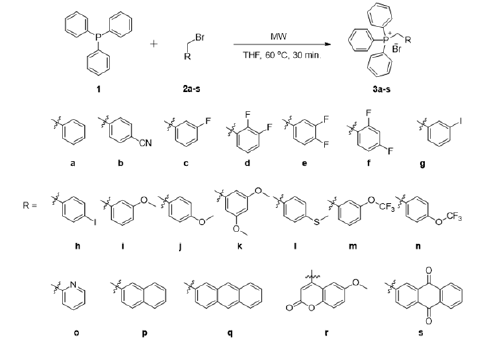
ABSTRACT
Here, preparation of Wittig reagents (substituted-benzyltriphenylphosphonium bromide) using a simple and efficient microwave (MW) irradiation method was established from substituted-benzylhalides and triphenylphosphine in good to quantitative yields (87-98%). Optimization of the reaction condition reveled that in presence of THF at 60 ºC for 30 min. is the appropriate condition for the synthesis of Wittig reagents.
Keywords: Wittig Reagents; Benzyltriphenylphosphonium Bromide; Microwave Irradiation; Phosphonium Salts
Abbreviations: ROS: Reactive Oxidative Species; DPPH: 2, 2-diphenyl-1-picrylhydrazyl hydrate
Introduction
Wittig reagent is one of the most important precursors for the synthesis of large number of natural and bioactive molecules thereby one of the keystones in the field of organic chemistry [1- 3]. Usually, the common reaction conditions such as heating at high temperature or refluxing are used to apply to synthesize the desired Wittig reagent. A detailed literature survey revealed that in the conventional method (CM) heating was applied in the presence of various solvents, such as THF [4-5], CH2Cl2 [6], CHCl3 [7], toluene [8-18], CAN [19] etc. Besides conventional methods, two microwave irradiation (MW) methods in neat [20] and with xylene [21-22] were performed by Kiddle and Cvengros in 2000 and 2004 respectively, for the synthesis of triphenylphosphonium bromide salts and another one synthesized by detected by NMR only [23]. Even though they have obtained excellent yields, but due to the lack of temperature control, a similar condition under CM, and uses of only xylene as well as neat conditions promoted us to optimize the reaction conditions varying temperature, pressure, solvents, voltage as well as mole equivalent of the reagents. Nowadays, microwave-assisted synthesis has become a well-established method for chemists as chemical reactions mixtures are heated instantly and reaction products obtained in good to excellent yields. The major advantage of microwave heating is the reduction of chemical reaction times from days and hours to minutes. Here, we report a simple and efficient method for the preparation of Wittig reagents, (substituted)-benzyltriphenylphosphonium bromide salts using MW irradiation from (substituted)-benzylbromides and triphenylphosphine in quantitative yields (87-98%) in the presence of THF at 60 ºC for 30 min.
Materials and Methods
General Preparation Procedure of Phosphonium Salts Using Mw Irradiation
A mixture of triphenylphosphine (1, 10.5 g, 40 mmol) and benzyl bromide (2a, 3.42 g, 20 mmol) in THF (20 mL) in a carboncoated quartz ampoule was heated under Microwave irradiating at 60 °C with 800 Watt and 1 bar pressure for 30 minutes. The ampoule was opened inside a fume hood, and the precipitate was filtered. Recrystallization was performed in CH2Cl2, obtained a 97 % yield of benzyltriphenylphosphonium bromide (3a). Similar procedures were adopted for other phosphonium salts (3b-s).
Benzyltriphenylphosphonium Bromide (3a)
Colourless powder. Yield 97%, Melting point: 296 ºC (MP. 295 - 298 ºC) [5]. 1H NMR (500 MHz, CDC13): δ 5.39 (d, JHP = 15 Hz, 2H, –CH2), 7.18 - 7.21 (m, 2H), 7.24 - 7.27 (t, J = 8 Hz, 2H), 7.34 - 7.37 (m, 1H), 7.77 - 7.81 (m, 6H), 7.85 - 7.90 (m, 6H), 7.94 - 7.99 (m, 5H) ppm.
