ABSTRACT
Intraoperative OCT is becoming an established technology in ophthalmic surgeries. Its detailed cross-sectional images provide valuable morphological information during surgery, and several studies proved that it influences greatly the surgeon’s intraoperative judgment and decisions. In this mini review, we are going to address anterior segment OCT in ophthalmic surgeries, mainly corneal surgeries.
Abbreviations: OCT: Optical Coherence Tomography; AS-OCT: Anterior Segment Optical Coherence Tomography; AC: Anterior Chamber; PKP: Penetrating Keratoplasty; iOCT: Intra-Operative Optical Coherence Tomography; DSAEK: Descemet Stripping Automated Endothelial Keratoplasty; DALK: Deep Anterior Lamellar Keratoplasty; DMEK: Descemet Endothelial Keratoplasty; DM: Descemet Membrane
Mini Review
Introduction
Optical Coherence Tomography (OCT) is a non-contact imaging technology that produces detailed cross-sectional images, using low-coherence interferometry in biological tissues. [1] Anterior Segment OCT (AS-OCT) is an innovative tool for evaluation of the cornea, conjunctiva, sclera, anterior chamber (AC), anterior chamber angle, intraocular lens, and adjacent anterior segment structures. It has been helpful for the diagnosis and management of conjunctival diseases, anterior segment tumors, Corneal diseases, and AC inflammation. [2] Standard clinical OCT systems are large and stationary. In the past few years, the OCT became an important tool for selected ophthalmic surgeries, and adaptations had to be made to convert the OCT to a tabletop system. Today there are several commercial systems of microscope-integrated intraoperative OCT (iOCT), which focuses on OCT video visualization in highresolution, maximum integration with the microscope, and some of them integrate a three-dimensional heads-up display system for maximum convenience of the surgeon while visualizing the surgical field. [3]
Intra-Operative Oct
The iOCT is a rather new technology and it is currently applied
in a wide variety of ophthalmic surgeries. The AS-OCT is used in
lamellar keratoplasty, Penetrating keratoplasty (PKP), cataract
surgeries, glaucoma surgeries, and other corneal procedures
(post-trauma, epithelial/fibrous growth). The posterior segment
OCT aids in cases of optic pit-related maculopathy, retinopathy of
premature, macular hole, retinal detachment, proliferative diabetic
retinopathy, epiretinal membrane, and posterior uveitis. [3] Over
the years, two major studies were conducted to test the benefits
of the intra-operative OCT (iOCT)- the PIONEER study and the
DISCOVER study. Both studies collected Clinical characteristics and
iOCT imaging was obtained during surgical milestones as directed
by the operating surgeon. A surgeon questionnaire was issued to
each surgeon and completed after each case. The PIONEER study
was published in 2014 and enrolled 531 eyes- 275 anterior segment
cases and 256 posterior segment cases. In the anterior segment
surgeries, the most common procedure was Descemet stripping
automated endothelial keratoplasty (DSAEK, n=135), and the second most common was cataract extraction with an intraocular
lens implant.
Immediately after the surgery, the surgeon was required to fill
a feedback form. Overall following iOCT, 48% of the eyes revealed
persistent interface fluid requiring additional manipulations. In
deep anterior lamellar keratoplasty (DALK), 1 of 3 cases where
the surgeon did not believe the trephination was deep enough,
iOCT revealed the depth was optimal and did not require further
deepening. In 56% of the cases of DALK, iOCT prompted further
manual dissection to deepen the initial trephination. [4] The
DISCOVER study was published in 2015, enrolled 227 eyes, 91 of
them were anterior segment cases. The most common procedure
was DSAEK (43%), following DALK (9%). In this study, 8% of the
cases were Descemet endothelial keratoplasty (DMEK) cases (in
the PIONEER study there were no DMEK cases at all). According to
surgeons in the study, 44% of the total anterior segment cases were
changed or modified due to the iOCT findings. [5] Regarding the
influence of iOCT on surgical time, the DISCOVER study concluded
that in 47% of the cases, the iOCT minimized the surgical time by
eliminating unnecessary manipulations but did not measure the
minutes that were spared. Recent studies of iOCT use in anterior
segment procedures (Figure 1).
