ABSTRACT
Antibodies and aptamers play a crucial role to improve the analytical performance of biosensors for medical applications. Besides their natural compatibility with many antigens and pathogens, their biochemical structure selectively binds the analyte providing high sensitivity and selectivity. Accordingly, this minireview presents the recent approaches in the field of immunosensors and aptasensors for clinical diagnoses with a focus on the key features of sensors over the conventional diagnostic assays and the trends in this field of knowledge (point-of-care devices for in situ applications, label-free bioreceptors, real-time monitoring of analytes and outstanding transduction techniques).
Keywords: Biosensor; Medical Diagnosis, Aptamer; Antibody
Abbreviations: SELEX: Systematic Evolution of Ligands by Exponential Enrichment; AG: Any Protein; FAB: Fragment Antigen Binding; LOD: Limit of Detection; PCR: Polymerase Chain Reaction; ELISA: Enzyme-Linked Immunoassays; POC: Point-of-Care; HER2: Human Epidermal Growth Factor Receptor 2; SPEs: Surface of Screen-Printed Electrodes; MIP: Molecular Imprinting Polymers; EIS: Electrochemical Impedance Spectroscopy; CV: Cyclic Voltammetry; PSA: Prostate Specific Antigen
Introduction
Advances in the field of molecular biology and chemistry have driven the studies in biosensing to an important and necessary level. The increasing attention of the population to healthcare summed to the alterations in their alimentary and social habits significantly changed the needs for personal health. Miotto, et al. [1] mentioned that the current context of healthcare demands to “ensure that the right treatment is delivered to the right patient at the right time”. In this scenario, the study of biosensors has provided sufficient tools, especially in the last decade, to advise the science of sensitive, rapid and accurate medical diagnostics. Clark and Lyons [2] were the pioneer in the field with the development of an enzymatic biosensor for detection of glucose. Their technology based on the oxidation of glucose by the enzyme glucose oxidase produced gluconic acid, hydrogen peroxide and electrons. This technology inspired unlimited researches up to nowadays and the more known commercial devices are still based on biosensing of glucose (being the first commercially available biosensor for glucose fabricated by the company Yellow Spring Instruments) [3].
Once biological molecules are irreplaceable agents in living beings to make humans and animals to perfectly function, not surprisingly, scientists and research companies devote maximum efforts to mimic the biochemical reactions that naturally occur in the nature. This is the basis of a biosensor. A biological element of recognition is attached to the surface of an electrode material to detect a target molecule by means of their specific sites. Changings in chemical and/or physical properties of the transducer system are thus monitored and associated to the presence or to the concentration of the molecule of interest. Regardless the numerous possibilities of substrate materials, transduction modes and kind of molecules of interest, possibly the study of bioreceptors is represents the golden effort to achieve the two most important characteristics of a tool for diagnosis: sensitivity and selectivity. In light of this context, this work proposes a critical review of the literature on biosensing technologies for medical diagnosis with respect to two of the most important bioreceptors employed in highperformance sensors: antibodies and aptamers. A discussion on the global features of biosensors, their importance and application in medical diagnoses, key aspects of antibodies and aptamers to be employed as bioreceptors are provided herein. This knowledge is illustrated with the most recent trends in current works available in the specialized literature in order to contribute to the field of biosensors and clinical bioassays.
Biosensors and Units of Biorecognition
Sensors are part of our daily lives, inserted in the most diverse equipment’s with the most different functionalities. In general, a sensor is a device that transforms a certain physical or chemical property into an analytically measurable signal. In this way we can classify sensors where the variation of a biochemical property generates any signal, these devices we call biosensors, which can be defined according to IUPAC as being “device that uses specific biochemical reactions mediated by isolated enzymes, immune systems, tissues, organelles or whole cells to detect chemical compounds usually by electrical, thermal or optical signals” [4] A biosensor consists of two parts, one formed by the biological recognition element (receiver) and the other by the transducer, which can be electrochemical, optical, thermal, piezoelectric, capacitive and field effect. We can classify them, by the different methods of transduction, as well as according to the element’s receptor. Here, we will classify them only this. Bioreceptors can be selective or not, but recognition element plays a crucial role in the overall biosensor performance and selectivity toward a particular analyte [5]. Temperature, pH, contaminants, ionic strength, type of solution (buffer solution, body fluids, water) are factors that determine the performance of the biosensors [6,7].
Aptamers / Aptasensors
Aptamers are short and single-stranded nucleic acids (DNA
or RNA) with capacity to bind to target molecules with high
affinity and specificity [8]. First introduced in 1990, the process
of selecting an aptamer is called Systematic Evolution of Ligands
by Exponential enrichment (SELEX), from a large oligonucleotide
library [9,10]. Aptamers can be selected for a variety of targets,
including small molecules, proteins, nucleic acids, microorganisms,
cells, tissues, metal ions and chemical compounds [11-13]. With the
advantages of small size, high binding affinity, good stability and
easy synthesis, aptamers show potential for various applications,
such as targeted therapy, detection and clinical diagnoses [14-17].
After selection and characterization, aptamers can be customized
for developing sensors [18]. A large variety of aptamer-based
biosensors (aptasensors) with various detection strategies have
been developed and reported in the literature [19]. In comparison to
antibodies, aptamers are smaller units containing oligonucleotides
with sizes over 30 oligos [20].
They are similar to monoclonal antibodies in terms of binding
affinities, being called synthetic antibodies [21] in addition to other
advantages, such as chemical stability and regeneration of its threedimensional
structure even after several cycles of denaturation/
renaturation [22]. Its small size allows a greater density of
immobilized molecules. They are chemically synthesized, which
allows the flexibility of the conformation of their two-dimensional
structure, so it can be built for the detection of any antigen, from
small molecules, heavy metals, protein, enzymes, microorganisms
and cells, with the possibility of adjusting the sensitivity and
selectivity [23-28].
