ABSTRACT
Mosquito control faces constant setbacks due to their resistance to chemical insecticides. Thus, the need to develop bio-larvicides that targets the larval stages of mosquitoes before their emergence to adults. Hence, the study on larvicidal activity of the leaf extracts and powder of Millettia aboensis against larvae of Anopheles gambiae s. l. was conducted. Fresh leaves of M. aboensis were collected from Tudun Kauri, Lafia, Nasarawa State in which a part of the collection was subjected to extraction by maceration, using methanol and distilled water while the other part was air dried. The extracts and leaf powder were prepared into varying concentrations (25mg/ml, 50mg/ ml, 75mg/ml, 125mg/ml and 250mg/ml) for the bioassays. After 72 hours exposure period, the 250mg/ml concentration yielded the mortality rate of 100%, 95% and 41% for aqueous, methanolic and leaf powder treatments respectively which varied significantly (P < 0.0001) between concentrations in each of the treatment. The LC50 value obtained showed that the methanolic leaf extract would be the most effective to use. For the LC90 value recorded, the leaf powder would be most effective at 24 hours, while the methanolic leaf extract would be most effective to kill 90% of Anopheles gambiae larvae at 48 and 72 hours. In conclusion, the methanolic leaf extract should be explored for bio-larvicidal development since it showed a potent and better larvicidal efficacy.
Keywords: Anopheles gambiae larvae; Millettia aboensis; Aqueous Extract; Methanolic Extract; Leaf Powder; Lafia
Introduction
Mosquitoes are an important group of insects of public health
importance, which transmit human diseases like filariasis, Dengue,
Malaria, Japanese encephalitis and yellow fever, resulting in millions
of deaths worldwide every year [1]. Malaria is the most prevalent
mosquito-borne disease, globally affecting about 3.5 billion
persons per annum and the causative pathogen (Plasmodium)
is vectored by the Anopheles mosquito [2,3]. An. gambiae is an
effective vector of human malaria and lymphatic filariasis (el
ephantiasis). Despite several efforts in controlling this vector, the
medical and economic burdens caused by it continue to grow [4].
The failure in current control measures and the growing insecticide
resistance is necessitating the search for newer and more effective
control strategies [5]. One of the approaches for controlling this
mosquito borne disease is to interrupt the disease transmission
through mosquito control or avoiding mosquito bite. The primary
public health intervention for reducing malaria transmission at the community level is through vector control and it is the only
intervention that can reduce malaria transmission from very
high levels to close to zero [3]. Among the most preferred target
for mosquito control is the larval stage, because the larva has
low mobility, making treatment to be easily localized in time and
space as compared to the adult stage [6]. Many approaches have
been developed to prevent mosquito menace and the diseases
they spread. One of such strategies has been based on the use of
synthetic insecticides to interrupt the disease transmission cycle
by either targeting the mosquito larvae at breeding sites (through
spraying of stagnant water) or by killing or repelling the adult
mosquitoes [7]. Though effective, these have created problems like
toxicity to humans and non-target populations, long persistence
in environment and entry in the food chain [8]. These problems
have necessitated the need to develop environmentally safe,
biodegradable, economical and indigenous methods of vector
control that can be used with minimum care by individuals and
communities [9].
Plant products with potentials to act as insecticides or repellent
can play an important role in the interruption of transmission of
mosquito borne disease at the individual as well as community level
[10]. The secondary metabolites in different plants make up a vast
repository of compounds with a wide range of biological activities
[11]. Millettia aboensis has been used by traditional medicinal
practitioners to manage constipation, respiratory difficulties, colds
and headaches [12]. The ethanol extract of the root is also used
in the study of anti-inflammatory, antioxidant and antimicrobial
activity and also macerated root in alcohol is used to treat hernias
and jaundice [13]. This study aims to investigate the larvicidal
potential of Millettia aboensis against larval stages of Anopheles
gambiae.
