ABSTRACT
The ongoing outbreak of the existing and mutated strains of coronavirus has been a cause of great concern worldwide. It has posed critical challenges for medical researchers and public health. Though a staggering number of vaccines have been developed, few of which are still under various phases of clinical trials, the development strategy for the COVID-19 therapy is the need of the hour. Heterocyclic moieties are pivotal in the field of medicine. This review covers the different classes of pre-existing drugs constituting various heterocyclic analogs that have been repurposed for the possible treatment of the COVID-19 disease. Moreover, it can also help the pharmaceutical industry to develop a new class of advanced medicine to fight against Covid- 19.
Keywords: Coronavirus; COVID-19; Hydroxychloroquine; Remdesivir; SARS CoV2
Abbreviations: COVID-19: Coronavirus disease of 2019; SARS CoV2: Severe Acute Respiratory Syndrome coronavirus; MERS CoV: Middle East Respiratory Syndrome coronavirus; UK:4. United Kingdom; CoV: Coronavirus; RNA: Ribonucleic Acid; ACE 2: Angiotensin-Converting Enzyme; RdRp: RNA-dependent RNA polymerase; WHO: World Health Organization; LPV/r: Lopinavir/ritonavir; RDV: Remdesivir; RCV: Respiratory Syncytial Virus; NHC: National Health Commission; HCV: Hepatitis C Virus; HIV: Human Immunodeficiency Virus; CQ: Chloroquine; HCQ: Hydroxychloroquine; AZM: Azithromycin; IMP: Importin; IVIg: Intravenous Immunoglobulin; H1N1: Hemagglutinin type 1 and Neuraminidase type 1; GABA: Gamma-Aminobutyric Acid; CNS: Central Nervous System
Introduction
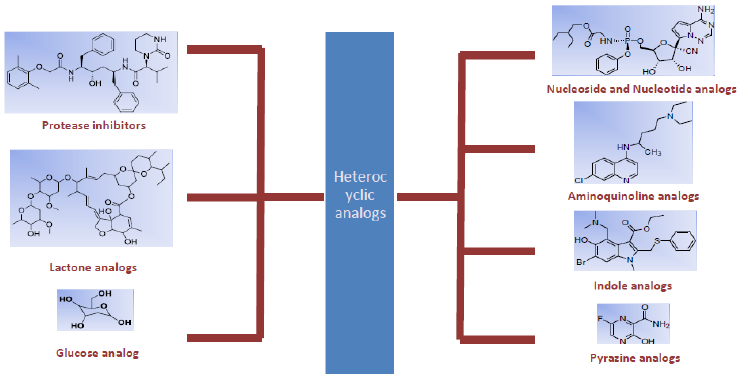
The global pandemic COVID-19 caused by the novel coronavirus named Severe Acute Respiratory Syndrom2 (SARS CoV2) has been a cause of great concern worldwide [1]. To date, no efficient therapy is available to treat this disease completely. Several heterocyclic drug moieties are being repurposed with adequate doses to treat the patients contracted with this virus. Heterocyclic compounds have an immense role in drug discovery and development. Among the various medical applications, heterocyclic compounds play an active role as anti-bacterial, anti- viral, anti-fungal, anti-tumor, and anti-covid-19 agents as well [2]. Coronavirus belongs to the family of viruses having a single stranded-RNA genome and capable of causing mild to severe symptoms of respiratory distress [3] (Figure 1).
Figure 1: Class of heterocyclic compounds used as prominent curable drugs in treatment of COVID -19 SARS-CoV-2
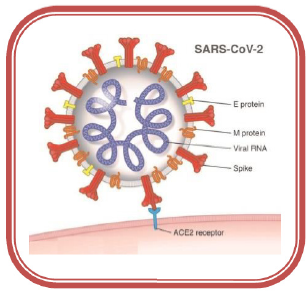
The novel coronavirus has 79% similarities in genome sequence with Severe Acute Respiratory Syndrome coronavirus (SARS CoV) and 50% similarities in genome sequence with the Middle East Respiratory Syndrome coronavirus (MERS CoV). The outer part of the virus has spike proteins that bind with Angiotensin- Converting Enzyme 2(ACE 2) of the host cell in the alveolar wall of the respiratory system. SARS CoV2 is endocytosed into the cytoplasm of pneumocyte where uncoating takes place by the lysosomal enzymes and the virus utilizes the host’s RNA-dependent RNA polymerase (RdRp) for viral genome replication [4]. They destroy the pneumocytes resulting in the high release of cytokines that causes systematic manifestations (Figure 2) [5]. The virus transmits through the droplets during respiration and symptoms can occur within 2-14 days. Furthermore, new coronavirus strains B.1.1.7 and B.1.351 have been identified in late 2020 in UK and South Africa, respectively that is more deadly (irrespective of the age, gender, and pre-existing health problems) than the previous circulating version of the coronavirus [6,7]. Reports show that these new strains having certain spike mutations replicate more quickly and spread more readily among humans [8,9]. The overall mortality rate of this disease is about 5.6% that surged up to 14% and more in many countries such as Italy, and the UK [10]. The reported complications include pneumonia, neurological complications, a multi- organ failure that may begin with fever, cold, cough, and fatigue. Senior citizens and patients with hypertensive, diabetic, or immune-compromised conditions are at higher risk [11] (Figure 3). Prominent pharmaceutical treatment options Though no effective treatment therapy has been designed yet, WHO has suggested some previously used drugs (Table 1) [12-18] for the possible treatment for COVID-19. This review represents various antiviral and antimalarial drugs that inhibit the viral replication or the virus entry into the host cells at different levels.
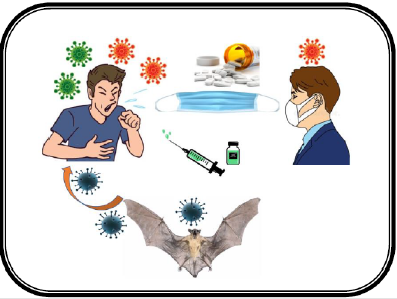
Figure 3: Transmittance of coronavirus. Bat coronavirus RaTG13 is highly similar to the severe acute respiratory syndrome Coronavirus 2 (SARS Cov-2). The virus transmits through the droplets during respiration when an infected person coughs, sneezes, talks, or breath. Therefore, masks are a common barrier to help prevent respiratory droplets from reaching others.
Nucleoside and Nucleotide Analogs
Synthetic analogs of pyrimidines and purines with modified sugar moiety are referred to as nucleoside and nucleotide analogs (Figure 4). These analogs are constantly being evaluated for their antiviral activities with lower toxicity [19].
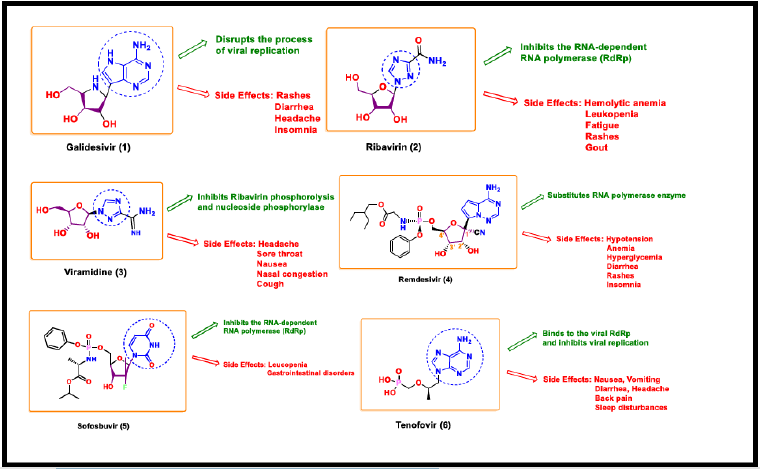
Galidesivir
The adenosine nucleoside analog Galidesivir is a potential antiviral drug for coronavirus treatment. Galidesivir (BCX4430) displays broad-spectrum antiviral activity against various pathogens including coronavirus. It is a nucleoside RNA polymerase inhibitor that disrupts the process of viral replication. It is metabolized to the active triphosphate form by ellular kinases. The nucleotide binds to the active site of the viral enzyme and gets incorporated into the growing viral RNA-strand leading to the chain termination [20,21]. Galidesivir is currently in the advanced development stage under the Animal Rule to combat multiple potential viral threats including coronavirus. It was safe and well-tolerated in Phase 1 clinical trials against coronavirus by both intravenous and intramuscular routes of administration in healthy subjects. In April 2020, Bio Cryst began enrollment of a randomized, double-blind, placebo-controlled clinical trial to assess the safety and antiviral effects of galidesivir in COVID 19 patients. However, galidesivir is under phase 2 human trial in Brazil and various other places in the world [22] and has not been deemed safe and effective by the FDA.
Ribavirin
It is a guanine nucleoside analog with activity against multiple RNA viruses including respiratory syncytial virus, SARS CoV and MERS CoV. Ribavirin inhibits the RNA- dependent RNA polymerase (RdRp) and hence inhibiting the viral protein synthesis [23]. Ribavirin was used extensively during the 2003 SARS outbreak. In vitro tests showed that ribavirin inhibits the beta coronavirus at higher concentrations [24]. It inhibits the natural guanosine generation by directly inhibiting inosine monophosphate dehydrogenase that is crucial for the production of guanine precursor to guanosine to promote the destabilization of viral RNA [25]. Ribavirin should be administered via intravenous infusion (500mg twice a day) in combination with interferon or LPV/r for up to ten days [26]. Intravenous ribavirin is suggested by China’s NHC for COVID 19 only as an add-on therapy to LPV/r or interferon [27]. Several adverse effects such as hemolytic anemia, leukopenia, fatigue, rash, and gout are generally observed from this medication (Scheme 1).
Viramidine
Viramidine or taribavirin is the most used derivative of ribavirin that shows broad-spectrum antiviral activity and immunomodulatory effects. Viramidine is a 3- carboxamidine prodrug of ribavirin [28,29]. Viramidine has better liver targeting property than ribavirin. Viramidine in combination with other drugs such as interferon is under clinical trial for the treatment of coronavirus disease.
Remdesivir
Remdesivir (RDV) was first developed for the treatment of Ebola virus. It is a phosphoramidate prodrug diastereoisomer (GS-5734)9a of a pyrrolo [1,2] (triazin-4- amino) adenine C - nucleoside analog [30]. Agostini et al. reported that remdesivir can potentially inhibit corona viruses such as SARS CoV1 and MERS CoV in vitro [31], with a half-maximal effective concentration (EC50) of 0.77μM. There are no published studies that justify the reactivity of remdesivir in animal models of SARS CoV2 in vivo. RDV is metabolized to an adenosine nucleoside analog via a number of steps. It is present as a monophosphate form extracellulary and as a triphosphate form intracellularly. The monophosphate form gets phosphorylated to the active nucleoside triphosphate form which is utilized by the viral RNA- dependent RNA polymerase (RdRp) to inhibit the viral replication through delayed chain termination [32,33 ]. Remdesivir possesses broad-spectrum antiviral activity against many viruses like Ebola [34], Nipah [35], and respiratory syncytial virus (RCV) family [36]. The structureactivity relationship studies pointed towards the criticality of the 1’-CN as compared to the unmodified nucleoside. The existence of the 3’-OH group allows the nucleophilic attack on the next coming nucleotide. 1’-CN substituent of remdesivir sterically clashes with RdRp (residue S861) upon further chain elongation (remdesivir + three additional nucleotides) distorting the position of RNA that prevents translocation [37]. Remdesivir is administered via intravenous injection (IV) with a loading dose of 200mg on the first day followed by a 100mg maintenance dose for up to ten days. Reports suggest that during the treatment no adverse effects were observed in association with the IV infusion. Daily administration of 10mg/kg of remdesivir yielded a short plasma half-life of the prodrug but sustained intracellular levels of the triphosphate form [38]. Recently, through a compassionate use indication, remdesivir has supportive evidence for yielding some clinical improvement in COVID 19 patients. Currently, remdesivir is not recommended by WHO and China’s NHC except for the hospitalized severe COVID-19 cases [39,40].
Sofosbuvir
Sofosbuvir is an anti-hepatitis C virus (HCV) drug that is clinically approved and also effective against other positive-strand RNA viruses. There are certain in-silico and in- vitro evidences that prove the effectiveness of sofosbuvir against SARS CoV-2. Sofosbuvir and its derivatives could potentially inhibit the SARS CoV-2 RdRp and it is safe and well-tolerated with daclatasvir at 400mg and 60mg respective doses daily. Sofosbuvir is converted into its nucleoside triphosphate active form through phosphorylation within the host cell. This terminates the RNA replication in the nascent viral genome through competition with the nucleotides of the virus [41]. Clinical trials show that more rapid recovery of COVID-19 patients was observed when dosed with sofosbuvir and daclatasvir in one arm with a significantly higher survival probability when compared with ribavirin in another arm [42]. However, this combination causes severe adverse effects like leucopenia and gastrointestinal disorders (GI). The clinical trials are still in progress to elucidate the antiviral efficacy against SARS CoV-2 (Scheme 2).
Tenofovir
Tenofovir is a reverse transcriptase inhibitor that is active against HIV-1, HIV-2, hepatitis B virus, and coronavirus. The active tenofovir diphosphate form binds to the viral RdRp and inhibits viral replication. It is able to bind tightly with SARS CoV-2 RdRp with binding energy -6.9 kcal/mol and contradicts the polymerase function [43]. Tenofovir in combination with Emtrcitabine could potentially increase its efficiency. However, no complete and conclusive clinical data is available suggestingthe exact mechanism of action of tenofovir and its analogs to treat COVID-19 patients. Common side effects of this antiretroviral drug include nausea, vomiting, diarrhea, headache, pain, back pain and sleep disturbances [44] (Scheme 3).
Protease Inhibitors
Protease Inhibitors (Figure 5) are substances with the ability to inhibit the enzymes’ proteolytic activity. They continue to appear for immediate use in the treatment of SARS CoV2 infections [45,46].
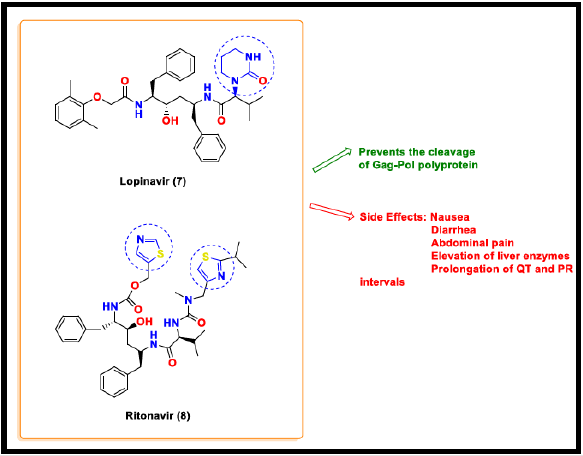
Lopinavir and Ritonavir
Lopinavir and Ritonavir (LPV/r) is a combination protease inhibiter that was firstly approved for the treatment of Human Immunodeficiency Virus (HIV) infections [47]. This combination shows potent antiviral activity against Severe Acute Respiratory Syndrome coronavirus (SARS CoV). Lopinavir binds to the viral protease and prevents the cleavage of Gag-Pol polyprotein, which produces immature non-infectious virus particles. Though Lopinavir does not affect the already infected cells, the main antiviral action of protease inhibitor is to prevent the subsequent infection of cells. Ritonavir is co-administered with Lopinavir to increase the plasma concentration of lopinavir by inhibiting the cytochrome P450 3A (CYP3A) metabolism and thus acts as its pharmacokinetics enhancer [48]. Lopinavir and Ritonavir (LPV/r) are shown to be effective for the treatment of MERS and SARS coronavirus disease [49]. COVID-19 patients receive either LPV/r (400/100) twice a day for 14 days, but this treatment therapy has not demonstrated much benefit to the patients when compared to the patients receiving standard care [50]. Moreover, this combination causes more side effects including nausea, diarrhea, abdominal pain, elevation of liver enzymes, and prolongation of QT and PR intervals [51]. China’s NHC has suggested LPV/r [52], but this combination has no proven clinical efficacy towards COVID-19. So, this combination drug therapy is not recommended by NIH [53].
Pyrazine Analogs
The volatile, nitrogen-containing heterocyclic pyrazines find various applications in the pharmaceutical industries [54]. Pyrazine derivatives (Figure 6) have been well reported as anti- bacterial, anti-diabetic, anti-viral, and anti-cancer agents [55].
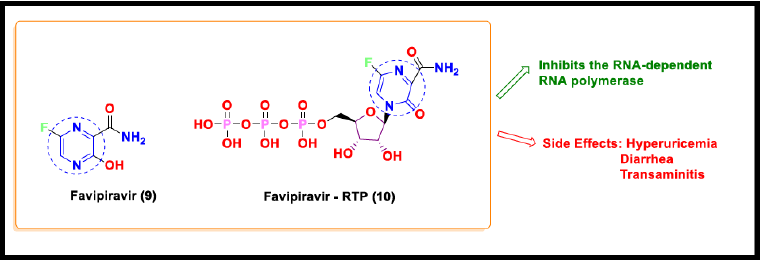
Favipiravir
Favipiravir (T-705, 6-fluoro-3-hydroxy pyrazin-2-carboxamide) is a prodrug of a purine nucleotide, which selectively inhibits the RNA-dependent RNA polymerase of the influenza virus [56]. Favipiravir exhibits acidic properties due to the presence of the hydroxyl group [57]. Favipiravir undergoes intracellular phosphor ribosylation to convert into an active form, Favipiravir-RTP (ribofuranosyl-5’-triphosphate) (11), which is recognized as a substrate by RdRp and inhibits the RNA polymerase activity [58]. Favipiravir also inhibits the other pathogenic RNA viral infections, such as flavivirus, arenavirus, bunyavirus, alphavirus, and norovirus Furuta et al. [59]. Less preclinical support has been established for favipiravir to treat SARS CoV 2 compared to remdesivir. The recommended dose of favipiravir is 1200mg to 1800mg every 12 hours [60]. In March 2020, the National Medical Products Administration of China approved favipiravir as the first anti- COVID-19 drug in China, since the clinical trials had demonstrated efficacy with minimum adverse effects.
Aminoquinoline Analogs
Aminoquinolines (Figure 7) are the quinoline derivatives that have inhibitory effects on DNA replication, cytokine production, and chemotaxis [61], Chloroquine (11) and Hydroxychloroquine (12) play a promising role in the management of SARS Cov2 outbreak by increasing the pH and conferring anti-viral effects [62].
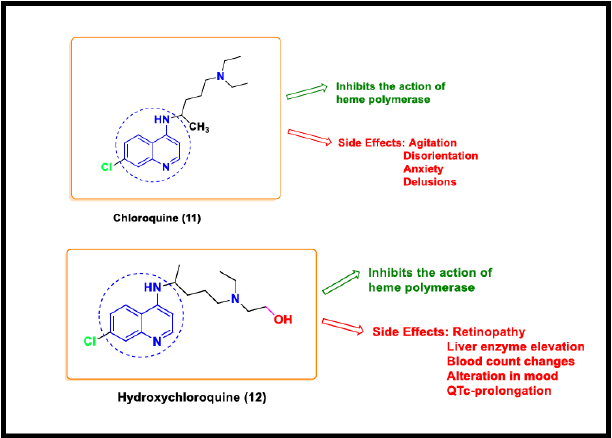
Chloroquine
Chloroquine (4-amino quinoline) is a classic anti-malarial and anti- autoimmune drug [63]. CQ can easily diffuse in the cytoplasm across the cell membrane to acidic vesicles in its unprotonated form. On protonation, it gets entrapped in the vesicles. Protonated CQ is not able to diffuse out of the lysosomes or endosomes, and hence remains retained in the cellular compartment with the hydrolases Savarino et al. [64]. Its effect of neutralizing the acidic endosomal pH supports broad-spectrum antiviral activity by blocking the endosome-mediated viral entry at the pre- and post-entry level. It blocks the virus infection and interferes with the glycosylation of cellular receptors of SARS CoV [65]. In Several clinical trials of China, the effectiveness of chloroquine phosphate has been proved against COVID-19. Chloroquine shows anti-viral effects, thus reducing the binding efficiency between ACE2 of host cells and the SARS Cov 2 spike protein [66].
Hydroxychloroquine
Hydroxychloroquine (HCQ) is an analogue of chloroquine and a less toxic metabolite of CQ. It was initially considered as an antimalarial drug that inhibits the entry of virus into the host cell, altering the synthesis of viral proteins [67]. This drug could be toxic and fatal if overdosed. It has several adverse effects such as retinopathy, liver enzyme elevation, blood count changes, alteration in mood, and QTc-prolongation [68]. Both HCQ and CQ could decrease the cytokine production. The mechanism of action of these two scaffolds is similar as both act as a weak base. The beneficial impact of HCQ (200mg three times a day for 10 days) or HCQ + azithromycin (AZM) (500 mg for day 1 followed by 250 mg/day for the next 4 days) on the viral negativity at day 6 was reported in a non-randomized open-label clinical trial in France enrolling COVID-19 patients treated with HCQ or untreated controls [69]. Since the COVID-19 outbreak, these drugs have shown an effective in-vitro antiviral effect on SARS CoV-2, particularly HCQ in combination with azithromycin [70].
Lactone Analog
The cyclic carboxylate esters called lactones (Figure 8) have a huge contribution in the development of antiviral drugs with lower toxicity. A rapid response against COVID-19 was reported when the patients contracted with coronavirus were dosed with the cyclic carboxylate ester (lactone) Ivermectin [71].
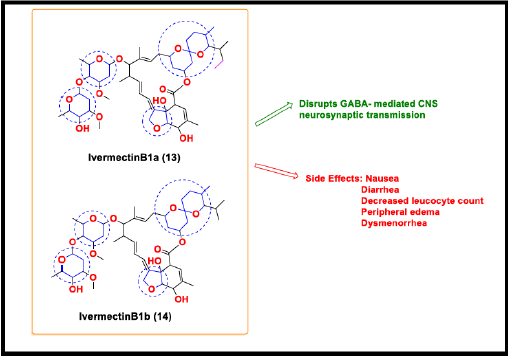
Ivermectin
Ivermectin, a macrocyclic lactone belongs to the family of Avermectins. It is a mixture of the two components H2B1a (90%) (13) and H2B1b (10%) (14). The structural difference between these two is the presence of one extra methyl group in Ivermectin B1a [72]. Ivermectin is an antiparasitic agent that shows invitro antiviral activity also against a wide range of viruses [73]. Ivermectin disassociates the preformed importin (IMP) alpha/ beta1 heterodimer which is responsible for the nuclear transport of viral protein cargos that disrupts the immune evasion mechanism of the virus [74,75]. Recent in-vitro studies prove that Ivermectin has the ability to reduce viral RNA up to 5,000-folds after two days of infection with SARS CoV-2 [76].
Indole Analog
Indole derivatives (Figure 9), being diverse in nature, display a wide range of promising biological activities [77,78]. They have been shown effective against the novel coronavirus.
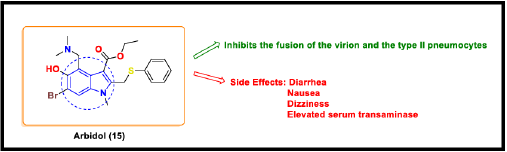
Arbidol (Umifenovir)
Arbidol (approved by Russia and China) is a synthetic antiviral drug used for the treatment of seasonal influenza. It is well-tolerated against SARS CoV with lower toxicity [79]. Arbidol is effective only when given in combination with other antiviral drugs such as LPV/r or interferon. Arbidol targets the S protein/ACE 2 interaction, thus inhibiting the membrane fusion of the viral envelope to the host cell. It particularly focuses on inhibiting the fusion of the virion and the type II pneumocytes [80]. Its major adverse effects include diarrhea, nausea, dizziness, and elevated serum transaminase [81].
Carboxylate Ester Analog
The carboxylic acid functional group (Figure 10) displays promising applications in the pharmaceutical industry as it adds to the hydrophilicity of the drug [82].
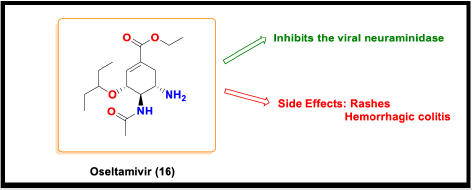
Oseltamivir
Oseltamivir is a flu drug that shows antiviral activity against
influenza A and B. It inhibits the viral neuraminidase but was
ineffective for the COVID-19 patients [83]. The combination of
oseltamivir with baloxavir also proved fruitless in curing the
patients with coronavirus disease.
However, a doctor’s team reported that if oseltamivir was
combined with lopinavir (7) and ritonavir (8), it could improve the
conditions of covid 19 patients significantly [84].
Glucose Analog
Glucose analogs provide active anti-covid candidates by mimicking the glucose structure [85].
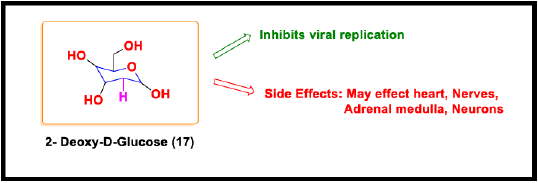
2-Deoxy-D-Glucose
2-Deoxy-2-Glucose has received much attention these days for its emergent use in anti-covid therapy. It is a non-metabolized analog of glucose [86] that has a hydrogen atom instead of the hydroxyl group at the second carbon position [87]. The mimicking property of 2-DG enables it to naturally exploit the glucose uptake mechanism, a universal characteristic of tumor cells and viruses [88]. SARS CoV-2 operates on the glucose metabolism for its replication and energy requirements [89]. This sugar analog enters the glycolysis cycle partially, interrupts the glucose phosphorylation by creating an inhibitory feedback effect on the hexokinase enzyme [90], causing the depletion of cellular ATP. This results in the inhibition of the cell cycle and hence leads to cell death ceasing the viral replication. So, besides its therapeutic use in seizures, cancer and, viral infections, clinical trials prove its efficient use in the treatment of COVID-19 helping the faster recovery of the hospitalized patients and reducing the dependence on supplemental oxygen. 2-DG is a non-selective and dose-dependent oral administrative drug that can cause several adverse effects on the heart, platelets, adrenal medulla, and neurons [91]. However, the reports showed that its cautious and prescribed use has resulted in the RT-PCR negative conversations in the COVID-19 patients (Figure 11).
Conclusion
Drug discovery is a challenging and time-consuming process. Several drugs have been suggested to treat COVID-19, but no proven studies are available which clarify the evidence to end the pandemic completely. Almost all the pharmaceuticals are under investigation and further researches via clinical trials. Repurposing of the preexisting drugs became an approach as a rapid response measure to the emergent pandemic. This review represents an overview of the current therapeutic drugs involving heterocyclic moieties that are suggested for the treatment of COVID-19 caused by SARS CoV-2. The literature showed that Remdesivir is moderately recommended by the NIH, that inhibits the viral replication and the anti-viral activity of CQ and HCQ has been identified in in-vitro studies though the evidence of these drugs is limited. Furthermore, the clinical trials depicted that the sugar analog 2-Deoxy-D-Glucose proved highly effective in the anti-covid therapy that works by inhibiting the viral replication in the host cell. So, it is anticipated that this glucose analog will bring immense relief to the COVID-19 patients.
Acknowledgement
Authors acknowledge Department of Chemistry, University of Delhi. Authors are thankful to Council for Scientific and Industrial Research, and University Grants Commission for providing fellowships and fundings.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- (2020) WHO Director-Generals opening remarks at the media briefing on COVID-19 - 11 March 2020.
- Mohammadian NT, Monajjemi M (2021) Heterocyclic compounds of somenatural products as anti-covid-19: Iranian plants and natural drugs of Biointerface Research in Applied Chemistry 11(3).
- M Negi (2020) Bioorganic Chemistry pp. 104
- Zhou P, Yang XL, Wang XG, Hu B, Zhang W, et al. (2020) A pneumonia outbreak associated with a new corona virus of probable bat origin. Nature 588(7836): E6.
- WG Whitford (1976) Sweating responses in the chimpanzee (Pan troglodytes). 53(4): 333-336.
- Daniel J Grint, Kavin Wing, Elizabeth Williamson, Helen I McDonald, Krishnan Bhaskaran, et al. (2021) Case fatality risk of the SARS CoV-2 variant of concern B.1.1.7 in England. medRxiv.
- Moyo-Gwete T, Madzivhandila M, Makhado Z, Ayres F, Mhlanga D, et al. (2021) SARS-CoV-2 501Y. V2 (B. 1.351) elicits cross-reactive neutralizing antibodies. BioRxiv.
- Patterson DS (2021) COVID Research Updates: One Mutation Could Explain a Coronavirus Variant’s Rampage. Nature.
- Liu Y, Liu J, Plante KS, Plante JA, Xie X, et al. (2021) The N501Y spike substitution enhances SARS-CoV-2 transmission. BioRxiv.
- (2020) World Health Organization. Corona virus disease 2019 (COVID-19): situation report, 77.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult in patients with COVID 19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229): 1054-1062.
- Rajter JC, Sherman MS, Fatteh N, Vogel F, SacksJ, et al. (2021) Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study. Chest 159(1): 85-92.
- Agrawal U, Raju R, Udwadia ZF (2020) Favipiravir: A New and Emerging Antiviral Option in COVID-19. Med. J. Armed Forces India 76 (4): 370-376.
- Nessaibia I, Siciliano D, Tahraoui A (2020) Why Nobody Discusses the Adverse Psychiatric Effects of Chloroquine in Case It Might Become the Future Treatment against COVID-19? Int J Health Plann Manage 35(6): 1311-1313.
- Abdul Aleem, JPK Remdesivir. StatPearls 2021.
- Zheng L, Zhang L, Huang J, Nandakumar KS, Liu S, Cheng K (2020) Potential Treatment Methods Targeting 2019-NCoV Infection. Eur J Med Chem 205: 112687.
- Lin CC, Philips L, Xu C, Yeh LT (2004) Pharmacokinetics and Safety of Viramidine, a Prodrug of Ribavirin, in Healthy Volunteers. J Clin Pharmacol 44 (3): 265-275.
- Türsen Ü, Türsen B, Lotti T (2020) Cutaneous Sıde-Effects of the Potential COVID-19 Drugs. Dermatol Ther 33(4): 13476.
- Chung K Chu, Srinivas Gadthula, Xin Chen, Hyunah Choo, SureyyaOlgen et al. (2006) Antiviral activity of nucleoside analogues against SARS-coronavirus(SARS CoV). Antiviral Chemistry and Chemotherapy 17(5): 285-289.
- Tchesnokov EP, Feng JY, Porter DP, Götte M (2019) Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 11(4): 1-16.
- Jonna B. Westover, Amanda Mathis, Ray Taylor, Luci Wandersee, et al. (2018) Galidesivir Limits Rift Valley Fever Virus Infection and Disease in Syrian Golden Hamsters. Antiviral Res 156: 38-45.
- Clinical trial number NCT03891420 for “A study to Evaluate the safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever on COVID-19” at Clinical gov
- (2015) Copegus tablet, film coated (ribavirin). South San Francisco, CA: Genentech USA
- