ABSTRACT
The cohesion between cells and dermal matrix is essential to ensure both mechanical resistance and barrier function of the epidermis so the presence of epidermal junctions is crucial. Since several years, tissue engineering allowed the emergence of cutaneous reconstructed tissues allowing to better understand the role and the functions of the epidermis. In this study, both reconstructed human epidermis and full thickness models presented all differentiated layers of a native epidermis. Indeed, the four typical epidermal layers are well differentiated with the presence of columnar keratinocytes in the basal layer, spinous layer constituted of 2-3 layers of polyhedral keratinocytes resting on the basal layer, the stratum granulosum, with characteristic keratohyalin vesicles and finally, the stratum corneum which is formed by a superposition of anucleated and completely keratinized cells, the corneocytes, forming very elongated lamellae. The barrier function integrity is insured by cell junctions from the basal layer with hemidesmosomes, focal adhesions and dystroglycans, to the upper layers with GAP junctions, desmosomes, adherens junctions, tight junctions and finally with corneodesmosomes. These different junctions can be observed by the detection of specific junction proteins involved in cell adhesion. In reconstructed human epidermis or full thickness tissue, at the dermoepidermal junction level, collagen XVII and β1 integrin involved in hemidesmosome or focal adhesion structure respectively were detected as well as the laminin 332 and the collagen IV. The claudin-1 and e-cadherin, detected by specific antibodies, were present in all suprabasal layers (spinous and granular) both in reconstructed epidermis and full thickness. The corneodesmosin was also detected in the stratum corneum in reconstructed human epidermis. These in vitro skin models are a perfect tool to study cell junction deficiency.
Keywords: Junction; Reconstructed Human Epidermis; Barrier Function; Full Thickness; Dermoepidermal Junction
Abbreviations: DEJ: Dermoepidermal Junction; HD: Hemidesmosome; FA: Focal Adhesion; AJ: Adherens Junction; TAMP: Tight junction Associated MARVEL Protein; ZO: Zonula Occludens; MUPP1: MUlti PDZ Domain Protein; CDSN: Corneodesmosin; SDS: Sodium Dodecyl Sulfate; NHK: Normal Human Keratinocytes; BPE: Bovine Pituitary Extract; DMEM: Dulbecco’s Modified Eagle Medium; FCS: Foetal Calf Serum; BSA: Bovine Serum Albumine; H&E: Hematoxylin & Eosin; PBS: Phosphate Buffer Saline; RHE: Reconstructed Human Epidermis; FT: Full Thickness; ECVAM: European Council of Validated Alternatives Method
Introduction
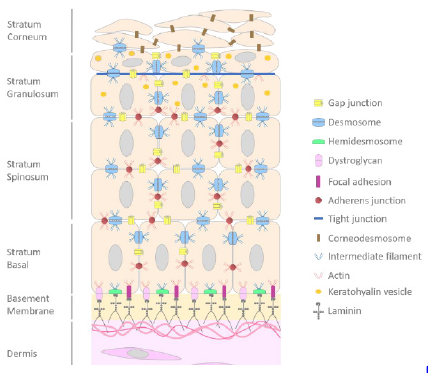
From several decades, reconstructed skin models have been developed both in the field of public research, in the cosmetics and pharmaceutical industries. All these approaches are based on the principle of isolation of primary cells, cultivation of cells in a monolayer and then specific differentiation of the tissue at the airliquid interface in order to obtain a multi-layered and specialized tissues [1,2]. Since the first experiments carried out by Rheinwald and Green in 1975 [3] on the isolation and culture of human primary keratinocytes on a monolayer of fibroblasts, techniques have evolved towards a culture of keratinocytes without a nourishing sub-layer. The technical changes were more focused on the culture media in order to obtain a tissue close to the characteristics of the in vivo skin. As a result, the epidermis is now cultivated on deepidermized dermis, inert filters or even more or less complex collagen matrices [1,4-8]. The growth and differentiation processes of the reconstructed epidermis, however, have changed little with a growth phase of keratinocytes in immersion and a differentiation phase induced with a medium concentrated in calcium ions [9,10]. These technical approaches improve the characteristics of reconstructed tissues with for example, better organization, structure, cohesion conferring them, a better integrity close to skin characteristics. The main role of the skin is to ensure a barrier function against external environmental stresses and to avoid water loss [11]. The maintenance of the skin integrity against stresses is due to the presence of numerous cutaneous junctions between cells and skin compartments [12,13]. In the skin, a stratified epithelium, from basal layer to stratum corneum we noticed the presence of focal adhesions (FA), dystroglycans and hemidesmosomes (HD) which ensure the adhesion to dermal compartment, cell-cell junction with GAP junctions, adherens junction, desmosomes then tight junctions in the granular layer and finally corneodesmosomes at the level of stratum corneum (Figure 1) [14]. The adhesion between the epidermis and the dermis is insured by a highly specialized zone called the Dermoepidermal Junction (DEJ).