Antibodies / Immunosensors
Antibodies (Abs) are proteins that can be employed as valuable
tools in laboratory and clinics [29]. Antibodies include those
secreted by a single clone of B lymphocytes, termed monoclonal
antibodies (mAbs), and those produced by a mixture of various B
lymphocyte clones, the polyclonal antibodies (pAbs) [30-32]. In
1975, Kohler and Milstein developed a system for the production
of monoclonal antibodies. Abs demonstrate high affinity and
specificity to target molecules and have been frequently selected
for a wide variety of applications including immunodiagnoses,
biomarker detection, immunological research and vaccine quality
control [33-35]. Abs can be used to develop a variety of sensors
(immunosensors) upon the formation of an antibody-antigen
complex [36]. Immunosensors are based on antigen-antibody
affinity, where an immunochemical reaction forms a very stable
complex. Every protein has an isoelectric point (point where the global electrical charge is equal to zero) that varies according to the
composition of the amino acids, thus determining the magnitude
and polarity of that point at a specific pH [37].
One can assume that any protein (Ag), with charge Ch1, and
its antibody pair with charge Ch2, the reaction of that system
(AgAb) results in a global charge Ch3 which can be described by the
following equation:
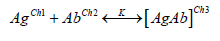
where K is the binding constant for this complex. This change in electrical charges can ideally be detected, depending on the antigen concentration and the transduction technique used. The ambivalence of this system still allows the use of a biosensor for the detection of an antigen, regarding the possibility of immobilizing an antigen and the antibody becomes the analyte. Abs possess a “Y” shaped structure consisting of two heavy and two light polypeptidic chains bound by S-S bonds with approximately 150 kDa and dimensions of 14 nm x 10 nm x 4 nm [38,39]. The base of this “Y” structure is called fragment crystallizable region (Fc) and is composed by the heavy chains. On the other two extremities, there are the antigen-binding sites, or epitopes, comprising the fragment antigen binding (Fab). The Fab branches exhibit different characteristics (such as the chemical composition, the physical structure and the isoelectric point) as a natural consequence of their properties to bind different analytes [39,40]. At the same time it is advantageous to orientate the immobilization of Abs by the Fc portion because it keep frees the active specific sites (Fab) to bind analytes, the extra protocol to allow this orientation makes the fabrication of oriented antibodies-based sensors more laborious and frequently more expensive.
Key Features on the Performance of Biosensors
The most important characteristics of a biosensor are its
selectivity, reproducibility, stability, sensitivity and linearity.
The combination of these parameters has been the focus of
many researchers specially in the last decade to develop high
performance devices for diagnosing molecules of medical interests.
These features can be defined as follows:
a. Selectivity: represents the ability of a sensor to present an
analytical signal exclusively due to the recognition of the
target analyte, not suffering the influence of interfering species
at a significant level. Morales and Halpern [41] mention that
selectivity is essential in the development of point-of-care
biosensors. This is because the testing biological samples are
typically very complex and can possess various interfering
molecules capable to compete for the bioreceptor sites of the
sensor;
b. Limit of Detection (LOD): is the minimum amount of analyte
able to generate an output signal distinguishable from the
blank signal (analyte absence) [42]. Depending on the level of
affinity between the biorecognition element and the analyte,
the biosensor can achieve low LODs and meets a broader
window of applications in the field of clinical diagnosis. This
affinity is expressed in terms of the dissociation constant “KD”
(reciprocal of the association constant “KA”), which relates
the concentration of free and bound molecules in a solution
to provide a sense of strength of these interactions. In this
regard, the lower KD is, the higher is the affinity between the
bioreceptor and the analyte and, consequently, the lowest
concentrations can be detected by the biosensor. IUPAC
recommends the use of the equation LOD = 3S/m to calculate
LOD, where “S” corresponds to the standard deviation derived
from the black measurements and “m” represents the slope of
the calibration curve;
c. Sensitivity: despite it is still very common to observe the
misuse of this term to designate the LOD, the sensitivity
actually refers to the variation of the analytical signal due to the
variation of the target analyte. In other words, it is calculated
as the slope of the calibration curve and has the unit of the
transduction signal (e.g. Ampères, Ohms, Volts, degrees, Celsius
degrees, Hertz, etc) divided by the unit of concentration [43].
Briefly, the higher is the sensitivity, the higher is the response
of a biosensor when it binds an analyte;
d. Stability: capability of keeping the analytical signal robust
enough to not suffer the influence of extrinsic agents, such as
environmental disturbances, loss of bioreceptors’ affinity to
the target, molecules degradation over time, etc [38];
e. Linearity: corresponds to the obeyance of the calibration
curve to a mathematical expression. Once the linearity is
set known, the concentration of the molecule of interest in
a certain medium can be predicted and this is the working
principle of quantitative accurate biosensors;
f. Reproducibility: can be defined as the ability to provide
similar responses under similar conditions of detection.
In addition to those basic analytical properties, some authors
also defend the evaluation of the linear range of detection and
the response time to validate the performance of a biosensor. The
former represents the concentration range of the analyte at which
the sensor generates linear output signals, which is important to
define whether the working range meets the requirement for a
certain application besides helping to calculate the LOD and the
sensitivity. The latter is an important reference mainly in medical
applications. The response time of a sensor is the time required by the device to generate the analytical output signal as a consequence
of the recognition of the target molecule. It is also frequent in the
literature to find this definition as the time required to obtain
95% of the data resulting from the detection [38]. In the context
of clinical diagnoses, fast responses of biosensors allow doctors
to manage the diseases at early stages, avoiding the spreading of
infections and the worsening of the clinical picture of patients.