(4-Cyanobenzyl)-Triphenylphosphonium Bromide (3b)
Colourless powder. Yield 94%, Melting point: 328 ºC (MP: 326 - 329 °C) [5]. 1H NMR (500 MHz, CDCl3): δ 5.23 (d, JHP = 15 Hz, 2H, –CH2), 7.21 - 7.18 (m, 2H), 7.35 (m, 2H), 7.64 - 7.57 (m, 12H), 7.71-7.75 (m, 3H) ppm.
(3-Fluorobenzyl)-Triphenylphosphonium Bromide (3c)
Colourless powder. Yield 98%, Melting point: 315 ºC (MP: >250 ºC) [24-25]. 1H NMR (500 MHz, CDCl3): δ 5.54 (d, JHP = 14.5 Hz, 2H, –CH2), 6.72 - 6.74 (d, 1H), 6.84 - 6.87 (m, 1H), 7.02-7.08 (m, 2H), 7.58-7.61 (m, 6H), 7.72-7.77 (m, 9H) ppm.
(2,3-Difluorobenzyl)-Triphenylphosphonium Bromide (3d)
Colourless powder. Yield 98%, Melting point: 293 ºC (MP: 292.7 ºC) [26]. 1H NMR (500 MHz, CDCl3) δ 5.52 (d, JHP = 14.5 Hz, 2H, -CH2), 6.89 (m, 1H), 7.97 – 7.05 (m, 1H), 7.29 (pt, J = 6 Hz, 1H), 7.64 - 7.58 (m, 6H), 7.71-7.78 (m, 9H) ppm.
(3,4-Difluorobenzyl)-Triphenylphosphonium Bromide (3e)
Colourless powder. Yield 97%, Melting point: 315 ºC (MP: 315 ºC) [27]. 1H NMR (500 MHz, CDCl3) δ 5.67 (d, JHP = 15 Hz, 2H, – CH2), 6.81 (pq, J = 9.5, 8.5 Hz, 1H), 6.96 (dt, J = 11, 8, 2 Hz, 1H), 7.00- 7.05 (m, 1H), 7.55-7.60 (m, 6H), 7.69-7.80 (m, 9H) ppm.
(2,4-Difluorobenzyl)-Triphenylphosphonium Bromide (3f)
Colourless powder. Yield 97%, Melting point: 258 ºC (MP: 258 ºC) [5]. 1H NMR (500 MHz, CDCl3): δ 5.48 (d, JHP = 14 Hz, 2H, – CH2), 6.53 - 6.56 (m, 1H), 6.69 - 6.72 (m, 1H), 7.59 - 7.63 (m, 7H), 7.73 - 7.77 (m, 9H) ppm.
(3-Iodobenzyl)-triphenylphosphonium Bromide (3g)
Colourless powder. Yield 87%, Melting point: 291 ºC (MP: 295- 298 ºC) [5]. 1H NMR (500 MHz, CDCl3): δ 5.46 (d, JHP = 15 Hz, 2H, –CH2), 6.83 - 6.86 (t, J = 8 Hz, 1H), 7.04 (s, 1H), 7.37 (d, 1H), 7.48 (d, 1H), 7.58 - 7.65 (m, 6H), 7.89 - 7.72 (m, 9H) ppm.
(4-Iodobenzyl) triphenylphosphonium Bromide (3h)
Colourless powder. Yield 95%, Melting point: 254 ºC (MP: 255- 256 ºC) [28, 29]. 1H NMR (500 MHz, CDCl3) δ 5.52 (d, JHP = 15 Hz, 2H, –CH2), 6.90 (dd, J = 8.5, 2.5 Hz, 2H), 7.39 (dd, J = 8.5, 1 Hz, 2H), 7.57 - 7.62 (m, 6H), 7.71 - 7.78 (m, 9H) ppm.
(3-Methyoxybenzyl)-triphenylphosphonium Bromide (3i)
Colourless powder. Yield 88%, Melting point: 262 ºC (MP: 261.7 ºC) [5]. 1H NMR (500 MHz, CDCl3): δ 3.45 (s, 3H, –OCH3), 5.22 (d, JHP = 14.4 Hz, 2H, –CH2), 6.57 (m, 1H), 6.99 (m, 2H), 6.94 (t, J = 8 Hz, 2H), 7.56 (td, J = 8, 4 Hz, 6H), 7.67-7.72 (m, 9H) ppm.