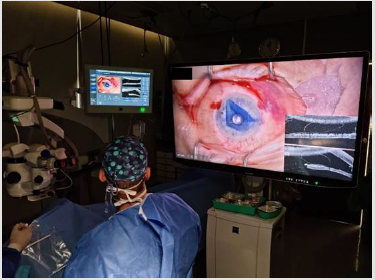
Figure 1: Corneal staff in Hadassah Hospital during DMEK procedure, using iOCT, ARTEVO 800 of produced by ZIESS.
DALK
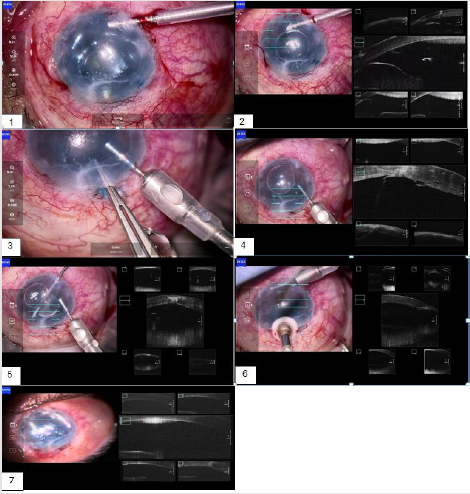
The DALK procedure gained popularity as an alternative to PKP. The “big-bubble” technique uses a forceful injection of air into the deep stroma, to create cleavage and to separate the Descemet membrane (DM) from the overlying stroma. The reported rate of successfully achieving a big bubble is 66% to 90%. For novice surgeons, the step of big-bubble generation is the most common surgical step at which perforation of DM occurs. [6] According to Myerscough et al, there are two main advantages of iOCT in DALK procedure - the first is assessing the depth of the cannula tip before performing pneumatic dissection, and the second is confirming that pneumatic dissection has indeed occurred. [7] Liu et al presented a new approach for DALK surgery in which a low-energy FSL created an anterior stromal dissection and a pre-Descemet intrastromal tunnel for the air injection in one step, to a preprogrammed depth, with the guidance of the iOCT and to facilitate big-bubble creation. 14 eyes were included: 11 eyes with keratoconus and no evidence of a history of acute hydrops and 3 eyes with corneal scars. In all cases, a big bubble was successfully achieved without intraoperative complications related to lasers (Figure 2).
Figure 2: A 30-year-old woman with advanced keratoconus, in DALK procedure using iOCT.
1. Picture number 1: separating the stroma layer from the DM, with the cannula corneal plane clearly visible.
2. Picture number 2: During air injection (Big bubble), the air reached the stroma, and caused an emphysema.
3. Picture number 3: The emphysema in the stroma is seen, and there is an additional air bubble underneath the stroma,
separating it from the pre-Descemet. This separation succeeded after six attempted injections of air. The iOCT was a crucial
factor is the success of this procedure.
4. Picture number 4: Removing the stroma. The pre-Descemet is exposed.
5. Picture number 5: after full removal of the stroma.
6. Picture number 6: After suturing the graft- it is visible in the OCT that it is attached properly to the DM
DSAEK
Most of the data about the DSAEK procedure still relies on the data drawn from the PIONEER study, due to its relatively large cohort. The two main surgical techniques of DSAEK differ in the air infused to the anterior chamber- one uses an active air infusion system while the other manually introduce air into the anterior chamber for graft positioning. Hallahan, et al analysed the fluid dynamics and clinical outcomes for iOCT-assisted DSAEK from the PIONEER study. They used a few features measured with the iOCT and discovered that high amounts of interface fluid significantly correlated with graft non-adherence rates within the first postoperative week, following placement and optimization of intraoperative lenticule adherence. Also, the iOCT revealed a significant difference between the area, volume, and thickness of maximum fluid pockets between the two surgical techniques- the manual technique had higher values, but in both techniques, there was a significant reduction of interface fluid during the procedure [8].
