antitumor potential.
Keywords: Citrus Paradisi; Essential Oil; Antioxidant Activity; Antitumor Activity; MTT; HeLa; MCF-7
Abbreviations: CPEo: Citrus Paradisi Essential Oil; DPPH: Diphenyl Picryl Hydrazine; ABTS: Azino-Bis-3-Ethylbenzothiazoline- 6-Sulfonic Acid; FRAP: Ferric- Reducing Antioxidant Power; TCA: Trichloroacetic Acid; FCS: Foetal Calf Serum; SD: Standard Deviation
Introduction
Phytomedicine has been of increasing interest in recent years.
Over 65 % of the world’s population relies on traditional and medical
approaches to treating diseases [1]. The return to phytotherapy
is more than using an herb to treat an illness or a crude herbal
preparation rather than a specific isolated component. Plants are
considered essential resources for researchers to prove and develop
new drugs. Generally, using plants in the treatment of disease has
a long history [2,3]. Thus plants have been primary resources
for producing traditional drugs effective in treating cancer and
inflammatory diseases [4]. In addition, plants containing essential
oils have bioactivity against a host of bacteria, fungi, viruses
[5,6]. Currently, essential oils of medicinal plants are increasingly used thanks to their antibacterial, anti-inflammatory, antiseptic,
antiviral, antifungal, antioxidant, and antitumor potential [7].
Essential oils are also used in other areas of economic interest,
such as cosmetics, perfumes, and aromatherapy [7,8]. Fragrant and
aromatic plants such as members of the Asteraceae, Lamiaceae,
Rutaceae, and Verbenaceae produce essential oils, which historically
have been important in traditional medicines [5]. Citrus species
have beneficial pharmacological activities, including antibacterial,
vascular protectant, antispasmodic, analgesic, antipyretic, antiinflammatory,
and antitumoral effects [9-11].
Citrus peel essential oils have also been searched for their natural
antioxidant and antimicrobial properties [12,13]. Furthermore,
studies have shown the synergistic effect of two or more ingredients
of essential oils against various human pathogens. It is necessary to
know more about Citrus paradisi essential oil (CPEo) antioxidant,
cytotoxic, and antitumor activity. Therefore, the DPPH, ABTS, FRAP,
and NO• techniques studied the antioxidant potential in a chemical
system. Furthermore, to check the antitumor activity, MTT was
realized using HeLa and MCF-7 tumor cells.
Material and Methods
Samples
In this study, the grapefruit (citrus paradisi) was collected in March 2018 from Sfax, Tunisia. Sfax is one of the biggest coastal cities in Tunisia. It is located in the eastern part of the country. The climate in this area is arid to semiarid with irregular and torrential precipitations [14]. After the collection, it was washed, peeled off and cut into small pieces.
Hydrodistillation
The zest of citrus paradisi was hydro distilled using a Clevengertype apparatus to recuperate the essential oils for 2h to produce the volatile constituents. The distilled essential oils were dried over anhydrous sodium sulfate; then separated from the distillate by liquid-liquid extraction using cyclohexane solvent. The recuperated oils were stored at + 4 °C.
Antioxidant Activity: Chemical System
DPPH Radical Scavenging Assay: Different aliquots from the stock solution (200μl in 1 ml Me OH) of essential oils were mixed with 500μl of 0.2mM diphenyl picryl hydrazine (DPPH) final volume brought to 1mL. The mixtures were vigorously shaken and allowed to stand in the dark for 30min at room temperature. The absorbance was measured by spectrophotometry (LKB BIOCHROM® ULTROSPECPUS 4054 UV/VIS) at 517 nm against a control sample without DPPH. The percentage of radical scavenging activity was calculated using the following equation:
DPPH scavenging effect (%) = (A0- A1)/A0 × 100
A0: The absorbance of the control at 30min
A1: The absorbance of the sample at 30min.