(4-Methyoxybenzyl)-triphenylphosphonium Bromide (3j)
Colourless powder. Yield 88%, Melting point: 248 ºC (MP: 234- 235 ºC) [5, 30]. 1H NMR (500 MHz, CDCl3): δ 3.69 (s, 3H, -OCH3), 5.25 (d, JHP = 14 Hz, 2H, –CH2), 6.62 (d, J = 9 Hz, 2H), 6.98 (t, J = 9, 3 Hz, 2H), 7.60 (td, J = 8, 4 Hz, 6H), 7.66 - 7.76 (m, 9H), ppm.
(3,5-Dimethyoxybenzyl)-triphenylphosphonium Bromide (3k)
Colourless powder. Yield 88%, Melting point: 267 ºC (MP: 264- 265 ºC) [31]. 1H NMR (500 MHz, CDCl3): δ 3.47 (s, 6H, 2 × -OCH3), 5.22 (d, JHP = 14.0 Hz, 2H, –CH2), 6.24 (q, J = 2.3 Hz, 1H), 6.28 (t, J = 2.5 Hz, 2H), 7.61 - 7.56 (m, 6H), 7.75 - 7.67 (m, 9H) ppm.
(4-Methylthiobenzyl)-triphenylphosphonium Bromide (3l)
Colourless powder. Yield 88%, Melting point: 234 ºC (MP: 232.9 ºC) [5]. 1H NMR (500 MHz, CDCl3): δ 2.36 (s, 3H, –CH3), 5.34 (d, J = 14.5 Hz, 2H, –CH2), 6.92 (d, 2H), 6.99 – 7.02 (dd, 2H), 7.61 - 7.56 (m, 6H), 7.68-7.74 (m, 9H) ppm.
(3-Trifluoromethoxybenzyl)-triphenylphosphonium Bromide (3m)
Colourless powder. Yield 97%, Melting point: 294 ºC (MP: 309- 310 ºC) [26]. 1H NMR (500 MHz, CDCl3) δ 5.65 (d, JHP = 14.5 Hz, 2H, –CH2), 6.78 (s, 1H), 7.02 (d, J = 8 Hz, 2H), 7.15 (d, J = 8 Hz, 2H), 7.34 (dd, J = 8 Hz, 2H), 7.57 - 7.62 (m, 6H), 7.71 - 7.79 (m, 9H) ppm.
(4-Trifluoromethoxybenzyl)-triphenylphosphonium Bromide (3n)
Colourless solid. Yield 97%, Melting point: 314 ºC (MP: 309- 310 ºC) [32]. 1H NMR (500 MHz, CDCl3) δ 5.67 (d, JHP = 14.5 Hz, 2H, –CH2), 6.95 (d, J = 9 Hz, 2H), 7.26 (dd, J = 9, 3 Hz, 2H), 7.63 - 7.58 (m, 6H), 7.81 - 7.73 (m, 9H) ppm.
(Pyridine-2-yl-methyl)-triphenylphosphonium Bromide (3o)
Light yellow powder, Yield 26%, Melting point: 116.2 ºC (MP: 116 ºC) [5]. 1H-NMR (500 MHz, CDCl3): δ 6.13 (d, JHP = 15.0 Hz, 2H, –CH2), 7.64 (td, J = 8, 4 Hz, 6H), 7.71 (d, J = 8 Hz, 1H), 7.75 - 7.90 (m, 6H), 8.09 (t, J = 7.4 Hz, 1H), 8.22 (d, J = 7.4 Hz, 1H), 8.32 (d, J = 1.6 Hz, 1H), 8.34 (d, J = 6.2 Hz, 2H), 8.65 (d, J = 6 Hz, 1H) ppm.
(Naphthalen-2-ylmethyl)-triphenylphosphonium Bromide (3p)
Colourless powder. Yield 90%. Melting point: 254 ºC (MP: 248- 251 ºC) [5]. 1H NMR (500 MHz, CDCl3): δ 5.49 (d, JHP = 14.5 Hz, 2H –CH2), 7.10 (td, 1H), 7.30 - 7.40 (m, 2H), 7.48 (d, 3H), 7.53-7.58 (m, 6H), 7.65 (d, 1H), 7.74 - 7.68 (m, 9H) ppm.
(Anthracen-2-ylmethyl)-triphenylphosphonium Bromide (3q)
Light yellow powder. Yield 13%. Melting point: 306 ºC. 1H NMR (500MHz, CDCl3): δ 6.34 (d, JHP = 14.2 Hz, 1H, –CH2), 7.10 (dd, J = 8.3, 7.2 Hz, 1H), 7.22 (t, J = 7.5 Hz, 1H), 7.44 (td, J = 8, 3.5 Hz, 3H), 7.56 (m, 3H), 7.62 (t, J = 7.1 Hz, 2H), 7.87 (dd, J = 9 Hz, 2H), 8.34 (d, J = 3.5 Hz, 1H) ppm.
(7-methoxy coumarin-4-yl-methyl)- triphenylphosphonium Bromide (3r)
Colourless powder. Yield 73%. Melting point: 279 ºC. 1H NMR (500MHz, CDCl3): δ 3.74 (s, 1H, –OCH3), 5.99 (d, J = 4.3 Hz, 1H, – CH2), 6.08 (d, JHP = 16.8 Hz, 1H), 6.42 (d, J = 2.5 Hz, 1H), 6.44 (d, J = 2.5 Hz, 1H), 6.46 (d, J = 2.5 Hz, 1H), 7.53 (td, J = 8, 4 Hz, 1H), 7.66 (m, 1H), 7.79 (d, J = 9 Hz, 1H), 7.96 (m, 1H) ppm.
(Anthraquinone-2-yl-methyl)-triphenylphosphonium Bromide (3s)
Colourless powder. Yield 73%. Melting point: 279 ºC. 1H-NMR (500MHz, CDCl3): δ 5.89 (d, JHP = 15.4 Hz, 1H, –CH2), 7.58 (t, J = 2 Hz, 1H), 7.63 (td, J = 8, 3.5 Hz, 3H), 7.72 (m, 1H), 7.77 (m, 2H), 7.84 (ddd, J = 13, 8, 1 Hz, 3H), 8.00 (d, J = 8.0 Hz, 1H), 8.06 (m, 1H), 8.13 (m, 1H) ppm.
Synthesis of Wittig reagents (substitutedbenzyltriphenylphosphonium bromide) from substitutedbenzylhalides and triphenylphosphine was straightforward as depicted in scheme 1. Initially, the condition was optimized using triphenylphosphine (1) and benzyl bromide (2a) as starting materials [equation (i)] (Figure 1).
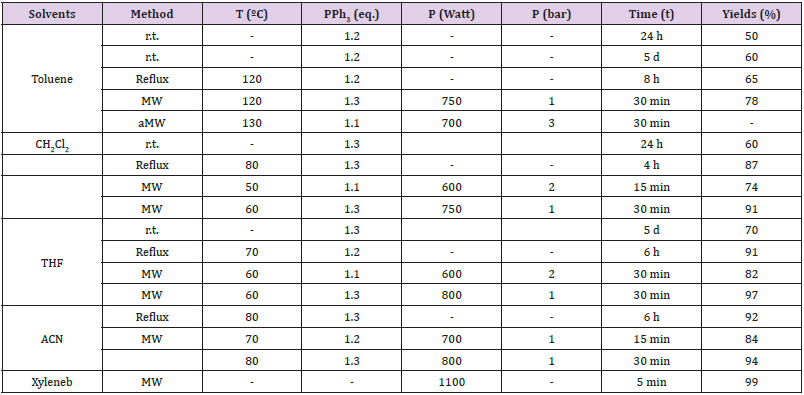
Various conditions, such as room temperature (°C), heating, reflux, different solvents, different molar ratios, powers (watt), pressures (bar), and times (t) were applied to optimize the reaction conditions and summarized in Table 1. From the optimization study table (Table 1), it appears that yields were increased when microwave irradiation was applied to the reaction mixture. Moreover, it saves time as the duration of the reaction was reduced to only 30 minutes. THF acts as the best solvent as it can dissolve triphenylphosphine well, and it also has dielectric properties that are crucial for microwave irradiation reactions. Under these optimal reaction conditions, phosphonium salts (3a-s) were synthesized (Scheme 1). The product formation was considered when a precipitate was observed.