DMEK
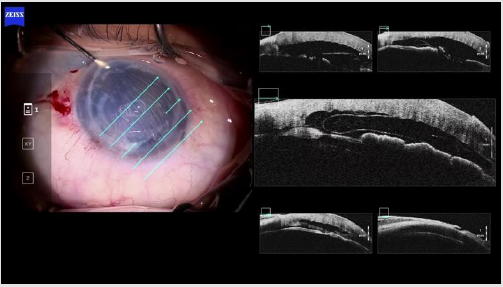
DMEK is currently the preferred procedure to replace diseased endothelium and is superior to DSAEK and PKP for visual recovery and a lower graft rejection rate. On the other hand, the surgeon must be skilled to perform this procedure. The difficult steps are preparation of the donor lamella, transfer of the graft into the AC, unfolding and orientation of the graft, and successful attachment after air filling. Another major challenge is operating through a hazy and/or scarred cornea, which makes the visibility obscure. [9-10] Muijzer, et al. examined 38 eyes which underwent DMEK procedure. iOCT was used during the surgery and its main steps. In 42% of the cases, the iOCT altered the surgical decision-making process. In 21% of the surgeries, OCT revealed interface fluid or a minor detachment of the graft, findings that were not noted using the en face surgical microscope view; In 32% of surgeries, the iOCT image provided crucial information regarding the graft orientation [10] (Figure 3).
Figure 3: A 61-year-old woman who suffered from a graft failure after PKP, during DMEK using iOCT. In the cross section of the imaging the cornea appears thick and edematous. In the en face surgical microscope view the graft is barely visible. The iOCT enables to identify the location and orientation of the graft, and it is possible that the outcomes of the surgery would be less satisfying without the use of the OCT.
Traumatic/Post-Operative Damage
Epithelial downgrowth is a rare complication of intraocular surgery or trauma characterized by the invasion of surface epithelial cells into the AC of the eye. Fibrous downgrowth is a similar but somewhat less aggressive condition characterized by fibrovascular connective tissue invading into the eye. Those pathologies may have a devastating sequela. There are a few case reports published that emphasized the utility of iOCT in those uneventful cases. Shazly, et al. discussed a case of a woman that underwent a DSAEK procedure in an eye with fibrous ingrowth and 2 glaucoma shunt devices. The iOCT provided a clear dissection plane of the fibrous membranes and a clear view of their relation to the iris and corneal endothelium. This proved to be valuable given poor visibility through the opacified cornea. In addition, it allowed determining the extent and location of the interface fluid gap between the DSAEK graft and the host cornea. [11] Ruland, et al. published a case of a woman with primary open angle glaucoma and corneal decompensation of the right eye secondary to tube shunt presented for a 3-month followup of PKP. The patient had a membrane connecting the iris to the host cornea and underwent a biopsy and excision of the membrane assisted by iOCT. The poor view of the peripheral anterior chamber secondary to recent corneal transplantation was aided using iOCT, especially in the manipulation and acquisition of material for biopsy [12] (Figure 4).
Figure 4: A 23-year-old patient who suffered an eye trauma 10 years ago. He underwent vitrectomy with intraocular lens
implantation successfully. After 10 stable years, his intra-ocular pressure increased uncontrollably, and an Ahmed valve was
eventually implanted with a suspicion of epithelial downgrowth. Later, the patient underwent an ingrowth membrane removal
with 5-Fluorouracyl treatment and followed by eight Methotrexate injections to the AC twice a month. Then he was referred
to remove the remained tissue, opening the Ahmed valve and go through a DMEK procedure.
1. Picture number 1: During the insertion of the maintainer the surgeon came across a tissue which cover the whole corneal
endothelium and the Ahmed valve opening.