Figure 1: Schematic representation of cell-cell and cell-matrix junctions.
Note: Scheme of the epidermis and its junctions from basal layer to stratum corneum. Dermoepidermal junctions are also
represented.
The DEJ confers a fine-tuned architecture to the skin useful for the maintain of the skin homeostasis. The DEJ regulates cell adhesion, cell differentiation and motility, and plays an important role in the communication between the epidermis and the dermis [15]. The DEJ also influences the basal keratinocyte polarity and defines the basal surface where proliferating epidermal cells are attached [16]. The DEJ is a highly complex structure composed to hemidesmosomes, focal adhesions and dystroglycans. Hemidesmosomes are found in different tissues such as the cornea, the skin [17] allowing the maintain of these tissues. HD have important role in cell adhesion, wound healing, tissue morphology allowing the maintenance of tissue structure and integrity. HD, half of desmosome, are small structure about less than 0.5μm consisting of a tripartite plaque with an inner and outer plaque separated by a less dense zone [18]. HD consist of α6β4 integrin, plectin (P1a), tetraspanin CD151, BPAG1 (or BP230) and collagen XVII (or BP180 or BPAG2) [19].
These junctions link anchoring intermediate filaments in the
epidermal compartment and fibrils on the extracellular matrix
side among which the following proteins are mainly found keratins
K5, K14, collagen VII and IV and other proteins like laminin 332
(laminin 5) [20]. In addition to hemidesmosomes, focal adhesions
are dynamic adhesions allowing also keratinocyte junctions
to the extracellular matrix through the connection of α3β1
integrin transmembrane proteins to the actin cytoskeleton and
on the opposite to laminin 332 to the extracellular matrix [21].
FA are involved in different processes like cell communication,
proliferation, migration, apoptosis, spreading, wound healing and
differentiation. The FA is a protein complex composed about more
50 proteins divided into three groups:
i) The structural components (talin, vinculin, kindlins also
named FERMT1-3)
ii) The enzymatic components (Focal Adhesion Kinase (FAK),
Integrin-Linked Kinase (ILK) and Tyrosine-Protein Kinase
SRC-1 (SRC))
iii) Adaptors (paxillin, P130, LIMS1…) [21-23].
The dystroglycans, another complex present in DEJ, were
shown as expressed by keratinocytes and fibroblasts in human
skin [24] and localized in the epidermal basement membrane. The
dystroglycans allow a closed-link with the actin cytoskeleton of
epidermal basal keratinocytes and with the extracellular matrix in
human skin [24,25]. The integrity of the epidermis compartment
is ensured by cell-cell junctions present between epidermal cells
in all layers including stratum corneum. Among all junctions, GAP
junctions link the cytoplasm of two cells allowing intercellular
exchange of ions and small molecules [26]. This intercellular
communication is important for the maintenance of skin
homeostasis, including keratinocyte growth and differentiation
[27], regulation in melanogenesis [28]. In fact, GAP junctions
are channels assembled from connexin subunits (26, 32 and 43)
belonging to connexin family about 21 members. The assembly of
6 connexins forms an oligomer called connexon, transported to the
plasma membrane [29].
The connexon docks with a connexon of adjacent cell and form a
GAP junction channel. These GAP junctions are regrouped into GAP
junction plaque. In addition to GAP junction, desmosomes form the
intercellular junctions (0.2–0.5μm in diameter) allowing the link of
intermediate filaments to the plasma membrane giving a resistance
to mechanical stress in the skin and other tissues [30]. The
desmogleins (Dsc1-3) and desmocollins (Dsg1-4), transmembrane
proteins of the desmosome, belong to the cadherin family of
calcium-dependent adhesion molecules. The cytoplasmic tails of
desmosomal cadherins are associated with the desmosomal plaque
proteins: plakoglobin and desmoplakin belonging to the armadillo
and plakin family of linker proteins respectively [31]. The tethering
of cytoskeleton is insured by interaction of desmoplakins with the
keratin intermediate filaments giving rise to inner dense plaque
[32,33]. A third cell junction complex is adherens junctions (AJs)
which have conserved plasma-membrane structures that mediate
cell–cell adhesions organized into two complexes of proteins:
nectin/afadin and cadherin/catenin.
