Abstract
Recently, the Persulfate (PS) based oxidation process (AOP) has received wide attention due to its excellent performance. However, PS activation as the crucial point for AOP has become a hot research topic in recent years. Due to environmentally friendly characteristics (wide sources and low economic expenditure), biomass carbon (BC) and its composites have gradually attracted significant attention in activating PS. Therefore, the present work systematically summarizes the recent research progress of BC and its composite material used for PS activation. The current preparation technologies of BC and its composite materials are demonstrated, with a focus on their application in the practical treatment of organic wastewater.
Keywords: Biomass Carbon; Composites; Persulfate; Advanced Oxidation; Degradation
Abbreviations: PS: Persulfate; BC: Biomass Carbon; AOP: Advanced Oxidation Process; PDS: Perdisulate; NZVI: Nano-Scale Zero-Valent Iron; PFR: Persistent Radicals; FMO: Frontier Molecular Orbital Theory; DD: Double Descriptor Method; ZVI: Zero-Valent Iron; SMX: Sulfonymethoxazole
Introduction
Nowadays, the continuous growth of the world population, the ever-increasing depletion of fossil fuel reserves [1], and the rapid pace of urbanization and mechanization, have led to the wanton discharge of a significant number of toxic organic and inorganic pollutants, thus, such urgent issue should be reasonably resolved, otherwise it will do great harm to us [2]. How to better deal with the wastewater containing refractory organic pollutants has aroused wide concern. Advanced Oxidation Process (AOP) [3-5] is a new technology for water treatment to effective removal of organic pollutants [6]. In general, many other organic pollutants that can be treated by this method will eventually be mineralized into small inorganic molecules, like CO2 and H2O, thus eventually achieving complete removal. So far, AOPs has developed into a method for fundamentally degrading pollutants [7-9]. In this method, the persulphate (PS) oxidation technology is widely used considering its excellent degradation performance, a more obvious oxidation reduction potential (2.6 V-3 V) and a broader pH processing range (4-9) [10]. Therefore, the persulfate-based advanced oxidation process in the future wastewater treatment has very broad application prospect [11].
In most cases, the major oxidant species in persulfate systems are the sulfate radical(•SO4-) and the hydroxyl radical (•OH). Persulphates can be divided into two categories, namely, persulfate (PMS) and perdisulate (PDS). However, due to the unique structure, the O-O bond is difficult to break to form (•SO4-), so it needs to undergo a certain process to activate the persulfate. The current activation methods mainly include thermal activity method, alkali activation, transition metal ion activation, and ultraviolet activation [12]. However, the thermal activity method usually requires a high energy consumption, and the alkali activation and the transition metal ions activation often arise new problems in the cost and subsequent processing period. It is reported that carbon materials can effectively achieve activation of PS, but the cost is too expensive. Fortunately, BC have more advantages over other carbon materials [13], such as extensive source and lower cost. And recent works has reported that biomass carbon made from 300-500℃ calcination has good results on PS activation. The annual biomass waste production from various sources is very huge. As a more environmentally friendly carbon material, biomass carbon shows considerable potential for PS activation and much effort has been devoted to it. Other articles also summarize the application of biomass as a catalyst in some advanced oxidation processes of persulfate activation [14]. However, the effect of biomass catalyst in the advanced oxidation of sulfate radical remains to be systematically arranged. Hence, in this mini review, the recent mechanisms and application of biocarbon materials in activated persulfate are summarized and new insights into the future applications in activated PS are simultaneously provided. The present work also provides a direction for the better use of BC in the future.
Modification Method of BCs
BC possesses strong adsorption ability due to its high surface area and has a fairly high degradation rate owing to the presence of high porosity and large oxygen-containing functional groups [15]. Despite of the good performance, the annual utilization rate of BC is less than 10% in China. Its catalytic properties can be improved by activation and doping, which can increase the specific surface area and create mesoporous structure, thereby increasing active sites and oxygen functional groups, promoting π-electron transfer, and also regulating the electron density in biochar [16]. Transitional metal ion activation of PS has proved to be an effective method, but the use of this method is abandoned because it is difficult to recover and has the risk of secondary pollution towards the environment. However, the load of transition metal ions with BC has successfully solved this problem. Da Ouyang, et al. [17]. pyrolysis of pine needles under oxygen-limited conditions, obtaining BC, at different temperatures and dispersed in 50 ml 3.0 mM NH3·H2O while adding 20 mL ultrapure water dissolved 1.39 g FeSO4·7H2O and 1.01 g Fe(NO3)3·9H2O. After 45Hz 100W treatment 1h, the solution was separated, and the obtained biomass carbon was washed with ultrapure water until the washing liquid was neutral. The degradation experiment of PS on 1,4-dioxane was also conducted. Its highest degradation efficiency of 1,4-dioxane was 98.6%. Yan et al. [18]. pyrolyzed rice husk at 350℃ for 6 hours under the condition of limited oxygen, and dispersed it into 0.054 mol/L FeSO4·7H2O solution with pH=5, 250 ml. NZVI/BC loaded with nano-scale zero-valent iron (NZVI) was prepared by dripping 250 ml of 0.108 mol l-1 NaBH4 solution, and then PS was applied to trichloroethylene. The data showed that when the mass ratio of NZVI/BC was 1:5, the degradation rate was 99.4%.