Materials And Methods
Study Area
This study was conducted in the Federal University of Lafia, Nasarawa state. The City of Lafia, Capital of Nasarawa State has farming as the main occupation of its residents, and it boasts crops such as cassava, yam, rice, maize, guinea corn, beans, soya beans, asha, groundnut, vegetables, sugar cane and millet. Also present in the State are mineral resources like columbite, coal and aquamarine. Residents in this area are prone to mosquito-borne diseases as a result of their farming activities [14].
Plant Sample Collection and Identification
Millettia aboensis plant leaves were sought for and collected locally from farmlands around the Lafia metropolis, Nasarawa State, Nigeria. The plant collected was identified and authenticated botanically at the Federal College of Forestry, Jos, with Herbarium Number 235.
Preparation and Extraction of Plant Sample
Leaves of M. aboensis were washed and air dried at room temperature, devoid of sunlight. Dried samples were ground into powdered form using mortar and pestle and then sieved to get fine powder, which was seperated into two portions. One portion of the powdered plant material was further divided into two parts and then each part (400g each) extracted with methanol (4 litres) and distilled water (4 litres) by maceration [8]. The extract was filtered and allowed to evaporate to dryness at room temperature (31oC). The dried extract was then transferred into an air-tight container and preserved in the refrigerator, prior to use.
Qualitative/Quantitative Phytochemical Screening of Millettia Aboensis
The leaf extracts of M. aboensis was screened for phytochemical constituents at the National Research Institute for Chemical Technology (NARICT), Zaria, utilizing standard methods of analysis [15-18].
Mosquito Larvae Collection and Identification
Anopheles gambiae larvae were collected from rock pools made as a result of quarrying activity in Arikpa-Randa, Nasarawa Eggon, Nasarawa State, located at latitude 08°41.408’N and longitude 008°20.835’E. The collected larvae were colonized in the laboratory in Department of Zoology, Federal University of Lafia prior to larvicidal test, and species identified using taxonomic key by [10]. The larvae were fed by adding finely ground powdered yeast on the surface of the water.
Larvicidal Test
Bioassay was carried out according to the WHO standard procedures for laboratory testing of mosquito larvicides [19]. The methanol leaf extract of M. aboensis were evaluated using different concentrations of 25, 50, 75, 125 and 250 mg/ml. Distilled water only (100 ml) was used as negative control, while distilled water (100 ml) to which 1 ml methanol was added was used as positive control. Twenty larvae of each mosquito species were put into each of seven disposable 250 ml bowls (controls inclusive) containing 100 ml of distilled water, to which a measured concentration of the test solution was added. Larval mortality was counted at 24, 48 and 72 hours after treatment. Mortality was calculated at each time interval, replicated four times and the results used to determine the LC50 and LC90 values for the extract by Probit analysis. Larvae were considered either alive, if they were clearly moving normally, or dead when there is no movement and no response to gentle probing on the abdomen with a needle. The interpretation of the mortality rate of Anopheles larvae based on WHO [19] susceptibility tests was:
a) Mortality rate between 98 – 100% within the diagnostic
time, indicates susceptibility.
b) Mortality rate between 80 – 97% suggest possible
resistance.
c) Mortality rate < 80% indicates resistance.
The percentage mortality was calculated by employing the
formula as propounded by [19] as:
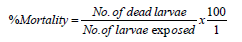
The Corrected percentage mortality was used when a proportion of the insects in the control batches died during the experiment. This was applied using Abbott’s formula [20], represented as:
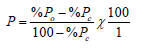
Where: P = Corrected Mortality
Po = Observed Mortality
Pc = Control Mortality, all expressed in percentages.
Determination of LC50 and LC90
Lethal concentrations (LC50 and LC90) were determined by Probit analysis as described by Finney [21] for both samples at the different concentrations and times used in this study. The LC50 and LC90 are the lethal concentrations of the extract that kills 50% and 90%, respectively of the larval population. It is important to note that the lower the LC50 and LC90, the more effective the larvicidal efficacy of the extract. Microsoft Excel regression probit analysis was employed. Percentage mortalities were converted to probits by looking up the percentage in Finney’s table. The log of concentrations is calculated. A graph of probits versus the log of concentration is plotted to fit a line of regression. Extrapolating the probit of 5 in the y-axis to the x-axis followed by taking the inverse of log of the extrapolated value on the x-axis gives the LC50. A similar procedure was used to determine the LC90.