The AJs form extracellular adhesive contacts between cells,
and intracellular links to the actin cytoskeleton. E-cadherin and
the catenin family members including p120-catenin, β-catenin,
and α-catenin are the main components of AJs [34]. Two types of
cadherins are expressed in the epidermis: P-cadherin expressed
in the basal layer and in hair follicles, and e-cadherin in all layers
of the epidermis. AJs are involved in several processes such as
cytoskeletal dynamics, cell polarity, cell adhesion, cell shape,
division, growth, apoptosis and barrier function [35]. At the upper
layer of the epidermis, another type of junctions is present. Indeed
the tight junctions are localized in the granular layer, thus ensuring
the barrier function, cell polarity and preventing epidermal water
loss and solutes [36]. Tight junctions are protein complexes
containing more than 40 proteins that form the semi-permeable
mechanical connections between cells.
The tight junctions consist of three main type of structural
transmembrane proteins that are common to all tight junctions:
claudins belonging to a family of 26 members, Tight Junctionassociated
MARVEL proteins (TAMP) as occludin or tricellulin
and junctional adhesion molecules (JAM-A, -B or -C) [37]. The
tight junctions are linked to the cytoskeleton through protein
adaptors called Zonula Occludens (ZO-1, -2, and -3) and MUPP1
(Multi-PDZ Domain Protein 1) forming the junctional plaque. Most
of the proteins forming these junctions are found in the stratum
granulosum including claudins 1, 4, 6, 7, 11, 12 and 18, occludin, ZO-1,
ZO-2, MUPP-1 and cingulin [38]. And finally in the stratum corneum,
composed of corneocytes responsible of the epidermis turnover and
conferring a regenerating power of the skin, corneodesmosomes
ensure the link to each other. Corneodesmosomes are a modified
form of desmosome, indeed they are formed upon integration of
corneodesmosin (CDSN) released by lamellar granules [39] during
the conversion of desmosome to corneodesmosome in the stratum
corneum of the epidermis [33]. CDSN glycoproteins embedded
within the desmoglea (the intercellular space of desmosomes) to
form the desmosomal plate [40].
Deposition of loricrin, a major component of the cornified
cell envelope, begins at the desmosomal plaques in the cytoplasm
of cell present in the upper layer of the stratum granulosum [39].
These junctions are degraded to allow the desquamation process
by proteases as kallikreins and cysteine proteases (cathepsins)
in contrast to protease inhibitors as LEKTI counterbalance to the
stratum corneum formation [41]. In this article, we highlighted
the presence of cell-cell junctions and cell-matrix junctions both
in reconstructed epidermis and full thickness (combination of dermis and epidermis) and the integrity of the barrier function
demonstrated with the penetration of lucifer dye after chemical
stress (SDS).
Material and Methods
Ethical Compliance
Samples were obtained from anonymous human healthy donors. Surgical residues were harvested according to French regulation (agreement DC 2021-4617) and procurement of written informed consent from the patient.
Cell Culture of Normal Human Keratinocytes and Fibroblasts
Normal human primary epidermal keratinocytes (NHKs) were isolated from surgery (circumcision). An enzymatic digestion was used to dissociate the epidermis from the dermis indeed the biopsies were incubated in the Dispase II (Sigma, France) at 4°C overnight. Then a second enzymatic digestion was used to separate the epidermal keratinocytes with Trypsin-EDTA (Sigma, France) at 37°C for 10 minutes from epidermis cut into small pieces. The cells were centrifugated and the pellet was taken in a specific medium complemented with BPE (Gentaur, France). Cells were placed at 37°C in a humidified atmosphere containing 5% of CO2. In parallel, the dermis explant was placed in a Petri dish and incubated with DMEM with 1g/L of glucose (Lonza, Switzerland), 2mM L-glutamine (Lonza, Switzerland), Gentamycin (Euromedex, France) complemented with 20% FCS (Biowest, France). The explants were incubated at 37°C in a humidified atmosphere containing 5 % of CO2. At the appearance of fibroblasts, the dermis explant was removed and the culture medium was replaced by DMEM with 1g/L of glucose (Lonza, Switzerland), 2 mM L-glutamine (Lonza, Switzerland), Gentamycin (Euromedex, France) complemented with 10 % FCS (Biowest, France) and incubated at 37°C in a humidified atmosphere containing 5% of CO2.
Reconstruction of Epidermis
After keratinocyte isolation, the NHKs were seeded on a 0.5cm² inert polycarbonate membrane (Nunc, Thermo Fisher Scientific, USA) in a proprietary chemically-defined media and were placed at the air-liquid interface until 17 days at 37°C in a humidified atmosphere containing 5 % of CO2.