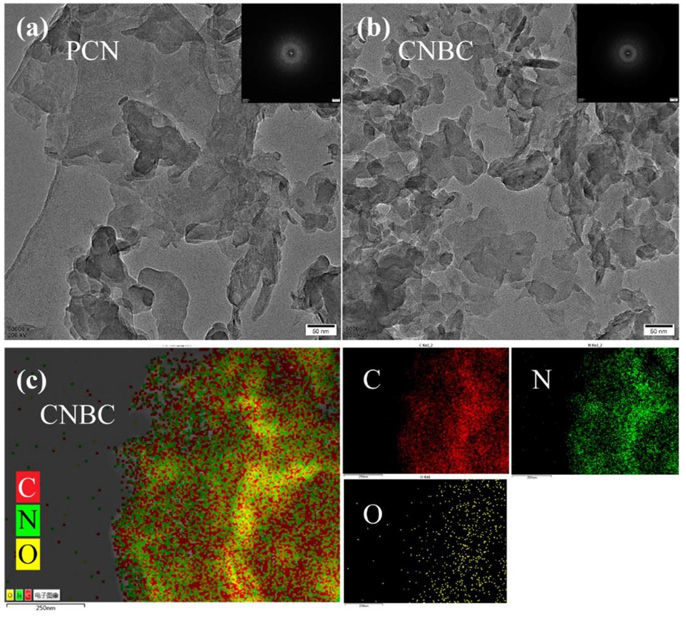
In addition to doping the transition metal ions, the physicochemical properties of biochar can be enhanced by acid or alkali treatment [19]. Acid activation method refers to the modification by soaking the biomass in or dispersed in a certain concentration of acid solution [20,21], including phosphoric acid, nitric acid, etc. Currently, phosphate acid activated biomass carbon technology has been widely use. The alkaline modification is performed by soaking or suspending the BC at different alkaline concentrations from room temperature to 100℃. The soaking and mixing time may last several hours or days (mostly 6-24 hours), depending on the raw materials used for modification, and the most commonly used bases are potassium hydroxide, sodium hydroxide, etc. But after the activation, it also costs a lot of consumables for washing. Atomic doping [22-24] is also a common method to modify the catalytic capacity of biomass carbon. For example, Wang, et al. [25], grinded melamine to the corn cob at various mass ratios, then poured the mixed precursor into the lid crucible, heated at 3℃ per min in the Marvel furnace to 550℃ and held for 4 hours. The obtained species were named CNBC1, CNBC2, CNBC3, CNBC4 and CNBC5, according to the mass ratio of corncore to melamine to activate PMS to degrade bisphenol A. The results showed that the efficiency of activated PMS processing BPA varied according to the mass ratio of BC and melamine, and it exhibited a BPA removal efficiency of 97.23% with mass ration of 1%. It should be noted that the removal performance, can be further enhanced with the suage of light. Figure 1a and Figure 1b are the contrast TEM diagrams before and after the doping modification, wherein it can be observed that both are sheet structures, meanwhile a smaller and looser distributed layered structure can be also observed in modified samples. Figure 1c is an EDS spectrum of CNBC, showing that CNBC contains large amounts of C, N and a small amount of O. LI, et al. [15]. successfully doped N in urea and Fe in iron salt into the BC by water thermal carbonization (HTC). The results showed that the best removal rate reached 98.2%.
Figure 1: TEM images of
a) (a-b) PCN
b) (c) the EDS diagram of the CNBC. Reprinted with permission from [25], Elsevier.
PSM Principle of Activation of BC
Activate PS Principle
The major element composition of biomass carbon is C, followed by H and O. Normally, biocarbon has 45% ~ 65% C, H 1%~5% and O 10%~40% [26]. After the high temperature carbonization, H gradually emerged, which caused the content of C to rise, and eventually led to the increased aromaticity of BCs. The excellent performance of biomass carbon materials is closely associated with its rich surface functional groups [27]. The main surface functional groups are carboxyl, phenolic hydroxyl, hydroxyl, group, lactone, carboxylic anhydride and cyclic peroxide group, respectively [12,26]. Among them, the BC has good redox properties and surface charges, which all originated from the carboxyl groups and phenolic hydroxygroups on its surface [28]. The presence of carboxyl, hydroxyl groups and can absorb organic pollutants [29]. This results in that the pollutants can also be firmly adsorbed to the BCs by forming hydrogen bonds to the surface polar functional groups. So, how does the biochar activate the PS? After the reaction of biochar, the ratio of -OH and -COOH decreased and -OH and -COOH can act as active sites in PS activation systems, where defect formation and increased graphitization generally strengthened the capacity of electron transfer [30]. However, there is no established theory, but four theoretical views exist: (1) its surface oxygen functional group can activate PS via defects caused by pyrolysis of ROS [15, 31]; (2) The defects and graphitized structure of biochar produced by pyrolysis can activate PS [32-34];(3) biocarbon can provide electrons and transfer electrons to PS to activate persistent radicals (PFR) in PS [35-37];(4) biochar may activate PS [38, 39].