ABTS+ Radical Scavenging Effect: The antiradical activity
was performed by the ABTS+ free radical decolorization
assay as developed by Re, et al. [15]. The 2,2-azino-bis-3-
ethylbenzothiazoline-6-sulfonic acid (ABTS) was prepared as an
aqueous stock solution (7mM). The ABTS radical cations (ABTS+)
were produced by the reaction of the ABTS stock solution with
2.5mM of ammonium persulfate methanolic solution. First,
the reaction mixture is incubated in the dark for 16h at room
temperature. Then, the solution is diluted to an absorbance of 0.7
± 0.02 at 734nm to form the working reagent. Next, the reaction
mixtures containing 100μl of the sample at different concentrations
and 900μL of reagent were incubated at 30 °C for 6min. Finally, the
antioxidant power of each sample was expressed as the inhibition
percentage calculated according to the following formula:
ABTS+ scavenging effect (%) = (A0- A1)/A0 × 100
A0: the absorbance of the control at 6min
A1: the absorbance of the sample at 6min.
Ferric-Reducing Antioxidant Power (FRAP): This method is
based on the plant’s ability to reduce ferric iron (Fe3+) to ferrous iron
(Fe2+). The mechanism is known to be a marker of electron donor
activity [16]. For 1mL of the sample at different concentrations,
2.5mL of a solution phosphate buffer (0.2M, pH 6.6) and 2.5mL of
1 % K3Fe (CN) 6 potassium ferricyanide solution were added. The
mixture is incubated at 50 °C for 20min and then cooled to room
temperature. Then, 2.5mL of 10 % trichloroacetic acid (TCA) is
added to stop the reaction, and then the tubes are centrifuged at
3000rpm for 10min. 2.5ml of the supernatant are then added
to 2.5mL of distilled water and 500μL of a 0.1 % solution of iron
trichloride (FeCl3, 6H2O) [16]. The absorbance reading is performed
against a blank at 700 nm using a spectrophotometer. Ascorbic acid
is used as a positive control. The increase in the absorption capacity
of the components indicates the increase in the reduction of iron.
Scavenging Activity of Nitric Oxide (NO·): NO scavenging
activity of the essential oils was determined as previously described
[17]. Briefly, 0.1mL of the essential oils (0–0.3mg/ml in DMSO) was
incubated with 0.5mL of sodium nitrite (0.01mg/mL in 100mM
sodium citrate pH 5) at 37 °C for 2h. After incubation, 0.5mL of
Griess reagent was added, and the absorbance was read at 540nm
using a spectrophotometer (Pharmacia, Uppsala, Sweden). The
equation obtained the percentage of RNS scavenging:
NO· Scavenging effect (%) = (A0- A1)/A0 × 100
A0: The absorbance of the control
A1: The absorbance of the sample.
Cytotoxicity Activity
Cell Lines and Culture Conditions: In this study, cancerous
cells were used: HeLa and MCF-7. HeLa is a transformed cell line
expressing the HPV18 virus (human papillomavirus) [18]. MCF-7
(Michigan Cancer Foundation-7) was isolated from a 69 years old
woman with metastatic disease [19]. Hela and MCF-7 cell lines
were supplied by ATCC (Manassas, VA, USA). All cells were grown in
RPMI 1640 medium (Gibco) supplemented with 10 % (v/v) foetal
calf serum (FCS) and 2mM L-glutamine in tissue culture flasks
(Nunc). They are incubated at 37 °C, 95 % air and 5 % CO2.
MTT: For the test of cytotoxicity activity, the MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
(Sigma-Aldrich, St. Louis, MO, USA) test was done [20]. First,
HeLa or MCF-7 cells in 96-well plates were exposed to different
concentrations of CPEo (3 wells for each concentration) and
incubated for 48h at 37 °C. Then, 20μl of MTT was added to each
well. After 4h of incubation at 37 °C, the supernatant was removed,
180μL of DMSO/Methanol (1V/1V) were added to each well to
solubilize the formazan crystals. Finally, the plates were shaken
15min at room temperature, and the absorbance was detected at
570nm with a spectrophotometric plate reader.