Reconstruction of Skin Equivalent (Dermis and Epidermis)
After fibroblast isolation, dermal equivalents were prepared using a neutralized solution containing bovine type I collagen (Collagen Solution, USA) diluted in complete DMEM [(1g/L of glucose, 2mM L-glutamine; Lonza, Switzerland), 10 % FCS (Biowest, France) and gentamycin (Euromedex, France)]. The mixture was dispensed onto 12-well tissue culture plates and incubated 24 hours at 37°C to allow the polymerization. After polymerization, complete DMEM medium was added to each well. Dermal equivalents were maintained at 37°C in a humidified atmosphere containing 5 % of CO2. After 4 days of contraction, the matrix was transferred in the proprietary chemically defined medium at the air-liquid interface. The NHKs were seeded on the dermal matrices and placed at 37°C under 5 % CO2. After 3 days of culture, the full thickness was placed at the air-liquid interface at 37°C in a humidified atmosphere containing 5 % of CO2.
Histology and Immunohistochemistry
For histological analysis, the reconstructed human epidermis
and skin equivalent were fixed in the 10% formalin buffer (Sigma,
France). After successively dehydration, the tissues were then
embedded in paraffin. Paraffin section (4μm) were deposited
on glass slides, deparaffinized and then successively rehydrated
in xylene baths, alcohol of different percentages and water. The
Hematoxylin & Eosin staining (H&E) staining was performed by
placing the sections in a hematoxylin bath for 3 minutes. The sections
were rinsed with water for 5 minutes at room temperature. The
slides were then placed in an eosin bath for 2 minutes. The tissues
were then dehydrated in successive baths of absolute alcohol and
xylene. After mounting a coverslip with Eukitt* (O. Kindler), the
photos were acquired with a Qimaging* Retina 2000R Fast1394
camera and processed by using the Q-Capture Pro 7 (QImaging,
England) acquisition software.
For immunohistochemistry (IHC), the reconstructed human
epidermis and skin equivalent were fixed in the 10% formalin buffer
(Sigma, France), then embedded in paraffin. Paraffin sections (4μm)
were deposited on glass slides, deparaffinized and rehydrated with
successive bath of xylene, absolute alcohol and water. The exposure
of the antigens was realized by treatment of the sections with 0.01
M citrate buffer pH6 and 0.25 % pepsin or 0.05 % trypsin (Zymed,
Invitrogen, Thermo Fisher Scientific, USA) for 15 minutes at 37°C.
After the fixation, the saturation of nonspecific sites was performed
with 5% BSA buffer (Sigma, France) for 30 minutes, primary
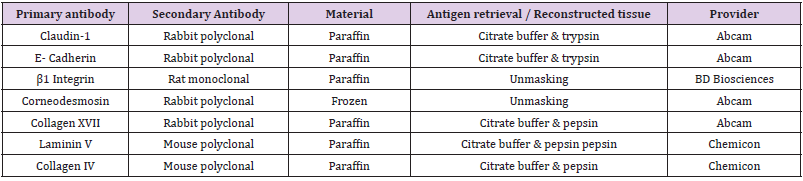
antibody was applied at room temperature for 1 hour (Table 1).
The sections were rinsed in PBS and the secondary antibody (Alexa
Fluor Donkey anti-rabbit, Alexa fluor Goat anti-mouse, Alexa Fluor
Goat anti-rat, (Invitrogen, Thermo Fisher Scientific, USA) was
deposited at room temperature in dark for 1 hour. Finally, the slides
were incubated with 0.3μM 4’,6’diamidino-2-phenylindole (DAPI,
Molecular Probes, USA) for 5 minutes at room temperature in the
dark and mounted with Fluoromount-G® (Electron Microscopy
Sciences, USA). The photos were taken with a Qimaging EXI blue
camera coupled to Volocity acquisition software (Improvision,
England).
Barrier Integrity Assay
To observe the barrier integrity, a Sodium Dodecyl Sulfate (SDS) (Sigma, France) stress (concentration 0.1 to 0.75 % w/v diluted in PBS 1X) was applied topically for 3 hours. Then epidermises were rinsed 3 times with PBS 1X and then Lucifer yellow 1mM was applied topically for 2 hours to visualize the diffusion of this passive dye throughout the epidermis. Epidermises were rinsed 3 times with PBS 1X then tissues were fixed with formalin solution neutral buffer 10 % and embedded in paraffin. Paraffin sections (4μm) were deposited on glass slides, deparaffinized and rehydrated with successive bath of xylene, absolute alcohol and water. The slides were incubated with 0.3μM 4’,6’diamidino-2-phenylindole (DAPI, Molecular Probes, USA) for 5 minutes at room temperature in the dark and mounted with Fluoromount-G® (Electron Microscopy Sciences, USA). The photos were taken with a Qimaging EXI blue camera coupled to Volocity acquisition software (Improvision, England).