Principles of Catalytic Degradation
Commonly, the present catalytic mechanism is divided into two parts: free radical pathway and non-radical pathway [40]. The O-O bond in PS activated by BC breaks to form (•SO4-), and the generated (•SO4-) will further react with H2O to generate (•OH) and SO42-, which then mineralize and degrade the pollutants. The non-radical degradation pathway achieves mainly through electron transfer, and the generation of 1O2 [41]. During non-radical oxidation, it occurs mainly on the catalyst surface. Due to the low oxidation performance of non-free radicals, the non-free radicals able to oxidize the pollutants in the early stage of the reaction, with the reaction progressing forward, the free radicals made the pollutants oxidized into completely mineralized into CO2 and H2O. However, in most cases, the whole reaction process is collaboration the result of the above two pathways [42,43].
Application of BCs in Activating PS
The Activation of PS by Original BC
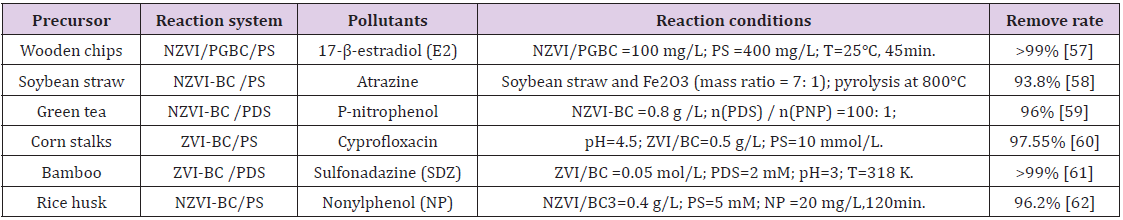
Herein, we mainly introduce the activation effect of BC with plants and sludge as the precursor on PS, and recent research progress on the performance of different catalysts is summarized in Table 1 below.
Table 1: Application of the primitive biocarbon catalyst in activating the PS for contaminants removal.
The Activation of PS by BC Doped with Transition Metal Ion
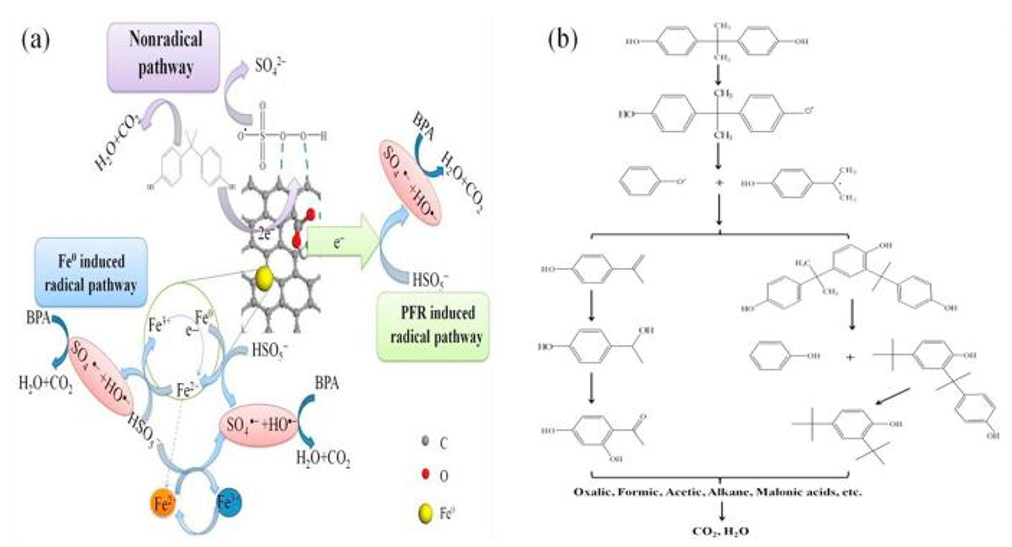
The Activation of PS by BC Doped with Iron Salt /ZVI: It has been reported that BC fail to reuse and possess poor stability, and therefore the degradation efficiency gradually decreases with continuous utilization. In order to solve such issues, doping transition metal ions has been adopted to activate PS of the degradation of contaminants. Among them, iron ions have become the research hotspot to activate PS on BC due to their non-toxic and low cost [51]. Jiang et al. [52]. chose sawdust as the precursor in a flask of the FeCl36H2O solution and then oscillated the mixture at 200 rpm for 300 minutes in a thermostatic oscillator. Subsequently, the water in the mixture was removed by evaporation, and the obtained residue was dried overnight at 80℃. BC-Fe was made and used to activate PS for the degradation of BPA. The results confirmed that Fe-BC-700 could completely remove 20 mg/L BPA in 5 minutes with 0.2 g/L PMS and 0.15 g/L catalysts within 5 minutes. Its activation and degradation mechanism is shown in Figure 2: (1) Fe0 can activate PMS to generate (•SO4-) and Fe2+, and the generated Fe2+ further catalyzes PMS to generate (•SO4-) (2) Fe3+ can react with PMS to generate (•SO5-) and Fe2+ (3) As a relatively weak transient substance, (•SO5-) can further self-react to form ps.