Statistical Analysis
The statistical studies are carried out using the program SPSS (19.0). The t-Student test carried out the comparison between the averages. The results are represented as mean ± standard deviation (SD).
Results
The Yield of Grapefruit Essential Oils
The yield of the extraction is defined as the ratio between the
mass of essential oil obtained and the mass of the plant material.
Y (%) = A/A1 ×100
A: Quantity of extracts recovered in g.
A1: Quantity of dry vegetable matter used for extraction
expressed in g.
CPEo yield was 1.32g Eo / 100g dry matter. This result proves
that the zest of the fruit was rich in Eo.
Antioxidant Effects
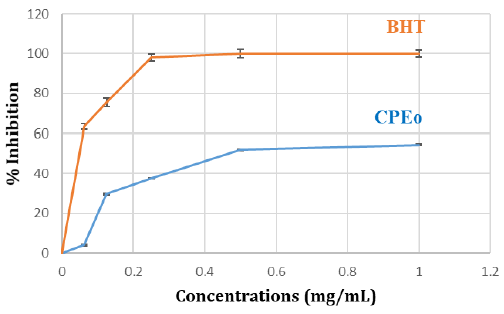
DPPH Radical Scavenging Assay: The curve relating to DPPH radical scavenging shows that the inhibition percentage of the free radical increases with the increase of concentration, either for BHT or CPEo (Figure 1). In effect, Citrus paradisi essential oil has a significant antiradical effect attending 54 % and can be qualified such having an important antioxidant activity.
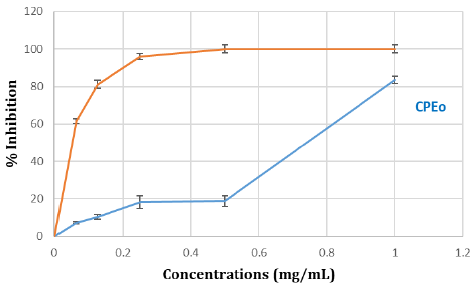
ABTS+ Radical Scavenging Effect: The results illustrated in the curve show that the percentage of inhibition gradually increases with the concentration of grapefruit essential oil. At a concentration of 1mg/mL, the inhibition rate reaches 83 % (Figure 2).
Ferric-Reducing Antioxidant Power (FRAP): The FRAP assay is usually used to measure the capacity of the sample to reduce the ferric complex to the ferrous form. The FRAP assay confirmed the antioxidant activity of CPEo, with values equal to 66 % (Figure 3).
Figure 1: Antiradical activity against the radical DPPH in the percentage of inhibition CPEo. Aliquots of various concentrations of CPEo (0, 0.2, 0.4, 0.6, 0.8, 1mg/mL) and standard BHT were mixed with DPPH and incubated in the dark. The antioxidant activity of CPEo was measured using a spectrophotometer at 517nm. Results were expressed as mean inhibition percentage (%) ± standard deviations (n = 3). BHT was used as the reference standard.
Figure 2: Anti-radical activity against the radical ABTS in percentage of inhibition of CPEo. Various concentrations of CPEo (0 to 1mg/mL) and acid ascorbic were mixed with FRAP reagents. The reduction of ferric ion (Fe3+) to ferrous form (Fe2+) by CPEo produces an intense blue light revealed as a change in absorption at 700nm. Results were expressed as mean inhibition percentage (%) ± standard deviations (n = 3). Ascorbic acid at various concentrations (0 to 1mg/mL) was used as standard.
Figure 3: Inhibition (%) of standard Vit C and CPEo by Ferric Reducing Antioxidant Power (FRAP) assay. Various concentrations of CPEo (0 to 1mg/mL) and acid ascorbic were mixed with FRAP reagents. The reduction of ferric ion (Fe3+) to ferrous form (Fe2+) by CPEo produces an intense blue light revealed as a change in absorption at 700nm. Results were expressed as mean inhibition percentage (%) ± standard deviations (n = 3). Ascorbic acid at various concentrations (0 to 1mg/mL) was used as standard.
