ABSTRACT
Objective: Splenic artery aneurysm exclusion from blood flow while preserving the
spleen has been described in the elective setting. In emergency setting, only one case
report of spleen preserving open aneurysm resection has been found. We can confirm
the safety of the procedure.
Design: We present the case of a male patient with a ruptured SAA and
hemodynamical instability.
Results: Open surgical treatment of ruptured SAA through aneurysm resection was
successful and the spleen could be preserved with sufficient perfusion. The procedure
was safe even with a hemodynamical instable patient.
Conclusions: Complications after splenectomy are well described. We recommend
a spleen preserving open surgical treatment of ruptured SAA even with hemodynamical
instability.
Introduction
Visceral artery aneurysms represent a rather rare disease
with documented incidence of 0,1 - 0,2 %. The actual incidence
is underestimated, since most aneurysms remain asymptomatic
[1]. Of all abdominal vessels, the splenic artery is the third most
common affected branch after aortic and iliac arteries [2]. Of the
visceral arteries, the splenic artery is the most common affected
(60-70%), followed by the hepatic artery (20%) and the celiac/
mesenteric arteries (10%) [3].
Most SAA affect the distal third of the artery and are typically
solitary and saccular shaped lesions. In one-third of SAA cases
concomitant aneurysms were found at other localizations
[4]. Predisposing factors for aneurysm are the well-known
cardiovascular risk factors like atherosclerosis (32%), medial
degeneration (24%) and inflammatory diseases (10%); previous
abdominal trauma was often stated (22%). Less common are
high blood flow conditions (e.g., pregnancy, portal hypertension)
or fibroconnective tissue diseases. The diagnosis of SAA is made
either after rupture or incidentally [4]. After rupture, a spontaneous
stabilization can occur if the bursa omentalis temporarily contains
the bleeding through compression. The natural consequence, if
untreated, is the haemorrhagic shock.
Management of SAA depends on the timing of initial diagnosis.
Acute ruptured aneurysms show mortality rates of 10 - 70% and
are therefore a surgical emergency [3]. Incidental aneurysms
with diameters < 2.5 cm rarely rupture spontaneously, as shown
by the Mayo Clinic and the Cleveland Clinic [5]. Size > 3cm as well
as symptomatic SAA and all pseudoaneurysms should be treated
urgently [6]. In contrast to real aneurysms, only specific wall layers are affected in pseudoaneurysms. Abdominal pseudoaneurysms
are often consequence of trauma or iatrogenic injury with faster
enlargement and higher rupture rates.
The aim of the treatment is to exclude the aneurysmatic sac
from blood flow without compromising the distal perfusion. This
can be accomplished with a surgical or endovascular approach [2].
Treatment of asymptomatic aneurysm should be performed in an
elective setting and an endovascular treatment should be discussed.
Depending on end-organ perfusion but also on the size and location
of the aneurysm along the splenic artery, the need for splenectomy
must be evaluated. In most cases end-organ perfusion can be
guaranteed by collateral arteries and other perfusion sources (i.e.,
Aa. gastricae breves, Aa. caudae pancreatis), therefore the need for
this procedure remains an exception [7].
Case
We present the case of a 40-year-old male patient transferred
from a regional hospital. At first contact severe, acute, left
abdominal pain since a few hours were stated. Previous illnesses or
surgical treatments were denied. His mother died due to a ruptured
intracranial aneurysm, no cases of connective tissue diseases were
known in the family. He had nicotine abuse as a risk-factor. Initially
the patient was hemodynamically stable (BP 138/100mmHg, P
80/min, SO2 100%, T 36.8°C) with signs of peritonitis to the left
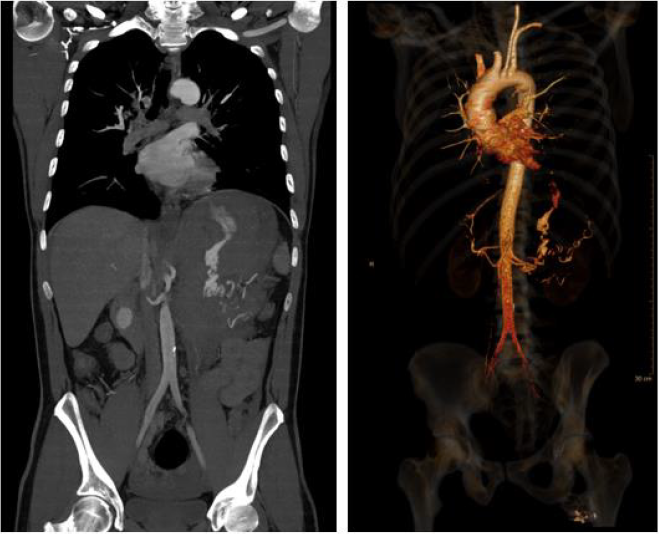
abdomen. Sonography showed excessive free fluid. A CT scan
identified a ruptured aneurysm of the splenic artery (Figure 1) and
the transfer to the shock room followed.
No other bleeding sites or aneurysms could be identified.
Initially haemoglobin (Hb) was 15 g/l, after one hour it dropped
to 13 g/l. 1g Tranexamic acid (TXA) was injected. Upon arrival in
the shock room GCS was 15 and the patient was hemodynamically
stable. Hb was 11.7 g/l, INR 1.1, thrombocytes 215 G/l and fibrinogen
2.1 g/l. An interventional management was initially discussed.
Due to progressive hemodynamical instability and no response
to fluid therapy (BP 100/60mmHg, P 105/min) we performed an
emergency median laparotomy because of hemorrhagic shock. The
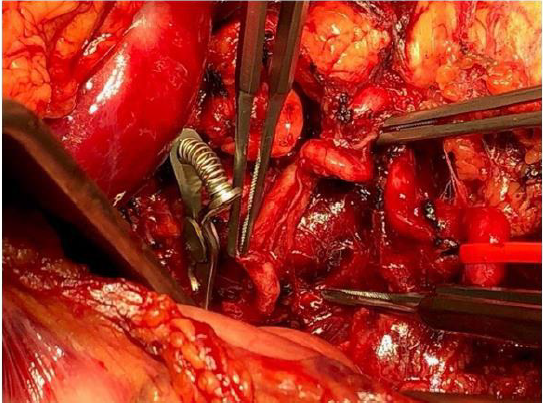
bursa omentalis was opened, about 1.5 L of blood was evacuated
(total blood loss 2.5 L), the splenic artery was identified and clipped
(Figure 2).
As the active bleeding reduced, the aneurysm sac was identified.
After controlling of residual bleeding, the spleen did not show any
ischemic sufferance. An accessory arterial branch guaranteed the
splenic perfusion, and we performed a spleen preserving aneurysm
resection. The aneurysmatic lesion (length 8.5cm) was removed.
A drain was inserted above the pancreas tail. Before closure,
there was no sign of hypoperfusion of the spleen. Intraoperative
transfusion of two red cell concentrates (600ml) and 600ml of own
blood through Cell Saver followed. The minimal postoperative Hb
after transfusions was 9.9 g/l.
The postoperative monitoring in ICU was uneventful.
Prophylactic broad-spectrum antibiotic was stopped after 72
hours. A POPF Grad A (postoperative pancreas fistula, biochemical
leak) was detected. The drain was removed on POD 7 after a CT
scan, the spleen showed normal perfusion. The discharge was
on POD 8. The histological aetiology of the lesion was a chronic
arteriosclerosis. The 30-days follow-up showed an asymptomatic
Patient with normalized Hb. A brain MRI excluded concomitant
intracranial aneurysms. A CT scan 3 months after surgery showed
a normal splenic perfusion with pancreo splenic and gastro-splenic
collaterals. A genetic analysis to rule out genetic connective tissue
disease was done. No pathological findings were reported.
Discussion
Ruptured SAA represent a surgical emergency and show
mortality rates of 10 - 70% [8]. The treatment should be performed
open surgically whenever possible [4,8]. Endovascular approaches
in the emergency setting have shown fewer desirable outcomes,
including the risk of postembolization syndrome and incomplete
aneurysm exclusion [3,8]. In elective setting, laparoscopic or
endovascular approaches are preferred [6]. A retrospective
analysis of a series of 94 patients undergoing aneurysm repair
showed morbidity and mortality rates in open approach (n=74)
respectively at 9.4% and 1.3%. The endovascular approach (n=20)
showed morbidity rates of 10% with no mortality [9]. We report a
spleen preserving open aneurysm resection as surgical treatment
of ruptured SAA in haemorrhagic shock since the spleen did not
show any ischemic sufferance. Spleen preüserving management
of SAA is well described in elective settings, in emergency settings
just by endovascular treatment. We only found one similar case
report (in English) [10]. We confirm that this procedure can be
performed in open surgical emergency treatment of ruptured
SAA. 80% of patients presenting with aneurysms of the splenic
artery are over 50 years old [5]. The prevalence of splenic artery
aneurysm in patients with liver cirrhosis and portal hypertension
is 7-20% [4]. Concomitant aneurysms, which can be found in one
third of the patients with SAA [4], should be excluded through brain
MRI and thoracic-abdominal CT scan. A genetic testing to exclude a
connective tissue disease is suggested.
We confirm that the clinical guidelines should be followed in
decision making. Open surgery remains the gold standard in the
treatment of ruptured SAA and a spleen-preserving management
should always be pursued.
Conflict of Interest
No conflict of interest with any institution/organization.
References
- Cordova AC, Sumpio BE (2013) Visceral Artery Aneurysms and Pseudoaneurysms-Should They All be Managed by Endovascular Techniques? Ann Vasc Dis 6(4): 687-693.
- Hogendoorn W, Lavida A, Myriam Hunink MG, Moll FL, Geroulakos G, et al. (2014) Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. Vol. 60, Journal of Vascular Surgery. Mosby Inc: 1667-1676.e1.
- Stanley JC, Wakefield TW, Graham LM, Whitehouse WMJ, Zelenock GB, et al. (1986) Clinical importance and management of splanchnic artery aneurysms. J Vasc Surg 3(5): 836-840.
- Berceli SA (2005) Hepatic and splenic artery aneurysms. Semin Vasc Surg 18(4): 196-201.
- Abbas MA, Stone WM, Fowl RJ, Gloviczki P, Oldenburg WA, et al. (2002) Splenic artery aneurysms: two decades experience at Mayo clinic. Ann Vasc Surg 16(4): 442-449.
- Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, et al. (2020) The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg 72(1S): 3S-39S.
- Messina LM, Shanley CJ (1997) Visceral artery aneurysms. Surg Clin North Am 77(2): 425-442.
- Ferrero E, Viazzo A, Ferri M, Robaldo A, Piazza S, et al. (2011) Management and urgent repair of ruptured visceral artery aneurysms. Ann Vasc Surg 25(7): 981.e7-981.e11.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, et al. (2011) Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 25(7): 936-946.
- Lo WL, Mok KL (2015) Ruptured splenic artery aneurysm detected by emergency ultrasound-a case report. Crit Ultrasound J 7(1): 7-10.

 Case Report
Case Report