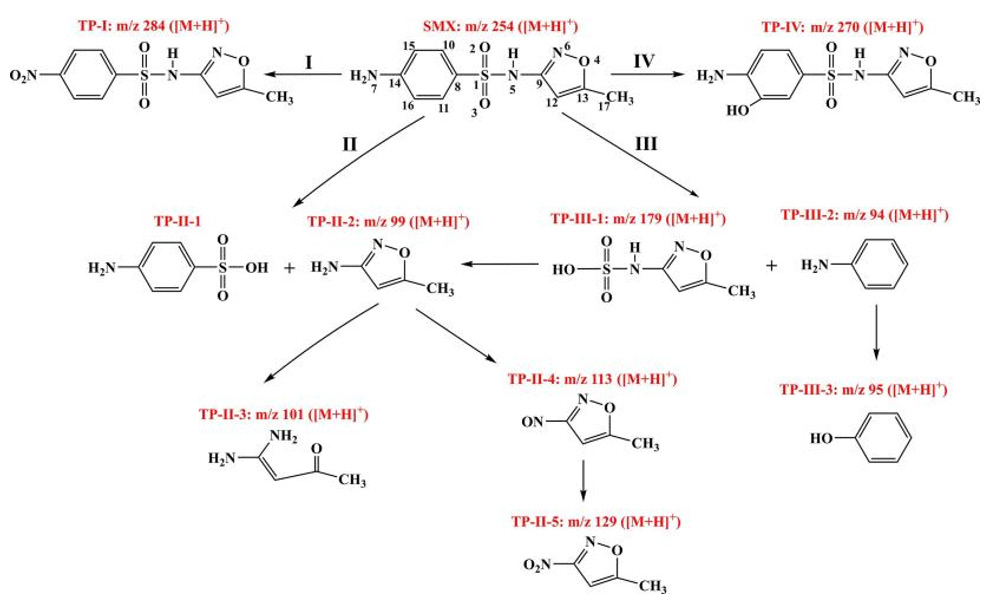
Similarly, Wang, et al. [53]. used wheat straw as the precursor to prepare BC under oxygen restriction conditions at 500℃, with the addition of BC, PS, trivalent iron to sulfamethoxazole. The study combined Frontier Molecular Orbital Theory (FMO) theory and Double descriptor method (DD) method to give four possible degradation mechanisms as shown in Figure 3. The research results showed that when BC=0.75g/L, PS=0.75g/L, T=25℃, pH=5, the removal rate of sulfamethoxazole by BC-Fe activated PS was 93.3%. However, several works found that Fe2+ was very easily leaching in the BC/PS process, generating a new pollutant for the water environment. Therefore, the stability of BC doped with transition metal ions still needs to be highly improved. Recently, zero-valent iron (ZVI) has received increasing attention in activated PS studies with high reaction performance, due to its high activation efficiency and multi-phase catalytic function [54,55]. ZVI can be activated by acidification, oxidation to Fe2+, by generating (•SO4-) or by direct electron transfer with PS. However, due to their high surface energy and magnetic interaction, ZVI particles tend to aggregate into micron class particles, leading to reduced reactivity [56]. Therefore, in recent years, research on how to load ZVI to BC and apply it to activate PS for the treatment of organic sewage has attracted significant attentions. Table 2 shows the recent application of ZVI/BC in wastewater treatment in PS.
Figure 2: (a) Fe-BC-700 activates the proposed mechanism for the PMS. (b) Potential degradation pathway of BPA in the Fe- BC-700 / PMS system. Reprinted with permission from[52], Elsevier.
Figure 3: The possible degradation pathway of SMX by BC/Fe (III) / PS. Reprinted with permission from[53], Elsevier.
Other Doping of Transition Metal Ions: Apart from applications of metal salt and ZVI, doping applications have also been reported for other metal materials. For example, Co2+ has been widely used to activate PS, but problems such as toxicity pose potential risks to the environment. Recent studies have shown that the leaching rate of Co is low and thus has become a hot spot on the catalysis. Yang et al. [63]. successfully synthesized Co-BC by loading Co to BC through a pyrolysis process, and applied it for the activation of PS for the degradations of amaranth and acetaminophen. The results showed that the degradation efficiency could reach 90% when CoIB= 50 mg/L, PMS = 400 mg/L, ACE = 5 mg/L, pH=6. Chen, et al. [64]. prepared the BC, which showed that the total removal rate of 5 heavy metals and 15 antibiotics was 57.4% and 42.3% in the presence of Cu, respectively. Although the transition metal ions perform well in the catalytic process, there is still a risk of leaching, and how to solve this risk completely remains to be studied in the near future. To sum up, in the activation of biocarbon material doped with transition metal ions to PS, as well as in the redox potential applied by transition metal ions on PS, the functional groups on the surface of biocarbon materials also play a pivotal role in transmitting electrons [12]. Therefore, in the subsequent work, we should clarify the role and path of BC in the transition metal ion activation process of PS, so that we can properly regulate the appearance and nature of BC to promote the degradation efficiency.
The Doping of Miscellaneous Atoms
Atomic doping can avoid the risk of leaching problems, and at the same time has an excellent ability to transport electrons. The majority of reported works are related to S, N doping. PMS degradation of BPA was activated using wood chips doped with N, as a precursor by Xu, et al. [65]. The results showed that under conditions of BPA = 10 mg/L, PMS = 2.0 mM, N-BC = 0.5 g/L, pH = 6.28 and T = 25℃, BPA was almost completely removed after 5min reaction. In another similar work, Xie, et al. [42]. prepared N-BC using molten salt-induced nitrogen-doped biomass nanosheets and studied the activation of PMS degradation of BPA. The data demonstrated that when BPA = 20 mg/L, PMS = 0.4 g/L, N-BC = 0.4 g/L, pH = 7 and T = 25℃, almost all BPA can be removed after 6 min reaction. An unbalanced electron density can be formed in the BC matrix by doping N, which provides a large number of transferable π electrons to the reaction, thus improving the catalytic efficiency of PMS activation for the degradation of organic matter [66]. In general, we argue that the graphite phase transition of carbon and nitrogen plays an important role in improving the reaction activity [67]. There are three forms of valence bonds for N in BC: pyridine N, pyrroN and graphite N, respectively. However, there is a big controversy about which kind of valence bond plays the major role, and this remains to be resolved in the future. Apart from N doping, other atomic doping was also applied to activate PS, but less reported information is available compared to N doping [68]. Xu, et al. [69]. successfully synthesized N, S-BC by one-step pyrolysis by using nanocellulose and thiourea to activate PMS for the degradation of sulfonymethoxazole (SMX) with a maximum degradation rate of 97.84%.
Application of Acid / Alkali Activated BC to Activate PS
Acid/alkali activation not only enables BC to display a negative and positive charge, respectively, but also simultaneously improves the hydrophilic and hydrophobic properties of BC. Acid activation can change the content of oxygen-containing functional groups on the BC surface. For instance, Li, et al. [70]. activated BC by hydrofluoric acid, increasing the carboxyl and phenol hydroxyl content by 23% and 50%, respectively. Unlike acid activation, alkali activation can result in a greater area and more porous structure of BC, because alkaline substances such as KOH and NaOH would etch the surface of carbon at high temperature, and then make the pore structure richly. This not only facilitates the transfer of electrons but also improves the pore volume of the BC and promotes the adsorption of pollutants on the BC. In the future, research can reasonably treat acid or alkali according to the mechanism of PS activation by BC, and then endow it ideal properties and structure to facilitate practical application.
Conclusion
In summary, this paper systematically outlines the application of various BCs in activation of persulfate, and expounds their synthesis process and physic chemical properties. In order to improve the degradation performance on organic pollutants, several modified methods, such as doping the transition metal ions and ZVI, acid and alkali activation, heteroatomic doping of the biomass, are adopted according to the actual needs. Furthermore, the action mechanism of BC in the PMS activation process and the AOP action mechanism of BC-PMS are subjected to in-depth investigation. The presence of oxygen-containing functional groups on the surface of BC, especially C=O, will activate PS. In addition, the collapse of biocarbon in pyrolysis and the improvement of graphitization also accounts for the activation of PS. Activated PS mineralized pollutants mainly through radical and non-radical reactions. Free radical reaction is mainly through the reaction of (•SO4-) and water to generate (•OH) to mineralize organic pollutants. While this reaction occurs, nonradical reactions are also ongoing. Non-radicals occur mainly on the BC surface by participating in the reaction through electron transfer and producing monolar oxygen. Although breakthrough progress has been made in activating PS to treat pollutants in the water medium, there are still some challenges faced with BC in the application of AOP. For example, how to use this advantage to treat pollutants in other systems such as soil, and how to prevent the leaching of transition metal ions. Moreover, the mechanism of electronic shuttle in PS activation by BC is still uncertain, which requires subsequently continuous research effort to address.
Acknowledgment
The work was supported by the National Natural Science Foundation of China (U1803114), Key Scientific and Technological Project of Henan Province (212102311069), Distinguished Foreign Scientist Workshop on Coal Green Conversion of Henan Province (GZS2020012), Henan Province Key Scientific Research Project Plan for Colleges and Universities (20A530003), Fundamental Research Funds for the Universities of Henan Province (NSFRF200308), the Research Fund of Henan Key Laboratory of Coal Green Conversion (CGCF201903) and the Doctoral Program of Henan Polytechnic University (B2019-46).
References
- Jiang F, Li T, Li Y, Zhang Y, Gong A, et al. (2018) Wood-Based Nanotechnologies toward Sustainability. Adv Mater 30(1): 1703453.
- Zhao Y, Yuan X, Jiang L, Li X, Zhang J, et al. (2020) Reutilization of cathode material from spent batteries as a heterogeneous catalyst to remove antibiotics in wastewater via peroxymonosulfate activation. Chemical Engineering Journal 400: 125903.
- Du J, Zhang B, Li J, Lai B (2020) Decontamination of heavy metal complexes by advanced oxidation processes: A review. Chinese Chemical Letters 31(10): 2575-2582.
- Serrano-Martínez A, Mercader-Ros MT, Martínez-Alcalá I, Lucas-Abellán C, Gabaldón JA, et al. (2020) Degradation and toxicity evaluation of azo dye Direct red 83:1 by an advanced oxidation process driven by pulsed light. Journal of Water Process Engineering 37: 101530.
- Horne GP, Zalupski PR, Daubaras DL, Rae C, Mezyk SP, et al. (2020) Radiolytic degradation of formic acid and formate in aqueous solution: modeling the final stages of organic mineralization under advanced oxidation process conditions. Water Res 186: 116314.
- Jiang L, Yuan X, Zeng G, Wu Z, Liang J, et al. (2018) Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant. Applied Catalysis B: Environmental 221: 715-725.
- Zhang J, Yuan X, Jiang L, Wu Z, Chen X, et al. (2018) Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J Colloid Interface Sci 511: 296-306.
- Jiang L, Yuan X, Zeng G, Liang J, Wu Z, et al. (2019) Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J Colloid Interface Sci 536: 17-29.
- Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, et al. (2018) Evaluation of advanced oxidation processes for water and wastewater treatment - A critical review. Water Res 139: 118-131.
- Zhao Y, Yuan X, Li X, Jiang L, Wang H (2021) Burgeoning prospects of biochar and its composite in persulfate-advanced oxidation process. J Hazard Mater 409: 124893.
- Yuan X, Jiang L, Liang J, Pan Y, Zhang J, et al. (2019) In-situ synthesis of 3D microsphere-like In2S3/InVO4 heterojunction with efficient photocatalytic activity for tetracycline degradation under visible light irradiation. Chemical Engineering Journal 356: 371-381.
- Wang J, Wang S (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chemical Engineering Journal 334: 1502-1517.
- Huang Q, Song S, Chen Z, Hu B, Chen J, et al. (2019) Biochar-based materials and their applications in removal of organic contaminants from wastewater: state-of-the-art review. Biochar 1(1): 45-73.
- Pan X, Gu Z, Chen W, Li Q (2021) Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci Total Environ 754: 142104.
- Li Z, Sun Y, Yang Y, Han Y, Wang T, et al. (2020) Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J Hazard Mater 383: 121240.
- Ruan X, Sun Y, Du W, Tang Y, Liu Q, et al. (2019) Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review. Bioresour Technol 281: 457-468.
- Ouyang D, Yan J, Qian L, Chen Y, Han L, et al. (2017) Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 184: 609-617.
- Yan J, Han L, Gao W, Xue S, Chen M (2015) Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour Technol 175: 269-274.
- Ahmed MB, Zhou JL, Ngo HH, Guo W, Chen M (2016) Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour Technol 214: 836-851.
- Zhou Y, Gao B, Zimmerman AR, Fang J, Sun Y, et al. (2013) Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chemical Engineering Journal 231: 512-518.
- Zhang M-m, Liu Y-g, Li T-t, Xu W-h, Zheng B-h, et al. (2015) Chitosan modification of magnetic biochar produced from Eichhornia crassipes for enhanced sorption of Cr(vi) from aqueous solution. RSC Advances 5(58): 46955-46964.
- Xue W, Zhou Q, Cui X, Jia S, Zhang J, et al. (2021) Metal–organic frameworks-derived heteroatom-doped carbon electrocatalysts for oxygen reduction reaction. Nano Energy 86: 106073.
- Li B, Yun S, Xing T, Wang K, Ke T, et al. (2021) A strategy for understanding the enhanced anaerobic co-digestion via dual-heteroatom doped bio-based carbon and its functional groups. Chemical Engineering Journal 425: 130473.
- Lee SJ, Theerthagiri J, Nithyadharseni P, Arunachalam P, Balaji D, et al. (2021) Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renewable and Sustainable Energy Reviews 143: 110849.
- Wang H, Guo W, Si Q, Liu B, Zhao Q, et al. (2021) Multipath elimination of bisphenol A over bifunctional polymeric carbon nitride/biochar hybrids in the presence of persulfate and visible light. J Hazard Mater 417: 126008.
- Sajjadi B, Chen W-Y, Egiebor NO (2019) A comprehensive review on physical activation of biochar for energy and environmental applications. Reviews in Chemical Engineering 35(6): 735-776.
- Qian K, Kumar A, Zhang H, Bellmer D, Huhnke R (2015) Recent advances in utilization of biochar. Renewable and Sustainable Energy Reviews 42: 1055-1064.
- Yuan Y, Bolan N, Prevoteau A, Vithanage M, Biswas JK, et al. (2017) Applications of biochar in redox-mediated reactions. Bioresour Technol 246: 271-281.
- Faheem, Du J, Kim SH, Hassan MA, Irshad S, et al. (2020) Application of biochar in advanced oxidation processes: supportive, adsorptive, and catalytic role. Environ Sci Pollut Res Int 27(30): 37286-37312.
- Zhou X, Zhu Y, Niu Q, Zeng G, Lai C, et al. (2021) New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes. Chemical Engineering Journal 416: 129027.
- Liu XQ, Ding HS, Wang YY, Liu WJ, Jiang H (2016) Pyrolytic Temperature Dependent and Ash Catalyzed Formation of Sludge Char with Ultra-High Adsorption to 1-Naphthol. Environ Sci Technol 50(5): 2602-2609.
- Huang D, Zhang Q, Zhang C, Wang R, Deng R, et al. (2020) Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline. Chemical Engineering Journal 391: 123532.
- Yu J, Tang L, Pang Y, Zeng G, Wang J, et al. (2019) Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chemical Engineering Journal 364: 146-159.
- Zhu S, Huang X, Ma F, Wang L, Duan X, et al. (2018) Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ Sci Technol 52(15): 8649-8658.
- Jiang Z, Li J, Jiang D, Gao Y, Chen Y, et al. (2020) Removal of atrazine by biochar-supported zero-valent iron catalyzed persulfate oxidation: Reactivity, radical production and transformation pathway. Environ Res 184: 109260.
- Guo L, Zhao J, Zhao L, Tang Y, Zhou J, et al. (2021) Persulfate activation by Cr2O3/BC derived from chrome shavings for antibiotics degradation. Chemical Engineering Journal 420: 127698.
- Zhu F, Wu Y, Liang Y, Li H, Liang W (2020) Degradation mechanism of norfloxacin in water using persulfate activated by BC@nZVI/Ni. Chemical Engineering Journal 389: 124276.
- Fang G, Gao J, Liu C, Dionysiou DD, Wang Y, et al. (2014) Key role of persistent free radicals in hydrogen peroxide activation by biochar: implications to organic contaminant degradation. Environ Sci Technol 48(3): 1902-1910.
- Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49(9): 5645-5653.
- Chen X, Oh W-D, Lim T-T (2018) Graphene- and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chemical Engineering Journal 354: 941-976.
- Zhang X, Feng M, Qu R, Liu H, Wang L, et al. (2016) Catalytic degradation of diethyl phthalate in aqueous solution by persulfate activated with nano-scaled magnetic CuFe2O4 /MWCNTs. Chemical Engineering Journal 301: 1-11.
- Xie Y, Hu W, Wang X, Tong W, Li P, et al. (2020) Molten salt induced nitrogen-doped biochar nanosheets as highly efficient peroxymonosulfate catalyst for organic pollutant degradation. Environ Pollut 260: 114053.
- Liu C, Chen L, Ding D, Cai T (2019) From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Applied Catalysis B: Environmental 254: 312-320.
- Ouyang D, Chen Y, Yan J, Qian L, Han L, et al. (2019) Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures. Chemical Engineering Journal 370: 614-624.
- He J, Xiao Y, Tang J, Chen H, Sun H (2019) Persulfate activation with sawdust biochar in aqueous solution by enhanced electron donor-transfer effect. Sci Total Environ 690: 768-777.
- Liu J, Jiang S, Chen D, Dai G, Wei D, et al. (2020) Activation of persulfate with biochar for degradation of bisphenol A in soil, Chemical Engineering Journal 381: 122637.
- Kemmou L, Frontistis Z, Vakros J, Manariotis ID, Mantzavinos D (2018) Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catalysis Today 313: 128-133.
- Huong PT, Jitae K, Al Tahtamouni TM, Le Minh Tri N, Kim H-H, et al. (2020) Novel activation of peroxymonosulfate by biochar derived from rice husk toward oxidation of organic contaminants in wastewater. Journal of Water Process Engineering 33: 101037.
- Chen Y-d, Duan X, Zhang C, Wang S, Ren N-q, et al. (2020) Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chemical Engineering Journal 384: 123244.
- Diao ZH, Dong FX, Yan L, Chen ZL, Qian W, et al. (2020) Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: Reactivity and mechanism. J Hazard Mater 384: 121385.
- Diao Z-H, Zhang W-X, Liang J-Y, Huang S-T, Dong F-X, et al. (2021) Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: Synergistic effect and mechanism. Chemical Engineering Journal 409: 127684.
- Jiang S-F, Ling L-L, Chen W-J, Liu W-J, Li D-C, et al. (2019) High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors. Chemical Engineering Journal 359: 572-583.
- Wang H, Guo W, Yin R, Du J, Wu Q, et al. (2019) Biochar-induced Fe(III) reduction for persulfate activation in sulfamethoxazole degradation: Insight into the electron transfer, radical oxidation and degradation pathways. Chemical Engineering Journal 362: 561-569.
- Diao ZH, Qian W, Lei ZX, Kong LJ, Du JJ, et al. (2019) Insights on the nitrate reduction and norfloxacin oxidation over a novel nanoscale zero valent iron particle: Reactivity, products, and mechanism. Sci Total Environ 660: 541-549.
- Zhu F, He S, Liu T (2018) Effect of pH, temperature and co-existing anions on the Removal of Cr(VI) in groundwater by green synthesized nZVI/Ni. Ecotoxicol Environ Saf 163: 544-550.
- Phenrat T, Saleh N, Sirk K, Tilton RD, Lowry GV (2007) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environmental Science & Technology 41(1): 284-290.
- Ding J, Xu W, Liu S, Liu Y, Tan X, et al. (2021) Activation of persulfate by nanoscale zero-valent iron loaded porous graphitized biochar for the removal of 17beta-estradiol: Synthesis, performance and mechanism. J Colloid Interface Sci 588: 776-786.
- Zhang Y, Jiang Q, Jiang S, Li H, Zhang R, et al. (2021) One-step synthesis of biochar supported nZVI composites for highly efficient activating persulfate to oxidatively degrade atrazine. Chemical Engineering Journal 420: 129868.
- Wang B, Zhu C, Ai D, Fan Z (2021) Activation of persulfate by green nano-zero-valent iron-loaded biochar for the removal of p-nitrophenol: Performance, mechanism and variables effects. J Hazard Mater 417: 126106.
- Du Y, Dai M, Naz I, Hao X, Wei X, et al. (2021) Carbothermal reduction synthesis of zero-valent iron and its application as a persulfate activator for ciprofloxacin degradation. Separation and Purification Technology 275: 119201.
- Ma D, Yang Y, Liu B, Xie G, Chen C, et al. (2021) Zero-valent iron and biochar composite with high specific surface area via K2FeO4 fabrication enhances sulfadiazine removal by persulfate activation. Chemical Engineering Journal 408: 127992.
- Hussain I, Li M, Zhang Y, Li Y, Huang S, et al. (2017) Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chemical Engineering Journal 311: 163-172.
- Yang MT, Du Y, Tong WC, Yip ACK, Lin KA (2019) Cobalt-impregnated biochar produced from CO2-mediated pyrolysis of Co/lignin as an enhanced catalyst for activating peroxymonosulfate to degrade acetaminophen. Chemosphere 226: 924-933.
- Chen J, Yu X, Li C, Tang X, Sun Y (2020) Removal of tetracycline via the synergistic effect of biochar adsorption and enhanced activation of persulfate. Chemical Engineering Journal 382: 122916.
- Xu L, Wu C, Liu P, Bai X, Du X, et al. (2020) Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chemical Engineering Journal 387: 124065.
- Huang L, Zeng T, Xu X, He Z, Chen J, et al. (2019) Immobilized hybrids between nitrogen-doped carbon and stainless steel derived Fe3O4 used as a heterogeneous activator of persulfate during the treatment of aqueous carbamazepine. Chemical Engineering Journal 372: 862-872.
- Duan X, Sun H, Wang S (2018) Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc Chem Res 51(3): 678-687.
- Huang W, Xiao S, Zhong H, Yan M, Yang X (2021) Activation of persulfates by carbonaceous materials: A review. Chemical Engineering Journal 418: 129297.
- Xu Y, Liu S, Wang M, Zhang J, Ding H, et al. (2021) Thiourea-assisted one-step fabrication of a novel nitrogen and sulfur co-doped biochar from nanocellulose as metal-free catalyst for efficient activation of peroxymonosulfate. J Hazard Mater 416: 125796.
- Li F, Xie Y, Wang Y, Fan X, Cai Y, et al. (2019) Improvement of dyes degradation using hydrofluoric acid modified biochar as persulfate activator. Environmental Pollutants and Bioavailability 31(1): 32-37.

 Mini Review
Mini Review