NO· Scavenging Assay
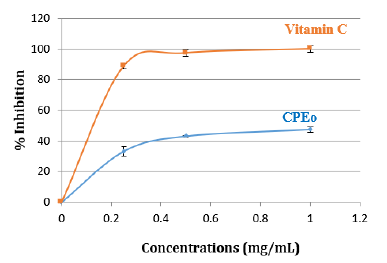
The capacity of citrus paradisi essential oils to scavenge NO was also measured. CPEo was checked for its inhibitory effect on nitric oxide production. The inhibition on free radical percentage increases with elevated concentrations of essential oil and Vitamin C (Figure 4).
Figure 4: Inhibition (%) of standard Vit C and CPEo by Ferric Reducing Antioxidant Power (FRAP) assay. Various concentrations of CPEo (0 to 1mg/mL) and acid ascorbic were mixed with FRAP reagents. The reduction of ferric ion (Fe3+) to ferrous form (Fe2+) by CPEo produces an intense blue light revealed as a change in absorption at 700nm. Results were expressed as mean inhibition percentage (%) ± standard deviations (n = 3). Ascorbic acid at various concentrations (0 to 1mg/mL) was used as standard.
Cytotoxicity Activity
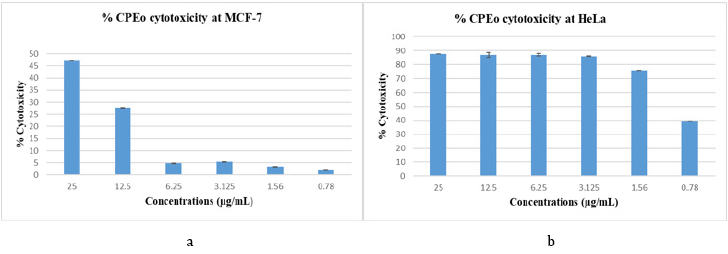
The MTT test studied the cytotoxic effect on HeLa and MCF-7 cells. Cells were cultured in 96-well plates for 48 h, in the presence and absence of the different concentrations of CPEo. The percentage of cytotoxicity was calculated. Our results showed an antitumor potential of the grapefruit essential oils on both cancer cells: Hela and MCF-7 according to the concentrations used: from 0.78 to 25μg / mL (Figure 5).
Figure 5:
(a) Study the cytotoxicity of the essential oil of the grapefruit outer wall in the HeLa
(b) and MCF-7 cell lines.
Cytotoxic activity of citrus paradisi essential oil on cancer cell lines. MTT assessed cell viability. The percent growth reduction
was calculated from the extinction difference between treated cell culture and the control. Results are the means of three
repetitions.
Discussion
The current study was designed to extract the essential oil from grapefruit. It was obtained by hydro distillation, which is the oldest method and the most used because it is very easy to achieve. Our results agree with studies, which confirmed that Citrus is rich in essential oil, and their yields varied from one plant species to another, ranging from 0.2 to 2.0 % [21]. Other work has shown that yield of Citrus paradisi essential oil collected in September in southern Taiwan was attending 0.37 %, and others were collected in October from Iran were 0.85 % [22,23]. So the difference in EOs yields may be related to the plant’s origin, the environmental conditions, time of collection, and extraction method [24]. The CPEo analyzed in this work showed potent radical scavenging activity. We have demonstrated that CPEo has an interesting antioxidant activity highlighted by the DPPH, ABTS+, FRAP, and NO. Our results agree with the literature, which showed that citrus EO possesses an anti-free radical activity DPPH of 17.7 to 64 % [7]. Other studies on grapefruit seed oil showed a high antioxidant potential (61 %) [25]. Furthermore, the effect of citrus paradisi essential oils on ABTS free radical was determined and exhibited a higher radical scavenging activity. Our results corroborate with other studies showing a significant antioxidant effect of C. paradisi EO against ABTS radicals [22]. In addition, the CPEo had a significant FRAP value, which was in accord with the results obtained by Jang et al. that confirmed the higher FRAP values in the peel of grapefruit [26]. The superoxide anion assay is commonly used to evaluate the superoxide anion radical-scavenging ability of plant extract. Therefore, CPEo was checked for their inhibitory effect on nitric oxide production in the present study, attending 47 %. Our results agree with studies that confirmed that the superoxide values of grapefruits varied from 36.83 % to 50.31 % in peels [27]. The antitumor potential of CPEo was tested on HeLa and MCF-7 tumor cells. Our results showed a considerable antitumor activity dependent on essential oil concentration. This result agrees with the work of Zu et al., which shows that essential oil from grapefruit exhibited an antiproliferative effect on MCF-7 cells [28]. Furthermore, Monajemi et al. studied the impact of different concentrations of essential oils of other species of the citrus family as Citrus limon, Citrus medica, and Citrus sinensis on MCF-7 and Hela [29]. They proved a significant decrease in viability in a dose-dependent manner in both tumor cells [29].