Statistical Analysis
Data obtained were analyzed using R Console software (Version 2.9.2). Mortality rate of the Anopheles larvae in relation to concentrations of extracts were compared using Pearson’s Chisquare test. P-value < 0.05 was considered statistically significant.
Results and Discussion
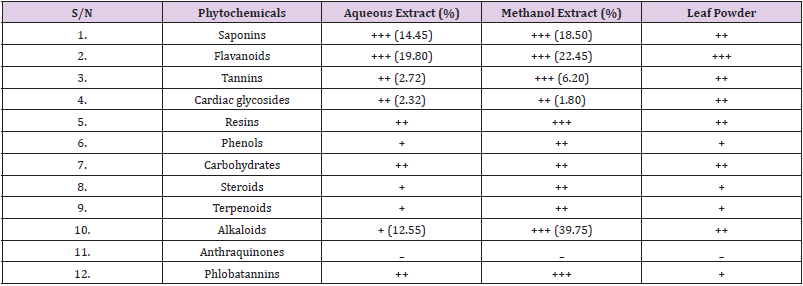
Results of qualitative and quantitative phytochemical screening of the leaves of M. aboensis showed that flavonoids was present in very high concentration from both extracts and leaf powder (Table 1). Flavonoids are hydroxylated phenolic substances synthesized by plants in response to microbial infection. They are also effective antioxidants, helping in the removal of oxidant free radicals [22,23]. They are health promoting compounds due to their active radicalscavenging potential [24].
Table 1: Results of Phytochemical Tests on Leaf Extracts and Leaf Powder of M. aboensis.
Key
+++ = Present in very high concentration
++ = Present in moderately high concentration
+ = Present in low concentration
- = Not Detected
Larvicidal Activity of Leaf Extracts and Powder of M. aboensis against Anopheles gambiae larvae
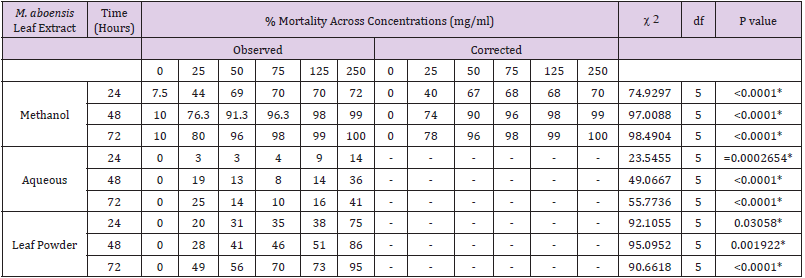
Results of mortality rates recorded against An. gambiae by methanol, aqueous and leaf powder extract at varying concentrations (25-250 mg/ml) are presented in Table 2.
Table 2: Percentage Mortality of An. gambiae Larvae exposed to Extracts Millettia aboensis in relation to Time.
*: Significant
Exposure to Methanol Extract
At 24 hours, 250 mg/ml concentration recorded highest mortality rate of An. gambiae larvae (70%) followed by 125 mg/ ml and 75 mg/ml (68%) then 50 mg/ml (67%), 25 mg/ml (40%), while no mortality was recorded at 0 mg/ml (Table 2). There was a very high significant difference (χ 2 = 74.9297, df = 5, P < 0.0001) in mortality rate of An. gambiae larvae in relation to concentrations of methanolic extract of the leaf of M. aboensis. The larvae were resistant at 24 hours to the larvicidal activity of methanolic extract of the leaf of M. aboensis. At 48 hours, 250 mg/ml concentration had the highest mortality rate of An. gambiae larvae (99%), 125 mg/ml (98%), 75 mg/ml (96%), 50 mg/ml (90%), 25 mg/ml (74%), whereas no mortality was recorded at 0 mg/ml (Table 2). Hence, there was a very high significant difference (χ 2 = 97.0088, df = 5, P < 0.0001) in mortality rate of An. gambiae larvae in relation to concentrations. The larvae were susceptible at 125- 250 mg/ml concentrations but showed possible resistance at 50- 75mg/ml concentration but were however resistant at 25 mg/ml concentration.