Results
The reconstruction of cutaneous tissues such as epidermis
or more complex tissue (dermis combinate with epidermis for
example) is not only a layering of keratinocytes but a cellular
stratification with the presence of specialized keratinocytes
with specific functions and characteristics according to the
corresponding epidermal layer such as basal, spinous, granular
layers or stratum corneum. To investigate the integrity of the tissue
due to the presence of cell junctions, immunodetections of specific
proteins related to junctions were realized on these reconstructed
tissues. Claudin-1 (a major protein involved in tight junction),
e-cadherin (a main protein involved in cell-cell junction), b1 integrin
(protein involved in cell-matrix adhesion and more specifically
to collagen fiber) and corneodesmosin (a protein involved in the
stratification of stratum corneum) were selected. Prior to realize
immuno-detection of specific proteins, a histological observation of
the human reconstructed tissue by hematoxylin and eosin (H&E)
staining was performed to compare the structure of reconstructed
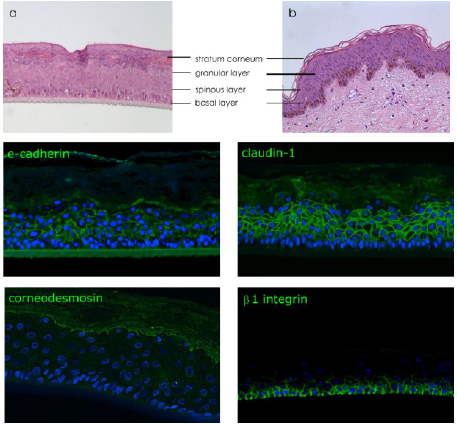
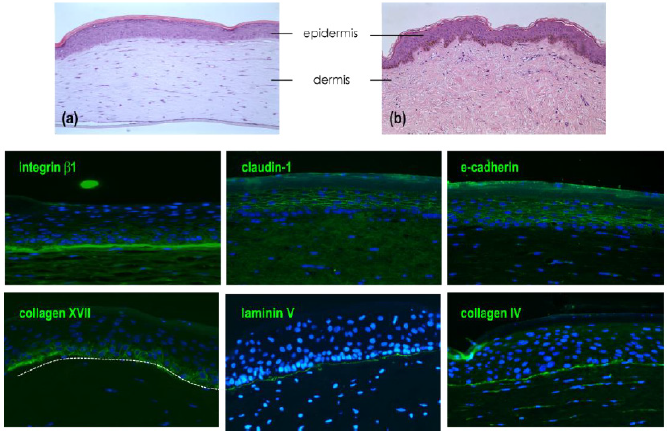
epidermis to native human skin (Figures 2a & 2b).
Figure 2: Characterization of reconstructed human epidermis.
Note:
a) Histological observation by H&E of reconstructed human epidermis
b) Native skin.
Immunodetection of e-cadherin and claudin-1 presents in all epidermal layers, corneodesmosin localized in the upper layers
(granular layer and stratum corneum) and β1 integrin expressed specifically in basal layer. Nucleus are stained in blue by DAPI.
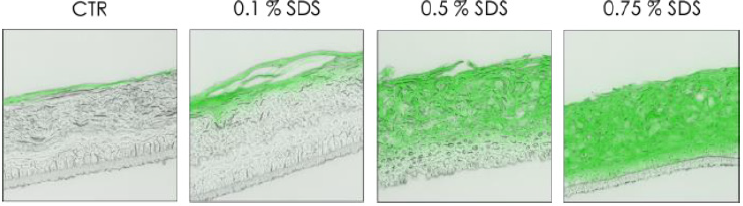
To validate this epidermal function, a passive dye was topically applied on the top of the RHE to observe the diffusion after a chemical stress by SDS at various concentrations (Figure 3). Treatment with SDS, a well know surfactant, disrupted cell junctions resulting in a deep penetration of dye Lucifer yellow inside the epidermis. This diffusion is dependent to the concentration of SDS, indeed at lowest concentration (0.1 %) the lucifer yellow was only present in the stratum corneum, and diffusion was not observed in viable cell layers. But on the opposite, at the highest tested concentration (0.75 %), lucifer yellow penetrated completely inside the reconstructed epidermis (Figure 3). After the reconstruction and the characterization of the in vitro epidermis, a more complex tissue combining dermal and epidermal compartment was studied. Prior to detect the presence of specific cell junction proteins, a histological staining by H&E was performed to compare the structure of full thickness to native skin (Figure 4).