Conclusion
Citrus paradisi essential oils are well known for their flavor and fragrance properties and numerous aromatherapeutic and medicinal applications. Accordingly, the essential oils of Citrus species exhibited intense antioxidant activity. In addition, the essential oil of Citrus paradisi showed an antitumor effect against both cancers cells, HeLa and MCF-7.
Acknowledgment
This work is dedicated to the memory of Pr. Saloua Lassoued Elamri. We will never forget her, and she is always in our hearts.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109(1): 69-75.
- Ben Amor I, Musarra Pizzo M, Smeriglio A, D'Arrigo M, Pennisi R, et al. (2021) Phytochemical Characterization of Olea europaea Leaf Extracts and Assessment of Their Antimicrobial and Anti-HSV-1 Activity. Viruses 13(6): 1085.
- Gargouri B, Mansour RB, Abdallah FB, Abdelfetteh Elfekih, Saloua Lassoued, et al. (2011) Protective effect of quercetin against oxidative stress caused by dimethoate in human peripheral blood lymphocytes. Lipids Health Dis 10: 149.
- Sewell RDE, Rafieian Kopaei M (2014) The history and ups and downs of herbal medicine usage. J HerbMed Pharmacol 3(1): 1-3.
- Edris AE (2007) Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review Phytother Res 21(4): 308-323.
- Martinez Velazquez M, Rosario Cruz R, Castillo Herrera G, J M Flores-Fernandez, A H Alvarez, et al. (2011) Acaricidal effect of essential oils from Lippia graveolens (Lamiales: Verbenaceae), Rosmarinus officinalis (Lamiales: Lamiaceae), and Allium sativum (Liliales: Liliaceae) against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). J Med Entomol 48(4): 822-827.
- Choi H S, Song H S, Ukeda H, M Sawamura (2000) Radical-scavenging activities of citrus essential oils and their components: detection using 1, 1-Diphenyl-2- picrylhydrazyl. J Agric Food Chem 48(9): 4156-4161.
- Zenasni L (2014) Etude de polymorphisme chimique des huiles essentielles de Thymus satureioides Coss et d’Origanum compactum Benth et du genre Nepeta et évaluation de leur propriété antibacté Thèse de doctorat. Université Mohammed VAgdal Rabat, pp.155.
- Graziano AC, Cardile V, Crascì L, Sivia Caggia, Paola Dugo, et al. (2012) Protective effects of an extract from Citrus bergamia against inflammatory injury in interferon-γ and histamine exposed human keratinocytes. Life Sci 90(25-26): 968-974.
- Singh J, Sood S, Muthuraman A (2014) In-vitro evaluation of bioactive compounds, antioxidant, lipid peroxidation and lipoxygenase inhibitory potential of Citrus karna L. peel extract. J Food Sci Technol 51(1): 67-74.
- Zhou Z, Xi W, Hu Y, Chao Nie, Zhiqin Zhou (2016) Antioxidant activity of Citrus fruits. Food Chem 196: 885-896.
- Tepe B, Daferera D, Sokmen A, Münevver Sökmen, Moschos Polissiou. (2015) Antimicrobial and antioxidant activities of the essential oil and; various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chemistry 90(3): 333-340.
- Viuda MM, Ruiz NY, Fernández LJ (2008) Antibacterial activity of different essential oils obtained from spices widely used in Mediterranean diet. International Journal of Food Science & Technology 43: 526-531.
- Boughariou E, Allouche N, Jmal I, Mokadem N, Ayed B, et al. (2018) Modeling aquifer behaviour under climate change and high consumption: Case study of the Sfax region, southeast Tunisia. J Afr Earth Sci 141: 118-129.
- Re R, Pellegrini N, Proteggente A, A Pannala, M Yang, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9-10): 1231-1237.
- Karagözler A, Erdaǧ B, Emek Y, Deniz Aktaş Uygun (2008) Antioxidant activity and proline content of leaf extracts from Dorystoechas hastate. Food Chemistry 111(2): 400-407.
- Kavoosi G, Purfard AM, Aram F (2012) Radical scavenging properties of essential oils from Zataria multiflora and Ferula asafoetida. Asian Pacific Journal of Tropical Biomedicine 2(3): S1351-S1356.
- Hatfull G, Bankier BG, Barrell P, PJ Farrell (1988) Sequence analysis of Raji Epstein-Barr virus DNA. Virology 164(2): 334-340.
- Gargouri B, Nasr R, Mseddi M, Riadh benmansour, Saloua Lassoued (2011) Induction of epstein-barr virus (EBV) lytic cycle in vitro causes lipid peroxidation, protein oxidation and DNA damage in lymphoblastoid B cell lines. Lipids in Health and Disease 10: 1-8.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2): 55-63.
- Tu NT, Onishi Y, Choi HS, Yusuke Kondo, Solomon Mitiku Bassore, et al. (2002) Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. Journal of Agricultural Food Chemistry 50(10): 2908-2913.
- Ming Chiu OU, Yi Hsin Liu, Yung Wei Sun, Chin Feng Chan (2015) The Composition, antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L) Osbeck. Evidence-Based Complementary and Alternative Medicine 80: 4091-4099.
- Alireza SD, Mohammad MS, Hassan Vatandoost, Mohammad Reza Abai (2016) Chemical Compositions of the Peel Essential Oil of Citrus aurantium and Its Natural Larvicidal Activity against the Malaria Vector Anopheles stephensi (Diptera: Culicidae) in Comparison with Citrus paradisi. Journal of Arthropod-Borne Diseases 10(4): 577-585.
- Hassiotis C, Lazari D, Vlachonasios K (2010) The Effects of Habitat Type and Diurnal Harvest on Essential Oil Yield and Composition of Lavandula Angustifolia Mill. In Fresenius Environmental Bulletin 19(8): 1491-1498.
- Atolani O, Omere J, Otuechere CA, Adewale Adewuyi (2012) Antioxidant and cytotoxicity effects of seed oils from edible fruits. Journal of Acute Disease 1(2): 130-134.
- Jang HD, Chang KS, Chang TC, Hsu Chuan Liang (2010) Antioxidant potentials of buntan pumelo (Citrus grandis Osbeck) and its ethanolic and acetified fermentation products. Food Chemistry 1118(3): 554-558.
- Xi W, Fang B, Zhao Q, Bining Jiao, Zhiqin Zhou (2014) Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chemistry 161: 230-238.
- Zu Y, Yu H, Liang L, Yujie Fu, Thomas Efferth, et al. (2010) Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 15(5): 3200-3210.
- Monajemi R, Oryan S, Haeri Roohani A, Alireza Ghannadid, Abbas Jafarian (2005) Cytotoxic Effects of Essential Oils of Some Iranian Citrus Peels. In Iranian Journal of Pharmaceutical Research 3: 183-187.

 Research Article
Research Article