2. Picture number 2: The tissue is demonstrated in the iOCT
3. Picture number 3: Excision of the tissue by pulling it through a limbal incision.
4. Picture number 4: The epithelial downgrowth is demonstrated in the iOCT- a bright finding appears in the center of the
cornea from the outer layer to the innermost layer and into the AC.
5. Picture number 5: Removing the hidden epithelial downgrowth tissue with a vitrectome.
6. Picture number 6: An additional cryotherapy was conducted in the area to prevent further growth of the epithelium.
7. Picture number 7: A DMEK procedure was conducted, the graft seals the limbal opening.
A year and a half after the surgery, there is no return of the epithelial growth, and the patient best corrected visual acuity is
0.5 (decimal).
Conclusion
Anterior segment IOCtl in ophthalmic surgeries is proven to be an efficient and important tool, which can impact surgeon decision in many cases, and perhaps surgical outcomes. Cases of opaque corneas which were almost impossible to operate are given hope due to this technology. Further prospective studies should be conducted on this issue.
References
- Chen T, Cense B, Pierce MC (2005) Spectral domain optical coherence tomography. Arch Ophthalmol 123(12): 1715-1720.
- Han SB, Liu YC, Noriega KM, Jodhbir S Mehta (2016) Applications of Anterior Segment Optical Coherence Tomography in Cornea and Ocular Surface Diseases. J Ophthalmol 2016: 4971572.
- Ehlers JP (2016) Intraoperative optical coherence tomography: past, present, and future. Eye 30(2): 193-201.
- Ehlers JP, Dupps WJ, Kaiser PK, Jeff Goshe, Rishi P Singh, et al. (2014) The prospective intraoperative and perioperative ophthalmic imaging with optical coherence tomography (PIONEER) study: 2-year results. Am J Ophthalmol 158(5): 999-1007.
- Ehlers JP, Goshe J, Dupps WJ, Peter K Kaiser, Rishi P Singh, et al. (2015) Determination of feasibility and utility of microscope-integrated OCT during ophthalmic surgery: the DISCOVER Study RESCAN Results. JAMA Ophthalmol 133(10): 1124-1132.
- Liu Y, Wittwer VV, Yusoff NZBM, Chan Nyein Lwin, Xin Yi Seah, et al. (2019) Intraoperative Optical Coherence Tomography-Guided Femtosecond Laser-Assisted Deep Anterior Lamellar Keratoplasty. Cornea 38(5): 648-653.
- Myerscough J, Friehmann A, Busin M, Didier Goor (2019) Successful Visualization of a Big Bubble during Deep Anterior Lamellar Keratoplasty using Intraoperative OCT. Ophthalmology 126(7): 1062.
- Hallahan KM, Cost B, Goshe JM, William J Dupps, Sunil K Srivastava, et al. (2017) Intreoperative interface fluid dynamics and clinical outcomes for intraoperative OCT-assisted DSAEK from the PIONEER study. Am J Ophthalmol 173: 16-22.
- Steven P, Le Blanc C, Velten K, Eva Lankenau, Marc Krug, et al. (2013) Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol 131(9): 1135-1242.
- Muijzer MB, Soeters N, Godefrooij DA, Chantal M van Luijk, Robert P L Wisse, et al. (2020) Intraoperative Optical Coherence Tomography-Assisted Descemet Membrane Endothelial Keratoplasty: Toward More Efficient, Safer Surgery. Cornea 39(6): 674-679.
- Shazly TA, To LK, Conner IP, Ladan Espandar (2017) Intraoperative Optical Coherence Tomography-Assisted Descemet Stripping Automated Endothelial Keratoplasty for Anterior Chamber Fibrous Ingrowth. Cornea 36(6): 757-759.
- Ruland K, Bouldin TW, Davis RM, David Fleischman (2018) Intraoperative optical coherence tomography-assisted retrocorneal fibrous membrane biopsy and excision. Am J Ophthalmol Case Rep 11: 101-104.

 Mini Review
Mini Review