Figure 3: Diffusion of passive dye after chemical stress (SDS).
Note: Observation of the passive diffusion of lucifer yellow applied topically (2h) after chemical stress induced by SDS (3h) at
various concentration 0.1%, 0.5% and 0.75% on RHE at day 17.
Figure 4: Characterization of skin full thickness (FT) model.
Note:
a) Histological observation by H&E of in vitro FT model
b) Compared to native skin.
Immuno-detection of markers expressed in full thickness. Detection of proteins expressed in dermal epidermal junction
(β1 integrin, collagen XVII, laminin 332 and collagen IV) and cell-cell junction in epidermal compartment (claudin-1 and
e-cadherin).
As we observed a similar structure of reconstructed human epidermis to the epidermis of native skin, the observation of the epidermal compartment in the full thickness model is similar with the presence of all differentiated layers; basal layer with columnar keratinocytes, spinous layer, granular layer with the presence of keratohyalin vesicles and finally a stratum corneum (Figures 4a & 4b). The global structure of reconstructed tissue is similar to native skin (except for the presence of dermal papilla) but due to the early stage of reconstruction (day 10), the stratum appeared thinner than the epidermal stratum observed in RHE at day 17. Cohesion of the epidermal compartment with the dermal compartment is fully present all along the tissue. The detection of b1 integrin is localized all along the dermoepidermal junction, collagen XVII, laminin 332 and collagen IV were also expressed at the DEJ. As observed on reconstructed epidermis alone, the detection of both claudin-1 and e-cadherin are localized in the membrane of cells constituting suprabasal layers, the detection of these proteins are less pronounced in the basal layer.
Conclusion
Reconstruction of in vitro skin models from human cells is a real
advantage for the research field and also it has been a revolution
for toxicological studies to avoid the use of animals. These RHE are
“real skin” and not only a superposition of cells which allowed the
validation of these models by ECVAM (European Council of Validated
Alternatives Methods) to use them for several toxicological studies
such as skin irritation or corrosion (respectively OECD TG439
(2021) and 431 (2019), [42,43]). To obtain these validations, the
“in vitro skin” must have similar properties to native skin and more
precisely in term of barrier function. This barrier function is ensured
by the stratum corneum with its structure in “bricks and mortar”
(bricks symbolize corneocytes and mortar the intercellular lipids).
In addition of the stratum corneum, to ensure this barrier function,
cohesion between epidermal cells in the viable layers is essential.
To ensure the tissue cohesion, there is several types of epidermal
junctions such as GAP junctions, tight junctions (essentially located in the stratum granulosum), desmosomes, corneodesmosomes
(in the stratum corneum) and hemidesmosomes, focal adhesions,
dystroglycans in the basement membrane.
In this study, both reconstructed human epidermis and full
thickness models presented all differentiated layers of a native
human epidermis. Indeed, four typical epidermal layers are well
differentiated with the presence of columnar keratinocytes in the
basal layer, spinous layer constituted of 2-3 layers of polyhedral
keratinocytes above the basal layer and the stratum corneum.
The cells of the epidermal layers come from the migration of cells
of basal layer. In the stratum spinosum, cells are provided with
spicules or thorns, hence their name of spiny cells. These spines
are in fact desmosomes to which microfilaments are attached.
The third layer, the stratum granulosum, with characteristic
cytoplasmic keratohyalin vesicles comprising loricrin, trichohyalin
and profilaggrin (the precursor of filaggrin), contributes to the
formation of interfibrillar cement by keratin filament aggregation.
Thus, the function of keratohyalin vesicles is to stabilize the
tonofibrils at the level of the corneocytes, by contributing to the
formation of the insoluble matrix of the stratum corneum that leads
to keratinization.
The cohesion of the spinous layers is also ensured by different
junctions such as tight junctions evidenced by claudin-1 and
desmosomes that are conversed in corneodesmosomes evidenced
by corneodesmosin. Finally, the stratum corneum is formed by
a superposition of anucleated and completely keratinized cells,
the corneocytes, forming very elongated lamellae. All epidermal
cells are firmly attached to each other, thus causing a mechanical
cell coupling inducing resistance of the epidermis to mechanical
stresses and a part of communication between cells are ensured by
the presence of GAP-type junctions. The barrier function integrity
can be observed by the detection of specific junction proteins
involved in cell-cell adhesion. Claudin-1 (tight junction) and
e-cadherin (adherens junctions), detected by specific antibodies,
were presents in all suprabasal layers (spinous and granular) both
in reconstructed epidermis and full thickness. It is interesting to
notice that the detection of these proteins was absent or very weak
in the basal layer.
This lower detection is due to the presence of proliferating cells
and stem cells which initiate the renewal of the epidermis, indeed
basal keratinocytes expressed high levels of β1 integrins and lower
levels of e-cadherin and claudin-1. Presence of these two proteins
is crucial because e-cadherin favors the incorporation of claudin-1
in the structure of tight junction and a decrease of claudin-1
plays a central role in dermatitis atopic [44]. The cohesion of
the epidermal compartment to the dermis is also ensured by
several proteins forming complex such as hemidesmosomes, focal
adhesions or dystroglycans. Our in vitro model presented proteins
belonging at the DEJ demonstrated by the presence of collagen
XVII (hemidesmosomes), laminin 332, collagen IV and β1 integrin
(focal adhesion). The study of the cell-cell junctions and cell-matrix
junctions are very important to better understand skin diseases.
The development of in vitro models deficient in specific proteins
can be a perfect tool in this investigation.
Indeed, for example hemidesmosome are very important bonds
because mutations in the genes encoding these proteins induce
serious pathologies such as bullous dermatosis which results from
the loss of interaction between plectin and collagen XVII [45], in
mice the deficiency of β1 integrin induced a resistance to skin
scleroderma resulting in reducing dermis thickness [46]. To develop
specific in vitro models, the combination between molecular biology
and tissue engineering allows to modify genetically the cells then
to reconstruct human tissues deficient in the expression of specific
proteins involved in cell-cell junctions or cell-matrix junctions to
mimic diseases.
Acknowledgment
This paper has been written thanks to the bibliographical research of Catherine Serre focused on epidermal junction and her availability.
References
- Prunerias M, Régnier M, Woodley D (1983) Methods for cultivation of keratinocytes with an air-liquid interface. J Invest Dermatol 81: 28s-33s.
- Bernstam LI, Vaughan FL, Bernstein IA (1986) Keratinocytes grown at the air-liquid interface. In Vitro Cell Dev Biol 22(12): 695-705.
- Rheinwald JG, Greene H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6(3): 331-343.
- Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T (1981). Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 211(4486): 1052-1054.
- Boyce S, Hansbrough J (1988) Biologic attachment, growth, and differentiation of cultured human epidermal keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery 103(4): 421-431.
- Tinois E, Tiollier J, Gaucherand M, Dumas H, Tardy M, et al. (1991) In vitro and post-transplantation differentiation of human keratinocytes grown on the human type IV collagen film of a bilayered dermal substitute. Exp. Cell Res 193: 310-319.
- Ponec M, Weerheim A, Kempenaar J, Mulder A, Gooris G, et al. (1997) The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J. Invest Dermatol 109(3): 348-355.
- El Ghalbzouri A, Siamari R, Willemze R, Ponec M (2008) Leiden Reconstructed human epidermal model as a tool for the evaluation of the skin corrosion and irritation potential according to the ECVAM guidelines. Toxicol in Vitro 22(5): 1311-1320.
- Mak VHW, Cumpstone MB, Kennedy AH, Harmon CS, Guy RH, et al. (1991) Barrier Function of Human Keratinocyte Cultures Grown at the Air-Liquid Interface. Journal of Investigative Dermatology 96(3): 323-327.
- Rosdy M, Pisani A, Ortonne JP (1993) Production of basement membrane components by a reconstructed epidermis cultured in the absence of serum and dermal factors. Br J Dermatol 129(9): 227-34.
- Elias PM (2008) Skin barrier function. Current allergy and asthma reports 8(4): 299-305.
- Agner T (2016) Skin Barrier Function. Indian J Med Res 147: 117-120.
- Basler K, Bergmann S, Heisig M, Naegel A, Zorn Kruppa M, et al. (2016) The Role of Tight Junctions in Skin Barrier Function and Dermal Absorption. Journal of Controlled Release 242: 105-118.
- Green KJ, Jaiganesh A, Broussard Joshua A (2019) Desmosomes: Essential contributors to an integrated intercellular junction network. F1000Res, p. 1-16.
- Hashmi S, Marinkovich MP (2011) Molecular organization of the basement membrane zone. Clin Dermatol 29(4): 398-411.
- Serre C, Plaza C, Meyrignac C, Capallere C, Botto JM (2019) The dermoepidermal junction: a key cutaneous structure for a healthy skin. Development of a bioinformatic interaction network and relevant bioengineered skin models. EURO COSMETICS 5: 16-20.
- Borradori L, Sonnenberg A (1999) Structure and Function of Hemidesmosomes: More Than Simple Adhesion Complexes. J Invest Dermatol 112(4): 411-8.
- Walko G, Castanon M, Wiche G (2015) Molecular architecture and function of the hemidesmosome. Cell and Tissue Research Volume 360(3): 529-544.
- Owaribe K, Kartenbeck J, Stumpp S, Magin TM, Krieg T, et al. (1990) The hemidesmosomal plaque. Characterization of a major constituent protein as a differentiation marker for certain forms of epithelia. Differentiation 45(3): 207-220.
- McGrath J, Uitto J (2016) Structure and Function of the Skin (9th Edn.)., Rook's Textbook of Dermatology.
- Hatzfeld M, Magin TM (2019) Crosstalk between Hemidesmosomes and Focal Adhesions: A Primer for Wound Healing, Blistering Skin Disease, and Skin Aging. J Invest Dermatol 139(9): 1854-1856.
- Lo H (2006) Focal adhesions: What's new inside. Developmental Biology 294(2): 280-291.
- Duperret EK, Ridky TW (2013) Focal adhesion complex proteins in epidermis and squamous cell carcinoma. Cell Cycle 12(20): 3272-3285.
- Herzog C, Has C, Franzke CW, Echtermeyer FG, Schlötzer-Schrehardt U, et al. (2004) Dystroglycan in Skin and Cutaneous Cells: β-Subunit Is Shed from the Cell Surface. Journal of Investigative Dermatology 122(6): 1372-1380.
- Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P (1998) Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem 46(4): 449-57.
- Beyer E, Berthoud V (2017) Gap junction structure: unraveled, but not fully revealed. Research 6: 1-10.
- Meşe G, Richard G, White TW (2007) Gap Junctions: Basic Structure and Function. Journal of Investigative Dermatology 127(11): 2516-2524.
- Divya Padma, Kapaettu Satyamoorthy, Kumar MR Bhat (2015) Role of gap junctions between keratinocyte and melanocyte in melanogenesis. Frontiers in Biology 10: 495-502.
- Churko JM, Laird DW (2013) Gap Junction Remodeling in Skin Repair Following Wounding and Disease. Physiology 28(3): 190 -198.
- Kowalczyk AP, Green KJ (2013) Structure, Function and Regulation of Desmosomes. Prog Mol Biol Transl Sci 116: 95-118.
- Delva E, Tucker DK, Kowalczyk AP (2009) The Desmosome. Cold Spring Harb Perspect Biol 1(2): a002543.
- Kowalczyk AP, Stappenbeck TS, Parry DA, Palka HL, Virata ML, et al. (1994) Structure and function of desmosomal transmembrane core and plaque molecules. Biophys Chem 50(1-2): 97-112.
- Garrod D, Chidgey M (2008) Desmosome structure, composition and function. Biochimica et Biophysica Acta 1778(3): 572-587.
- Hartsock A, Nelson WJ (2008) Adherens and Tight Junctions: Structure, Function and Connections to the Actin Cytoskeleton. Biochim Biophys Acta 1778(3): 660-669.
- Brandner JM, Haftek M, Niessen CM (2010) Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function. The Open Dermatology Journal 4: 14-20.
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, et al. (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156(6): 1099-1111.
- Vermette D, Hu P, Canarie MF, Funaro M, Glover J, et al. (2018) Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Medicine Experimental 6(1): 37.
- Brandner JM, Zorn Kruppa M, Yoshida T, Moll I, Beck LA, et al. (2015) Epidermal tight junctions in health and disease. Tissue Barriers 3: 1-2.
- Ishida Yamamoto A, Eady RA, Watt FM, Roop DR, Hohl D, et al. (1996) Immunoelectron microscopic analysis of cornified cell envelope formation in normal and psoriatic epidermis. J Histochem Cytochem 44(2): 167-175.
- Raknerud N (1975) The ultrastructure of the interfollicular epidermis of the hairless (hr/hr) mouse. III. Desmosomal transformation during keratinization. J Ultrastruct Res 52(1): 32-51.
- Marek Haftek (2014) Epidermal barrier disorders and corneodesmosome defects. Cell Tissue Res 60(3): 4 83-90.
- (2019) OECD. Test No. 431: In Vitro Skin Corrosion: Reconstructed Human Epidermis (RHE) Test Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France.
- (2021) OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France.
- Bergmann S, Von Buenau B, Vidal (2020) Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently. Sci Rep 10(1): 2024.
- Natsuga Wataru K (2017) Loss of interaction between plectin and type XVII collagen results in epidermolysis bullosa simplex. Human mutation 38(12): 1666-1670.
- Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A (2009) Loss of β1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis & Rheumatism 60(9): 2817-2821.

 Research Article
Research Article