A. gambiae larvae were predominantly killed (100%) by 250 mg/ ml concentration at 72 hours period followed by 125 mg/ml (99%) then 75 mg/ml (98%), 50 mg/ml (96%), 78 mg/ml (20%), but no mortality was recorded at 0 mg/ml (Table 2). Therefore, there was a very high significant difference (χ 2 = 98.4904, df = 5, P < 0.0001) in mortality rate of An. gambiae larvae in relation to concentrations. The larvae were susceptible at 75-250mg/ml concentration, and indicated possible resistance at 50mg/ml, but were resistant at 25 mg/ml concentration. The results obtained showed progressive increase in percentage mortality as concentrations increased. The findings are in agreement with studies by Dakum [25] and Abok et al. [26] who investigated the larvicidal potency of the methanol leaf extract of H. suaveolens against An. gambiae larvae, a possible reason being the similarity in the use of solvent for extraction, the same mosquito species tested, and the same Protocol applied for larvicidal testing. This agrees with the findings of Kholhring [27], who investigated the mosquitocidal activity of M. pachycarpa on the larvae and eggs of Ae. aegypti, recording peak mortality at the highest concentration of the extract, after total exposure time. A possible reason for this may be the similarities in the use of plant species from the same family and similar solvents used for extraction.
Exposure to Aqueous Extract
At 24 hours exposure time, highest mortality rate of An. gambiae
(14%) was observed at 250 mg/ml concentration followed by 125
mg/ml (9%), 75 mg/ml (4%), 50 mg/ml and 25 mg/ml (3%) while
no mortality was recorded at 0 mg/ml (Table 2). Therefore, there
was significant difference (χ 2 = 23.5455, df = 5, P=0.0002654) in
mortality rate of An. gambiae larvae in relation to concentrations of
aqueous extract of the leaf of M. aboensis. The larvae were resistant
at 24 hours exposure. The 250 mg/ml concentration had the highest
mortality rate of An. gambiae larvae (36%) at 48 hours followed by
25 mg/ml (19%), 125 mg/ml (14%), 50 mg/ml (13%) and 75 mg/
ml (8%), whereas no mortality was recorded at 0 mg/ml (Table 2).
Thus, there was a very high significant difference (χ 2 = 49.0667, df
= 5, P < 0.0001) in mortality rate of An. gambiae larvae in relation
to concentrations. The larvae at 48 hours were resistant. The mortality rate of An. gambiae larvae was highest (41%) at 250 mg/
ml concentration during the 72 hours exposure period followed by
25 mg/ml (25%), 125 mg/ml (16%), 50 mg/ml (14%) and 75 mg/
ml (10%), while no mortality was recorded at 0 mg/ml (Table 2).
Hence, there was a very high significant difference (χ 2 = 55.7736, df
= 5, P < 0.0001) in mortality rate of An. gambiae larvae in relation to
concentrations. The larvae were resistant at this hour.
After 24 hours of larval exposure to aqueous leaf extract, the
result demonstrated very slow increase in percentage mortality
as concentrations increased. At 48 hours exposure period, larval
mortality showed an irregular pattern as mortality was high at 25
mg/ml concentration, but started to decline steadily at 50-75 mg/
ml. There was a sudden increase in mortality as concentration was
increased to 125 mg/ml and peak mortality was recorded at 250
mg/ml. This gave an irregular pattern of larval mortality. After 72
hours exposure period, there was a similar irregular mortality rate
pattern. Larval mortality was high at 25 mg/ml concentration, but
started to decline steadily from 50-75 mg/ml. There was a sudden
increase in mortality as concentration was increased to 125 mg/
ml and peak mortality was recorded at 250 mg/ml. This irregular
pattern of larval mortality also gave an undulating mortality rate
curve. A possible explanation for the irregular mortality rates
recorded at the 24th and 72nd hour may be that the larvae developed
some resistance to initial dose of the aqueous extract and were
able to tolerate higher doses, but however lost their resistance
when concentration was increased to the maximum. This result
disagrees with the findings of Meenakshi and Jayaprakash [28] who
investigated the mosquito larvicidal efficacy of leaf extract from
mangrove plant Rhizophora mucronata against Anopheles and Aedes
species and recorded 100% larval mortality at all concentrations
after 48 hours exposure period.
Dakum et al. [25] and Abok et al. [26] who conducted similar
experiments, recorded peak mortality at all concentration of the
extract after 72 hours exposure time, which disagrees with the
findings from the current experiment. The variations in results
may be due to the conditions under which the experiments were
conducted and the locations from which the larvae were collected
and the variations in plant species used for extraction and larvicidal
testing.
Exposure to Leaf Powder
At 24 hours, 250 mg/ml concentration recorded highest
mortality rate of An. gambiae larvae (78%), followed by 125 mg/ml
(38%), 75 mg/ml (35%), 50 mg/ml (31%), 25 mg/ml (20%), while
no mortality was recorded 0 mg/ml (Table 2). Therefore, there
was a significant difference (χ 2 = 92.1055, df = 5, P = 0.03058) in
mortality rate of An. gambiae larvae in relation to concentrations of
the leaf powder of the leaf of M. aboensis. The larvae were resistant
at 24 hours. At 48 hours, 250 mg/ml concentration recorded
highest mortality rate of An. gambiae larvae (86%), followed by
125 mg/ml (51%), 75 mg/ml (46%), 50 mg/ml (41%), 25 mg/ml
(28%), while no mortality was recorded at 0 mg/ml (Table 2). There
was a significant difference (χ 2 = 95.0952, df = 5, P = 0.001922) in
mortality rate of An. gambiae larvae in relation to concentrations.
The larvae showed possible resistance at 250 mg/ml concentration
but were susceptible however at 25 – 125 mg/ml concentrations. At
72 hours, 250 mg/ml concentration recorded highest mortality rate
of An. gambiae larvae (95%), followed 125 mg/ml (73%), 75 mg/ml
(70%), 50 mg/ml (56%), 25 mg/ml (49%), while no mortality was
recorded at 0 mg/ml (Table 2). There was a very high significant
difference (χ 2 = 50.6667, df = 4, P < 0.0001) in mortality rate of
An. gambiae larvae in relation to concentration. The larvae showed
possible resistance at 250 mg/ml concentration but were however
resistant at 25 – 125 mg/ml concentrations.
The results obtained demonstrated progressive increase in
percentage mortality as concentrations increased. A possible
reason for susceptibility recorded may be the fact that the leaf
powder used up the dissolved oxygen available in the water, making
it difficult for the mosquito species to survive. Chukwujekwu et al.
[29]. who investigated the antiplasmodial diterpenoids from the
leaves of H. suaveolens recorded similar finding. Also, Ombugadu et
al. [30] recorded an increase in mortality of Anopheles larvae with
increase in dosage of Capsicum chinensis.
Comparison of the Efficacy of Three Treatments
The larvae of An. gambiae showed progressive increase in mortality after 24 hours exposure period. The methanol leaf extract showed greater efficacy than the aqueous leaf extract and the leaf powder. However, at 250 mg/ml, the leaf powder was more effective, recording the highest mortality than the methanol and aqueous leaf extracts. A possible explanation to this occurrence may be that larval mortality resulted from pollution of test habitat caused by the 250mg/ml concentration of the leaf powder, which may have caused a decline in the amount of dissolved oxygen available for the juvenile larval population. This event may have promoted larval mortality at that concentration and time. This result disagrees with the findings of Dakum et al. [25]. who recorded recorded higher mortality rates of An. gambiae exposed to concentrations of the methanol leaf extract of H. suaveolens than those exposed to the aqueous leaf extract of the same plant. After 48-hour exposure period, larval mortality showed progressive increase at all concentrations. The methanol leaf extract proved to be more effective than the aqueous leaf extract and leaf powder, with peak mortality recorded at 99% of the methanol leaf extract at 250 mg/ml. This is in agreement with the findings of Meenakshi and Jayaprakash [28], who also recorded higher mortality rates of An. gambiae and Ae. aegypti larvae exposed to the methanol leaf extract of Rhizophora mucronata.
After 72 hours exposure period, larval mortality showed progressive increase at all concentrations. The methanol leaf extract proved to be more effective than the aqueous leaf extract and leaf powder
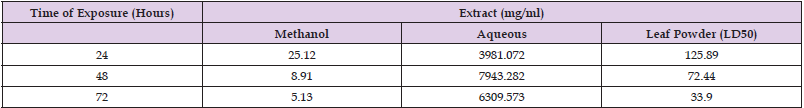
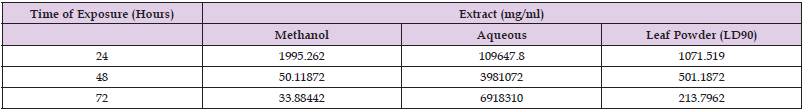
Lethal Concentration of Leaf Extracts and Powder of M. aboensis
LC50 and LC90 values for Anopheles species in relation to leaf extracts and powder of M. aboensis are presented in Tables 3 & 4 respectively. Table 3 shows that the methanol leaf extract would be the most effective to use in order to yield 50% mortality rate at 24, 48 and 72 hours against An. gambiae larvae. The leaf powder would be most effective in order to yield 90% mortality rate at 24 hours, while the methanol leaf extract would be most effective to kill 90% of Anopheles gambiae larvae at 48 and 72 hours Table 4.
Conclusion
Results obtained established that the methanol and aqueous leaf extracts, and leaf powder of M. aboensis can be used as an alternative to synthetic insecticides in control of Anopheles gambiae larvae at high concentrations. It is recommended that the extracts could be merged with various Pest Management programs. Repercautions of extracts on non-target organisms should also be investigated before its recommendation for use in vector control programme and commercial production.
References
- Das P, Amalraj D D (2007) Biological control of Malaria Vectors. Indian Journal of Medical Research 106: 174-197.
- Ohimain E I, Angaye T C N, Bassey S E (2014) Comparative Larvicidal Activities of the leaves, bark, stem and root of Jatropha curcas (Euphorbiaceae) against Malaria Vector Anopheles gambiae. Sky Journal of Biochemistry Research 3(3): 029-032.
- Roll Back Malaria (2011) Malaria Key Facts (Accessed May 25th, 2011).
- Wendy CV, Goddard J, Harrison B (2012) Identification Guide to Adult Mosquitoes in Mississippi.
- Radhika D, Ramathilaga A, Sathesh PC, Murugesan AG (2011) Evaluation of larvicidal activity of soil microbial isolates (Bacillus and Acinetobactor sp.) against Aedes aegypti (Diptera: Culicidae) - the vector of Chikungunya and Dengue. Proceedings of the International Academy of Ecology and Environmental Sciences, 1(3-4): 169-178.
- Kumar MS, Maneemegalai S (2008) Evaluation of larvicidal effect of Lantana camara Linn against mosquito species Aedes aegypti and Culex quinquefasciatus. Biological Research 2: 39-43.
- Corbel V, Ducon S, Zainm M, Hougand JM (2004) Dinotefuran: a Potential Neonicotinoid Insecticide against resistant Mosquitoes. Journal of Medical Entomology 41(4): 712-717.
- Borase HP, Patil CD, Salunkhe RB, Narkhede CP, Salunke BK, et al. (2013) Phyto-synthesized silver nanoparticles: a potent mosquito biolarvicidal agent. Journal of Nanomedicine and Biotherapeutic Discovery 3: 111.
- Mittal PK (2003) Biolarvicides in Vector Control: Challenges and Prospects. Journal of Vector Borne Diseases 40(1-2): 20-32.