Within the scenario of the ongoing pandemic of coronavirus
disease (COVID-19) for instance, authors defend that the
importance of a quick diagnosis lies on fact that SARS-CoV-2 has
exhibited higher contagiousness and infection rate if compared to
other coronaviruses infections [44]. Furthermore, early diagnosis
contributes to fast decisions on medical treatments and quarantine
strategies to slow down the spread of the transmission rate.
Traditional Analytical Techniques for Diseases Diagnosis
Diagnosis, detection and prognosis techniques have been studied for several years and many methods for fault detection and diagnosis have been developed [45]. Molecular diagnostics assays use in vitro biological techniques for detection. Polymerase chain reaction (PCR) and quantitative PCR are performed to detect and amplify a genetic material (DNA or RNA) from a specific organism, for instance, a virus [46,47]. The advantages of PCR include the high sensitivity, quick performance and the ability to detect lesscommon organisms. On the other hand, its disadvantages include the supply costs, machinery fees and training expenses [48,49]. At present, PCR assay is regarded worldwide to as the most accurate and reliable test to detect active COVID-19 infections [50,51]. Immunoassays, such as enzyme-linked immunoassays (ELISA) and point-of-care (POC) techniques can be used for detection of antigens or specific antibodies [52]. Currently, immunoassays play a prominent role in the analysis of many clinical laboratory analytes such as proteins [53]. A broad variety of tests detecting specific SARS-CoV-2 antigens and IgA, IgM and/or IgG antibodies were developed [54,55]. Although the classic immunoassays can provide very sensitive and accurate diagnoses, many of them possess some important limitations: high cost, they are time consuming, demand sophisticated equipment and high skilled staff [56].
Recent Trends in Biosensors for Detection of Analytes of Medical Interest
It is worthy notable that the field of biosensing through the design of assays to detect molecules of medical interest has attracted huge attention specially in the last year with the outbreak of COVID-19 around the world. Not exclusively due to the current pandemic, though, numerous researches have been devoted to some special improvements in the analytical sciences in order to ameliorate the performance of the already known technologies. Within the recent literature in this domain, one can easily recognize some trends in the newest biosensors of medical interest: the fabrication of point-of-care devices, the label-free detection, realtime measurements and the advance of electrochemical transducer mechanisms. Under all these trends, the use of antibodies and aptamers as bioreceptor agents seem to properly match the needs and expectations of current diagnoses.
Point-of-Care Biosensors
Point-of-care diagnoses collect several unquestionable
advantages over traditional laboratory setups. Not surprisingly, the
golden characteristic refers to the possibility of running the test
wherever the patient is, on-demand and onsite [57]. It makes the
sensors amenable for bedside monitoring, analysis in pharmacies
or even by the user himself. Consequently, this kind of device
tends to gain increasing visibility in the market. Eguilaz et al. [58]
highlight that these devices are even more relevant in resourcelimited
regions where the access to medical centers is difficult to
the majority of the population. Nonetheless, point-of-care devices
combine other interesting characteristics, such as (generally) the
rapid detection, fewer steps for data/results acquisition, friendly
interface, easy transport due to the reduced dimensions and light
weight and demand for small sample volumes [57,59]. Concerning
this last characteristic, though, there is a strategic point to be taken
in account. Depending on the application, the target molecule is
present at very low concentrations in the sample of analysis. Thus,
a small volume for testing can contain insufficient quantity of
analyte in such a manner that the biosensor would not be able to
detect it [58].
In this regard, antibody- and aptamer-based biosensors are
widely employed to overcome this drawback because of their high
sensitivity resultant from the high affinity and selectivity of these
molecules. Searching for overcoming the limitations of conventional
diagnoses, Ferreira, et al. [60] worked on the development of an
aptasensor for detection of breast cancer in undiluted human serum.
This kind of cancer is unfortunately responsible for thousands of
deaths annually. According to the authors, the diagnosis is mostly
based on the detection of tumor markers present in blood or
other corporal fluids at concentrations from 15 ng/mL to 75 ng/
mL (over the regular healthy range of 2- 15 ng/mL). Ferreira, et
al. [60] exploited two functionalization methods to attach Human
Epidermal Growth Factor Receptor 2 (HER2) aptamers to the
surface of screen-printed electrodes (SPEs). These devices are
widely recognized in the literature to serve as useful substrates for
designing portable electrochemical sensors, mainly because of their
good conductivity, electrical stability in typical electrolytes and
reduced dimensions [61]. In a list of 110 recent articles reviewed
by Ranjan, et al. [62] on point-of-care biosensors for breast cancer
diagnosis, 23% were described the use of antibodies and 5% the use of aptamers as bioreceptors, which symbolically represents the
large employment of these biomolecules in biosensors of medical
interests. In this same work, other elements of recognition were
described, e.g. enzymes, inorganic probes, DNA, proteins, receptorligand
complexes and molecular imprinting polymers (MIP).
Label-Free Detection
The topic of label-free sensing in the context of bioassays
generally converges to two points: the advantage of reducing
the consumption of reagents and the consequent lower number
of fabrication steps of biosensors in comparison to golden
standard techniques (e.g. ELISA and PCR). Label-free sensing
mechanisms consist in the direct detection of target molecules by
the bioreceptor attached to the transducer substrates, i.g., without
the needs for fluorescent chemicals, enzymes and so on [63]. Thus,
since the label-free biosensors do not demand extra labels to run
the detection, this characteristic nicely meets the requirement of
point-of-care biosensors for the simplest incubation protocols and
allows the use of unprepared samples at working environments.
Among other interesting features, Andryukov, et al. [63] point out
the following advantages over label-based similar analytical assays:
simpler pattern of detection, lower response time, lower cost of
analysis, opportunity to detect small molecules and possibility of
multiplexing.