Table 1: Optimization for the synthesis of benzyltriphenylphosphonium bromide salt 3a.
aDecomposition of products; b Temperature was not mentioned, starting materials were liquids
Conclusion
Conventional and microwave irradiation methods were applied for the preparation of eighteen Wittig reagents. Various temperature (°C), power (watt), time (t) and solvents were optimized for the method development of substituted-benzyltriphenylphosphonium bromides. We found, using THF at 60 ºC for 30 min at 800-watt substituted-benzylhalides and triphenylphosphine gave good to quantitative yields. Therefore, this method is simple, efficient and has an advantage over the reported method.
Acknowledgements
The authors gratefully acknowledge the grant from the Ministry of Science, Technology and Innovation, Malaysia (MOSTI) (Biotech Grant Number-30600007001).
References
- M M Heravi, V Zadsirjan, H Hamidi, M Daraie, T Momeni, et al. (2020) "Chapter three - recent applications of the wittig reaction in alkaloid synthesis," The alkaloids: Chemistry and biology, H.-J. Knölker (Editor), vol. 84, Academic Press, 2020: 201.
- M Ohba, I Natsutani (2007) Total synthesis of the proposed structure of macrocaffrine, Tetrahedron 63 (2007) 12689.
- S Tohyama, T Choshi, K Matsumoto, A Yamabuki, K Ikegata, et al. (2005) A new total synthesis of an indolo[3,2-j] phenanthridine alkaloid calothrixin b, Tetrahedron Letters 46 (32): 5263-5264.
- A Kaoukabi, L Belachemi, M Lahcini, MC V Massuard, C Croix, et al. (2019) Efficient synthesis of new 2h-chromene retinoid hybrid derivatives by suzuki cross-coupling reactions. J Heterocycl Chem 56(4): 1260-1274.
- M Mohideen, S Zulkepli, NS Nik-Salleh, L H Leong, K M Chan, et al. (2013) Design, synthesis, in vitro cytotoxicity evaluation and structure-activity relationship of goniothalamin analogs, Arch. Pharmacal Res. 36 (7) 812-831.
- T Suzuki, M N A Khan, H Sawada, E Imai, Y Itoh, et al. (2012) Design, synthesis, and biological activity of a novel series of human sirtuin-2-selective inhibitors. J Med Chem 55 (12): 5760-5773.
- P R D Murray, W M M Bussink, G H M Davies, F W van der Mei, A H Antropow, et al. (2021) Intermolecular crossed [2 + 2] cycloaddition promoted by visible-light triplet photosensitization: Expedient access to polysubstituted 2-oxaspiro[3.3]heptanes, Journal of the American Chemical Society 143 (10) : 4055-4063.
- R K Kawade, C Hu, N R Dos Santos, N Watson, X Lin, K Hanson, et al. (2020) Phenalenannulations: Three-point double annulation reactions that convert benzenes into pyrenes, Angew. Chem Int Ed 59(34): 14352-14357.
- X Yang, WY Kong, JN Gao, L Cheng, NN Li, et al. (2019) Rhodium catalyzed c-c bond cleavage/coupling of 2-(azetidin-3-ylidene)acetates and analogs. Chem Commun (Cambridge, U. K.) 55(84): 12707.
- X Zhang, R Guo, X Zhao (2015) Organoselenium-catalyzed synthesis of indoles through intramolecular c-h amination Org Chem Front 2 (10): 1334.
- J Singh, K W Fowler (2019) Preparation of aromatic, stilbene and related carboxylic acids for modulating ddah and adma levels, useful in treatment and prevention of diseases. Vasculonics LLC, USA. 2019, p: 169.
- L W Souza, R A Squitieri, C A Dimirjian, B M Hodur, et al. (2018) Enantioselective synthesis of indolines, benzodihydrothiophenes, and indanes by c-h insertion of donor/donor carbenes. Angew Chem Int Ed 57(46): 15213-15216.
- J A Smith, K D Moeller (2013) Oxidative cyclizations, the synthesis of aryl-substituted c glycosides, and the role of the second electron transfer step. Org Lett 15 (22): 5818-5821.
- S Mondal, R K Mohamed, M Manoharan, H Phan, I V Alabugin, et al. (2013) Drawing from a pool of radicals for the design of selective enyne cyclizations. Org Lett 15 (22): 56505653.
- R Umeda, S Miyake, Y Nishiyama (2012) Synthesis of dibenz[a,h]anthracenes by pd-catalyzed intramolecular double-cyclization of (z,z)-p-styrylstilbenes. Chem Lett 41 (3): 215-217.
- L Chen, H Min, W Zeng, X Zhu, Y Liang, et al. (2018) Transition-metal-free sulfuration/annulation of alkenes: Economical access to thiophenes enabled by the cleavage of multiple c-h bonds. Org Lett 20 (23): 7392-7395.
- R Mudududdla, R Sharma, S Abbat, P V Bharatam, R A Vishwakarma, et al. (2014) Synthesis of 2-phenylnaphthalenes from styryl-2-methoxybenzenes. Chem Commun 50 (81): 12076.
- M Chalal, D Vervandier-Fasseur, P Meunier, H Cattey, JC Hierso, et al. (2012) Syntheses of polyfunctionalized resveratrol derivatives using wittig and heck protocols. Tetrahedron 68 (20): 3899-3907.
- H Secinti, H Secen (2015) Synthesis of two natural furan-cyclized diarylheptanoids via 2-furaldehyde. Helv Chim Acta 98: 938.
- J Cvengros, S Toma, S Marque, A Loupy (2004) Synthesis of phosphonium salts under microwave activation - leaving group and phosphine substituents effects. Can J Chem 82 (9): 1365.
- J J Kiddle (2001) Microwave irradiation in organophosphorus chemistry. Iii. Moderate scale synthesis of reagents for olefin formation. Synth Commun 31(21): 3377-3382.
- J J Kiddle (2000) Microwave irradiation in organophosphorus chemistry. Part 2: Synthesis of phosphonium salts. Tetrahedron Lett 41(9): 1339-1341.
- J J Kiddle, A F Gurley (2000) Microwave irradiation in organophosphorus chemistry 1: The michaelis-arbuzov reaction, Phosphorus, Sulfur, and Silicon and the Related Elements 160(2000): 195.
- P Wyatt, A Hudson, J Charmant, A G Orpen, H Phetmung, et al. (2006) Synthesis and chemistry of enantiomerically pure 10,11-dihydrodibenzo[b,f]thiepines. Org Biomol Chem 4(11): 2218.
- Z L Zhou, J F Keana (1999) A practical synthesis of 4-(substituted-benzyl) piperidines and (+/-)-3-(substituted-benzyl) pyrrolidines via a wittig reaction. J Org Chem 64 (1999): 3763.
- D J Rawson, D Brugier, A Harrison, J Hough, J Newman, et al. (2011) Part 3: Design and synthesis of proline-derived α2δ Bioorg Med Chem Lett 21 (12): 3771-3773.
- M Sasaki, H Takatsu, K Takeuchi (1992) Preparation of fluorophenylcyclodexyl ethers fluorine-substituted compound containing ether bond. Dainippon Ink Chemical Industry Co., Japan. p: 25.
- J G Rodrı́guez, R Martı́n-Villamil, A Lafuente (2003) Π-extended conjugate phenylacetylenes. Synthesis of 4-[(e) and (z)-2-(4-ethenylphenyl) ethenyl] pyridine. Dimerization, quaternation and formation of charge–transfer complexes. Tetrahedron 59: 1021.
- M Cui, Z Li, R Tang, H Jia, B Liu, et al. (2011) Novel (e)-5-styryl-2,2'-bithiophene derivatives as ligands for β-amyloid plaques. Eur J Med Chem 46 (7): 2908.-2916.
- W Zhang, M L Go (2007) Quinone reductase induction activity of methoxylated analogues of resveratrol. Eur J Med Chem 42 (6): 841-850.
- B Sun, J Hoshino, K Jermihov, L Marler, J M Pezzuto, et al. (2010) Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem 18 (14): 5352-5366.
- S M Kelly, A Germann, R Buchecker, M Schadt (1994) Polar nematic methyl (e)-[trans-4-cyclohexyl-substituted] allyl ethers: Synthesis, liquid crystal transition temperatures and some physical properties. Liquid Crystals 16 (1): 67-93.

 Research Article
Research Article