- Potters MF, Beavers GM (2005) Public Health Pest Management: A Training Guide, University of Kentucky, College of Agriculture p. 35.
- Venketachalam MR, Jebasan A (2010) Larvicidal activity of Hydrocotyl javanica Thumb (Apjaceal) extract against Culex quinquefaciatus. Journal of Experimental Zoology India 4(1): 99-101.
- Neuwinger H D (2000) African Traditional Medicine. A Dictionary of plant use and Applications. 1st Edition medpharm scientific publishers, Stuttgart pp.
- Lock JM (1989) Legumes of Africa: A checklist. 1st edition Royal Botanical Garden, Kew.
- National Bureau of Statistics (2006) 2006 Population Census, Federal Republic of Nigeria. Archived from the original on 2009-03-25.
- Handa S S, Khanuja SPS, Longo G, Rakesh DD (2008) Extraction Technologies for Medicinal and Aromatic Plants, (1stedn), no. 66. Italy: United Nations Industrial Development Organization and the International Centre for Science and High Technology.
- Vishnoi NR (1979) Advanced Practical Chemistry. Ghaziabad-India: Yikas Publication House, PVT Ltd 447-449.
- Sofowora A (1993) Medicinal plants and Traditional medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria 289.
- Trease GE, Evans W C (1989) Pharmacognosy. 13th (edn.). ELBS/Bailliere Tindall, London, 345-346: 535-536: 772-773.
- World Health Organization (2013) Susceptibility Test Manual: Instructions for Determining the Susceptibility or Resistance of Mosquito Larvae to Insecticides. WHO/VBC/75.583.
- Abbott W B (1925) A method for computing the effectiveness of an Insecticide. Journal of Economic Entomology 18(2): 265-267.
- Finney D J (1971) Probit Analysis, 3rd ed. Cambridge University Press, Cambridge 333. 60(9): 1432-1432.
- Salah N, Miller NJ, Panange G, Tijburg L, Bolwell GP, et al. (1995) Polyphenolic flavonoids as scavenger of aqueous phase radicals as chai breaking antioxidant. Archives Biochemistry and Biophysics 322(2): 339-346.
- Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Science 74(17): 2157-2184.
- Hausteen B (1983) Flavonoids, a class of natural products of high pharmacological potency. Biochemical Pharmacology 32(7): 1141-1148.
- Dakum YD, Amajoh CN, Ombugadu A, Istifanus G, Agwom F, et al. (2021) Larvicidal Efficacy and GC-MS Analysis of Hyptis suaveolens Leaf Extracts against Anopheles species. International Journal of Biochemistry Research & Review 30(1): 8-19.
- Abok JI, Ombugadu A, Angbalaga GA (2018) Hyptis suaveolens Extract Exhibits Larvicidal Activity Against Anopheles gambiae Larvae. Tropical Journal of Natural Product Research 2(5): 245-249.
- Kholhring L (2011) Mosquitocidal activity of Millettia pachycarpa on the larvae and eggs of Aedes aegypti. Scholars Research Library. Annals of Biological Research 2(3): 217-222.
- Meenakshi SV, Jayaprakash K (2014) Mosquito larvicidal efficacy of leaf extract from mangrove plant Rhizophora mucronata (Family: Rhizophoraceae) against Anopheles and Aedes species. Journal of Pharmacognosy and Phytochemistry 3(1): 78-83.
- Chukwujekwu JC, Smith P, Combes PH, Mulholland DA, Vanstanden J, et al. (2005) Anti plasmodial diterpenoids from the leaves of Hyptis suaveolens. Journal of Ethno pharmacology 102(2): 295-297.
- Ombugadu A, Micah E M, Adejoh VA, Odey SA, Njila HL, et al. (2020) Capsicum chinensis (Hot Pepper) Powder Larvicidal Activity Against Mosquitoes Larvae in Lafia Local Government Area, Nasarawa State, Nigeria. Biomed Journal of Science & Technology Res 31(5)-2020. BJSTR. MS.ID.005162.

 Research Article
Research Article