Zhang and Liu [64] mentioned that the success of using DNA
in label-free devices based on optical biosensors has inspired the
same approach in the aptamer field. However, the authors explain
that aptamers can fold DNA and hide its bases, providing slow
kinetics of target binding, especially when the target is a small
molecule. Therefore and since aptamers possess lower affinity to
small molecules (KD around low micromolar units) than DNA (KD
approximately in picomolar or low nanomolar), the detection of
aptasensors tends to be more challenging, justifying the efforts on
label-free sensing to enhance its analytical response. On the other
hand, numerous works can be easily found in the field of labelfree
immunosensors for detection of analytes for highly sensitive
diagnoses [65-68].
Real-Time Measurements
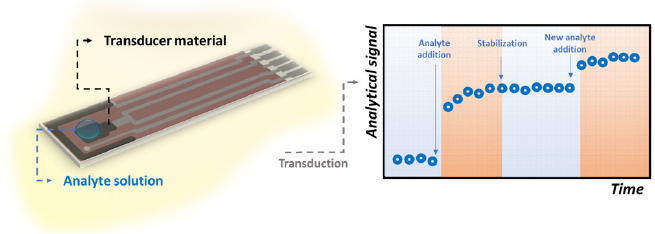
The key point of real-time biosensing is the necessity of the sensor to rapidly recognize the target molecule. If so, the output signal will be registered by the transducer source in short time intervals and a variation in its magnitude could be notable as illustrated in Figure 1. This need makes some important well recognized techniques such as ELISA and Luminex assay to fail as real-time methods for in vivo applications, since they require laborious and pre-defined longtime steps [69]. Cohen et al. [69] highlight that ELISA, for instance, depends on diffusion processes concerning the interaction between antibodies and antigens in a non-mixed solution, which is associated to a low binding equilibrium constant and makes the response time longer. Typically, this technique requires approximately 3 hours to be performed [70,71]. In this regard, Shengnan, et al. [72] reported the construction of an aptasensor for the real-time detection of vascular endothelial growth factor, one of the most important cytokines present in cancer patients (with average concentration of 434 pg/mL). The authors achieved a LOD of 0.1 pg/mL within a linear detection window from 2 pg/mL to 500 pg/mL. The mechanism of recognition was based on a Chronoamperometry test at the positive redox peak potential of ferrocene-labeled aptamer for 5,000 seconds.
Figure 1: Illustrative scheme referring to the fluctuations of the analytical signal of a biosensor as a consequence of rapid interaction between its bioreceptors and the target molecules.
Also taking advantages of the specificity of aptamers as bioreceptors, Soleimani, et al. [73] manufactured an aptasensor assisted by a computerized monitoring system to detect prostate specific antigen (PSA). To characterize their aptasensor and to construct the calibration curve towards PSA, the authors carried out Electrochemical Impedance Spectroscopy (EIS) and Cyclic Voltammetry (CV). Due to the steric hindrance of the analyte, the electrochemical signal of the transducer substrate increases over the time when aptamers bind the molecules of PSA. As a result, the findings showed that this kind of setup presented sensitive and rapid response fitting the real application aimed to the diagnosis of patients with prostate cancer.
Electrochemical Transducing
As per the examples of the previous sections, electrochemical
mechanisms have progressively illustrated the transduction modes
of many biosensors for medical applications. From 2017 to 2019, for
example, these devices represented 45% of the published articles
in the specialized literature of biosensors [74]. The reason is the
collaboration that electrochemical reactions provide to enhance
sensitivity, accuracy and response time.
Briefly, in this kind of biosensor the electrical properties of
biological molecules and their interaction with electro active
surfaces are exploited for assessing the changes in current,
potential, charge, impedance, conductivity, etc. Complementarily,
depending even on the dimensions of target molecules, the distance
from the electrode surface and needs for redox probes, the specific
electrochemical technique can be chosen to achieve highest
analytical performance [75]. The detection system consists of three
(or two) electrodes, of which one is the sensing surface (named
working electrode), one is the counter-electrode and the other is
a reference. These electrodes must be immersed in a conductivity
solution to allow redox processes to occur and charges transfer.
When the detection of the analyte happens and the electrical
properties of the surface is altered, an electronic system acts to
amplify and manage the resultant data. Traditionally, this last
step is performed by a potentiostat interfaced with a software
for control of the required parameters. Mishra, et al. [75] pointed
out details on the electrochemical aptasensors referring to design
strategies and functionalization. The researchers reported that
aptamers have been mostly immobilized to gold and carbon-based
electrodes via chemical cross-linking with particular attention
to ensure biochemical stability, surface coverture and optimal
binding affinity. Most common electrochemical techniques used for
fabrication of biosensors are CV [76,77], EIS [78-80], potentiometry
[81,82] and amperometry [83,84]. When real-time performance
is required, time-based assays (such as chronoamperometry,
chronocoulometry and chronopotentiometry) well fits medical
applications.
Efficiency of Immunosensors and Aptasensors
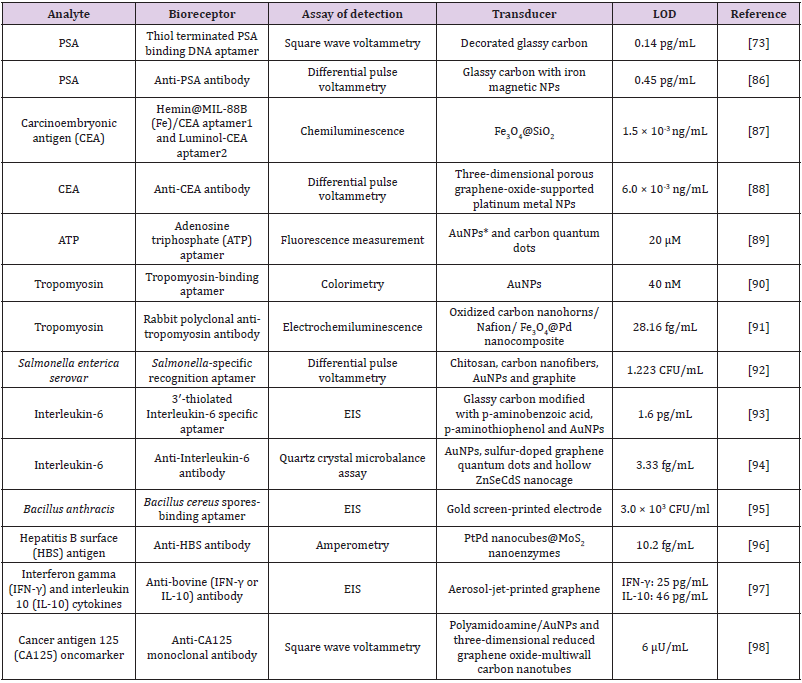
Cesewski and Johnson [85] point out that, in some cases, the high sensitivity of immunoassays are not enough to detect certain pathogens in the organism. In such circumstances, although these infectious agents are present, they do not generate enough available Abs in the blood, so the concentration of the Abs in the blood are lower than the LOD of the technique, failing the detection. According to the authors, this is a typical situation in which the employment of DNA-based systems is more useful. The biosensors consisting of nucleic acids, for instance, are usually able to recognize low concentrations of pathogens by themselves or through the indirect expression of toxins they release in the infected organism (e.g. toxins, other nucleic acids and raised cells. In this regard, Table 1 contains a list of recent researches in the literature of biosensors for medical applications using antibodies and aptamers as bioreceptors. It is worthy notable that these biomolecules facilitate the biosensing of analytes at concentrations as low as some femtograms per milliliter [86-90].
Table 1: Recent developments (from the last two years) in the field of aptasensors and immunosensor for assisting clinical diagnosis.
Note: *NPs = Nanoparticles
Regardless the obvious different protocols used to attach antibodies and aptamers to the different transducer substrates, it is worthy notable that the sensitivity of these devices are really high. Besides, in this recent literature is not rare to observe a trend in using label-free molecules to optimize the fabrication step and to allow accessible in-situ measurements [91-98]. Nonetheless, it is also evident that many authors have employed electrochemical techniques to ensure accuracy and high performance of biosensors, corroboration the previous discussion brought to this minireview in the section “Recent trends in biosensors for detection of analytes of medical interest”.
Conclusion
With the increasing humans needs for accurate, fast and
friendly methods for health control, biosensors for medical
applications have undergone important changes in the last decade.
The immobilization of antibodies and aptamers on transducer
substrates for high performance detection has been an exhaustive
strategy for the production of biosensors, especially due to the high
sensitivity of these bioreceptors. Articles published in the recent
literature exhibit LODs in the order of femtograms per milliliter.
To this end, added to the intrinsic advantages of antibodies and
aptamers, there is a notable trend to search for label-free devices,
with less functionalization steps, lower times for the formation
of bioreceptor-analyte complexes, under selective and sensitive
sensing modes. Thus, much is seen about the use of electrochemical
techniques such as CV, EIS and amperometry, although optical
and piezoelectric transduction techniques are also present in the
field of biosensors for various applications including the ones for
medical diagnostics.
It is believed that this specific application demands advanced
technologies, especially to shorten the detection time, since early
diagnoses are essential for the administration of first aids and
precise medications that can enhance the chances of cure and
survival of patients (especially those who have less access to health
centers). The main challenges in the area still seem to be related to
the commercial viability of these devices. Likewise, quite possibly,
the prospect of advances in technology is likely to be based on the
study of alternative materials and methods to make immunosensors
and aptensensors increasingly simple and inexpensive.
Acknowledgement
The authors gratefully acknowledge the Banco Nacional de Desenvolvimento Econômico e Social (BNDES) and MedicOnChip Desenvolvimento Tecnológico for the financial and technical supports.
References
- Miotto R, Wang F, Wang S, Jiang X, Dudley JT (2018) Deep learning for healthcare: review, opportunities and challenges. Brief Bioinfo 19(6): 1236-1246.
- Clark Jr LC, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann New York Acad Sci 102: 29-45.
- Mascini M, Tombelli S (2008) Biosensors for biomarkers in medical diagnostics. Biomark 13(7-8): 637-657.
- Nagel B, Dellweg H, Gierasch LM (1992) Glossary for chemists of terms used in biotechnology (IUPAC Recommendations 1992). Pure Appl Chem 64(1): 143-168.
- Turner APF, Karube I, Wilson GS (1987) Biosensors: fundamentals and applications. Oxford University Press.
- Kissinger PT (2005) Biosensors - a perspective. Biosens Bioelectron 20(12): 2512-2516.
- Salvo P, Dini V, Kirchhain A, Janowska A, Oranges T, et al. (2017) Sensors and biosensors for c-reactive protein, temperature and ph, and their applications for monitoring wound healing: a review. Sens 17(12): 2952.
- Zhenjian Z, Yuanyuan Y, Maolin W, Jie L, Zongkang Z, et al. (2017) Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int J Mol Sci 18(10): 2142.
- Guodong W, Jun L, Ke C, Yiling X, Bo L, et al. (2017) Selection and characterization of DNA aptamer against glucagon receptor by cell-SELEX. Sci Rep 7: 7179.
- Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Sci 249: 505-510.
- Tao W, Changying C, Leon ML, Roberto AB, Rakesh NV (2019) Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechn Adv 37(1): 28-50.
- Jun L, Huixia L, Kwame S, Bo L, Ying P, et al. (2012) Selection of Aptamers Specific for Adipose Tissue. PloS one 7: e37789.
- Prabodhika M, Zhiwen T, Sefah K, Ling M, Dihua S, et al. (2007) Aptamer Directly Evolved from Live Cells Recognizes Membrane Bound Immunoglobin Heavy Mu Chain in Burkitt’s Lymphoma Cells. Mol Cell Proteom 6: 2230-2238.
- Jürgen F, Tammo O, Ulf J, Michael B, Heinfried HR, et al. (1999) Novel Approach to Specific Growth Factor Inhibition in Vivo: Antagonism of Platelet-Derived Growth Factor in Glomerulonephritis by Aptamers. Am J Pathol 154: 169-179.
- Rebekah RW, Siqing S, Christopher PR, Geetha S, Mark WD, et al. (2003) Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci USA 100(9): 5028-5033.
- Ying P, Zhenxu L, Yi L, Peng Y, Jun L, et al.(2015) Using DNA aptamer probe for immunostaining of cancer frozen tissues. Analytical chemistry 87: 1919-1924.
- Zhenxu L, Yi L, Ying P, Jun L, Bo L, et al. (2015) Using aptamers to elucidate esophageal cancer clinical samples. Scientific reports 5: 18516.
- Ling SL, Fei W, Yonghe G, Pik KL (2021) Recent Developments in Aptasensors for Diagnostic Applications. ACS Appl Mater Interfaces 13(8): 9329-9358.
- Spurti UA, Charles JW (2018) Critical Review: DNA Aptasensors, Are They Ready for Monitoring Organic Pollutants in Natural and Treated Water Sources? Environ Sci Technol 52(16): 8989-9007.
- Strehlitz B, Nikolaus N, Stoltenburg R (2008) Protein detection with aptamer biosensors. Sens 8(7): 4296-4307.
- Poolsup S, Kim CY (2017) Therapeutic applications of synthetic nucleic acid aptamers. Curr Opin Biotechnol 48: 180-186.
- Liss M, Petersen B, Wolf H, Prohaska E (2002) An aptamer-based quartz crystal protein biosensor. Anal Chem 74(17): 4488-4495.
- Baker BR, Lai RY, Wood MS, Doctor EH, Heeger AJ, et al. (2006) An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J Am Chem Soc 128(10): 3138-3139.
- Qi Y, Ma J, Chen X, Xiu F-R, Chen Y, et al. (2020) Practical aptamer-based assay of heavy metal mercury ion in contaminated environmental samples: convenience and sensitivity. Anal Bioanal Chem 412(2): 439-448.
- Wang W, Chen C, Qian M, Zhao XS (2008) Aptamer biosensor for protein detection using gold nanoparticles. Anal Biochem 373(2): 213-219.
- Fraser LA, Kinghorn AB, Dirkzwager RM, Liang S, Cheung YW, et al. (2018) A portable microfluidic Aptamer-Tethered Enzyme Capture (APTEC) biosensor for malaria diagnosis. Bios Bioelectron 100: 591-596.
- Qin C, Wen W, Zhang X, Gu H, Wang S (2015) Visual detection of thrombin using a strip biosensor through aptamer-cleavage reaction with enzyme catalytic amplification. Analyst 140(22): 7710-7717.
- Liu G, Mao X, Phillips JA, Xu H, Tan W, et al. (2009) Aptamer−nanoparticle strip biosensor for sensitive detection of cancer cells. Anal Chem 81(24): 10013-10018.
- Surjit S, Nitish KK, Pradeep D, Jaykaran C, Rimplejeet K, et al. (2018) Monoclonal Antibodies: A Review. Curr Clin Pharmacol 13(2): 85-99.
- Marlies L, Coenraad FMH (2005) Critical steps in the production of polyclonal and monoclonal antibodies: evaluation and recommendations. ILAR J 46(3): 269-279.
- Anchal S, Ayush M, Anju V (2020) Antibodies: monoclonal and polyclonal. Models in Discovery and Translation. In: Animal Biotechn, pp. 327-352.
- Michael RK, Thomas DC, Brian KK (2012) Detection of biomarkers using recombinant antibodies coupled to nanostructured platforms. Nano Rev, p3.
- César M, Georges K (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495-497.
- Hugo M , Johannes FS, Markus JZ, Michelle K, Rene GO, et al. (2010) Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature 467: 591-595.
- Zidane Q, Yahia C, Hassan S, Zineb Qmichou (2021) Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection. Molecules 26(4): 1058.
- Shikha S, Hannah B, Richard JOK (2016) Antibodies and antibody-derived analytical biosensors. Essays in Biochemistry 60: 9-18.
- Schasfoort RBM, Bergveld P, Kooyman RPH, Greve J (1990) Possibilities and limitations of direct detection of protein charges by means of an immunological field-effect transistor. Anal Chim Acta 238: 323-329.
- Naresh V, Lee N (2021) Review on biosensors and recent development of nanostructured materials-enabled biosensors. Sens 21: 1109.
- Welch NG, Scoble JA, Muir BW, Pigram PJ (2017) Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerph 12: 02D301.
- Park M (2019) Orientation control of the molecular recognition layer for improved sensitivity: a review. BioChip J 13: 82-94.
- Morales MA, Halpern JM (2018) Guide to selecting a biorecognition element for biosensors. Bioconjug Chem 29(10): 3231-3239.
- Mehlhorn A, Rahimi P, Joseph Y (2018) Aptamer-based biosensors for antibiotic detection: a review. Biosens 8(2): 54.
- Gauglitz G (2017) Analytical evaluation of sensor measurements. Anal Bioanal Chem 410: 5-13.
- Aziz A, Asif M, Ashraf G, Farooq U, Yang Q, et al. (2021) Trends in biosensing platforms for SARS-Cov-2 detection: a critical appraisal against standard detection tools. Current Opinion Coll Interf Sci 52: 101418.
- Raparthi S, Ch Ratnam, Vana VR (2019) A Review on Fault Detection, Diagnosis and Prognosis, in Vibration Measurement through Wavelets on Machine Elements. Int J App Eng Res 14(2): 547-555.
- Hyonmin C, Noreen JH, Tiffany NM, Rocky ST (2015) Molecular Diagnostics. J Am Acad Orthop Surg 23(0): S26-S31.
- Choe H, Inaba Y, Kobayashi N (2014) Evaluation of the time period for which real-time polymerase chain reaction detects dead bacteria. Pol J Microbiol 63(4): 393-398.
- Cindy JS, Mark O (2009) Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol 67: 6-20.
- Leslie AP (2008) The Biotechnology Revolution: PCR and the Use of Reverse Transcriptase to Clone Expressed Genes. Nature Education 1(1): 94.
- Olivier V, Delphine M, Olivier R, Alex VB, Zisis K (2021) Considerations for diagnostic COVID-19 tests. Nature Rev Microbiol 19: 171-183
- Weissleder R, Lee H, Ko J, Pittet MJ (2020) COVID-19 diagnostics in context. Sci Transl Med 12: eabc1931.
- Berson SA, Yalow RS (1959) Assay of plasma insulin in human subjects by immunological methods. Nature 184(16): 48-49.
- Belanger L, Sylvestre C, DuFour D (1973) Enzyme-linked immunoassay for alpha-fetoprotein by competitive and sandwich procedures. Clin Chim Acta 48(1): 15-18.
- Ong DSY, Man SJ, Lindebom FA, Koeleman JGM (2020) Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clin Microbiol Inf 26(8): 1094.e7-1094.e10
- Elslande JV, Houben E, Depypere M, Brackenier A, Desmet S, et al. (2020) Diagnostic performance of 7 rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Inf 26: 1082-1087.
- Metkar SK, Girigoswami K (2019) Diagnostic biosensors in medicine-a review. Biocatal Agric Biotech 17: 271-283.
- Xu K, Zhou R, Takei K, Hong M (2019) Toward flexible surface-enhanced Raman Scattering (SERS) sensors for point-of-care diagnostics. Adv Sci 6: 1900925.
- Eguilaz MR, Cumba LR, Forster RJ (2020) Electrochemical detection of viruses and antibodies: a mini review. Electrochem Commun 116: 106762.
- Miesler T, Wimschneider C, Brem A, Meinel L (2020) Frugal innovation for point-of-care diagnostics controlling outbreaks and epidemics. ACS Biomater Sci Eng 6: 2709-2725.
- Ferreira DC, Batistuti MR, Junior BB, Mulato M (2021) Aptasensor based on screen-printed electrode for breast cancer detection in undiluted human serum. Bioelectrochem 137: 107586.
- Faria RAD, Douaud A, Soares RB, Heneine LGD, Matencio T, et al. (2020) Electrochemical Behavior of Screen-Printed Carbon Electrodes as Transducers in Biosensors. Corr 76(6): 553-561.
- Ranjan P, Parihar A, Jain S, Kumar N, Dhand C, et al. (2020) Biosensor-based diagnostic approaches for various cellular biomarkers of breast cancer: a comprehensive review. Anal Biochem 610: 113996.
- Andryukov BG, Besednova NN, Romashko RV, Zaporozhets TS, Efimov TA (2020) Biosens 10:11.
- Zhang F, Liu J (2021) Label-free colorimetric biosensors based on aptamers and gold nanoparticles: a critcial review. Anal Sens 1: 30-43.
- Tan Z, Dong H, Liu Q, Liu H, Zhao P, et al. (2019) A label-free immunosensor based on PtPd NCs@MoS2 nanoenzymes for hepatitis B surface antigen detection. Biosens Bioelectron 142: 111556.
- Kaushik A, Yndart A, Kumar S, Jayant RD, Vashist A, et al. (2018) A sensitive electrochemical immunosensor label-free detection of Zika-virus protein. Sci Rep 8: 9700.
- Yang Y, Liu Q, Liu Y, Cui J, Liu H, et al. (2017) A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped graphene quantum dots for carcinoembryonic antigen detection . Bios Bioelectron 90: 31-38.
- Guo M, Shu J, Du D, Haghighatbin MA, Yang D, et al. (2021) A label-free three potential ratiometric electrochemiluminescence immunosensor for cardiac troponin I based on N-(4-aminobutyl)-N-ethylisoluminol functionalized graphene quantum dots. Sens Actuators B Chem 334: 129628.
- Cohen N, Sabhachandani P, Golberg A, Konry T (2015) Approaching near real-time biosensing: microfluidic microsphere based biosensor for real-time analyte detection. Bios Bioelectron 66: 454-460.
- Uygun ZO, Şahin Ç, Yılmaz M, Akçay Y, Akdemir A, et al. (2018) Fullerene-PAMAM(G5) composite modified impedimetric biosensor to detect Fetuin-A in real blood samples. Anal Biochem 542: 11-15.
- Faria RAD, Houmard M, Rosário VAM, Lins VFC, Heneine LGD, et al. (2019) TiO2 so-gel coating as a transducer substrate for impedimetric immunosensors. Chem Biochem Eng Q 33(4): 437-447.
- Ni S, Shen Z, Zhang P, Liu G (2020) Enhanced performance of an electrochemical aptasensor for real-time detection of vascular endothelial growth factor (VEGF) by nanofabrication and ratiometric measurement. Anal Chim Acta 1121: 74-82.
- Soleimani S, Arkan E, Jalalvand AR, Goicoechea HC (2020) Fabrication of a novel electrochemical aptasensor assisted by a novel computerized monitoring system for real-time determination of the prostate specific antigen: A computerized experimental method brought elegancy. Microchem J 157:104898.
- Sanati A, Jalali M, Raeissi K, Karimzadeh F, Kharaziha M, et al. (2019) A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Microchim Acta 186: 773.
- Mishra GK, Sharma V, Mishra RK (2018) Electrochemical aptasensors for food and environmental safeguarding: a review. Biosens 8: 28
- Özgüra E, Uyanıkb HU, Şenelc S, Uzun L (2020) Immunoaffinity biosensor for neurofilament light chain detection and its use in Parkinson's diagnosis. Mat Sci Eng B 256: 114545
- Sumithra B, Jayanthi VSPKSA, Manne HC, Gunda R, Saxena U, et al. (2020) Antibody-based biosensor to detect oncogenic splicing factor Sam68 for the diagnosis of lung cancer. Biotechnol Lett 42: 2501-2509.
- Özcan B, Sezgintürk MK (2021) A novel and disposable GP- based impedimetric biosensor using electropolymerization process with PGA for highly sensitive determination of leptin: Early diagnosis of childhood obesity. Talanta 225: 121985.
- Uygun ZO, Yeniay L, Sağın FG (2020) CRISPR-dCas9 powered impedimetric biosensor for label-free detection of circulating tumor DNAs. Anal Chim Acta 1121: 35-41.
- Bussooa A, Hoare D, Kirimi MT, Mitra S, Mirzai N, et al. (2020) Impedimetric detection and electromediated apoptosis of vascular smooth muscle using microfabricated biosensors for diagnosis and therapeutic intervention in cardiovascular diseases. Adv Sci 7: 1902999.
- Himori S, Nishitani S, Sakata T (2021) Aptamer-based nanofilter interface for small-biomarker detection with potentiometric biosensor. Electrochim Acta 368: 137631.
- Bouri M, Zuaznabar-Gardona JC, Novell M, Blondeau P, Andrade FJ (2021) Paper-based potentiometric biosensor for monitoring galactose in whole blood. Electroanal 33(1): 81-89.
- Teengam P, Siangproh W, Tontsirin S, Jiraseree-amornkun A, Chuaypen N, et al. (2021) NFC-enabling smartphone-based portable amperometric immunosensor for hepatitis B virus detection. Sens Act B Chem 326: 128825.
- Zhang R, Liu L, Mao D, Luo D, Cao F, et al. (2020) Construction of electrochemical aptasensor of carcinoembryonic antigen based on toehold-aided DNA recycling signal amplification. Bioelectrochem 133: 107492.
- Cesewski E, Johnson BN (2020) Electrochemical biosensors for pathogen detection. Bios Bioelectron 159: 112214.
- Ehzari H, Amiri M, Safari M (2020) Enzyme-free sandwich-type electrochemical immunosensor for highly sensitive prostate specific antigen based on conjugation of quantum dots and antibody on surface of modified glassy carbon electrode with core-shell magnetic metal-organic frameworks. Talanta 210: 120641.
- Han R, Sun Y, Dai Y, Gao D, Wang X, et al. (2021) A chemiluminescence aptasensor for sensitive detection of carcinoembryonic antigen based on dual aptamer-conjugates biorecognition. Sens Act B Chem 326: 128833.
- Jing A, Xu Q, Feng W, Liang G (2020) An electrochemical immunosensor for sensitive detection of the tumor marker carcinoembryonic antigen (CEA) based on three-dimensional porous nanoplatinum/graphene. Micromach 11: 660.
- Ren L, Xu P, Zhang P, Qin Z, Zhang Y, et al. (2021) Label-free fluorescence aptasensor based on AuNPs and CQDs for the detection of ATP. AIP Adv 11(1): 015316.
- Pavase TR, Lin H, Soomro MA, Zheng H, Li X, et al. (2021) Visual detection of tropomyosin, a major shrimp allergenic protein using gold nanoparticles (AuNPs)-assisted colorimetric aptasensor. Mar Life Sci Tech 3: 382-394.
- Azam NFN, Mohd-Naim NF, Kurup CP, Ahmed MU (2020) Electrochemiluminescence immunosensor for tropomyosin using carbon nanohorns/Nafion/Fe3O4@Pd screen-printed electrodes. Microchim Acta 187: 456.
- Fathi S, Saber R, Adabi M, Rasouli R, Douraghi M, et al. (2021) Novel competitive voltammetric aptasensor based on electrospun carbon nanofibers-gold nanoparticles modified graphite electrode for Salmonella enterica serovar detection. Bioint Res App Chem 11(1): 8702-8715.
- Tertis M, Leva PI, Bogdan D, Suciu M, Graur F, et al. (2019) Impedimetric aptasensor for the label-free and selective detection of Interleukin-6 for colorectal cancer screening. Bios Bioelectron 137: 123-132.
- Atar N, Yola ML (2021) A novel QCM immunosensor development based on gold nanoparticles functionalized sulfur-doped graphene quantum dot and h-ZnS-CdS NC for Interleukin-6 detection . Anal Chim Acta 1148: 338202.
- Mazzaracchio V, Neagu D, Porchetta A, Marcoccio E, Pomponi A, et al. (2019) A label-free impedimetric aptasensor for the detection of Bacillus anthracis spore simulant. Bios Bioelectron 126: 640-646.
- Tan Z, Dong H, Liu Q, Liu H, Zhao P, et al. (2019) A label-free immunosensor based on PtPd NCs@MoS2 nanoenzymes for hepatitis B surface antigen detection. Bios Bioelectron 142: 111556.
- Parate K, Rangnekar SV, Jing D, Mendivelso-Perez DL, Ding S, et al. (2020) Aerosol-jet-printed graphene immunosensor for label-free Cytokine Monitoring in Serum. ACS Appl Mater Interf 12: 8592-8603.
- Pakchin PS, Fathi M, Ghanbari H, Saber R, Omidi Y (2020) A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Bios Bioelectron 153: 112029.

 Mini Review
Mini Review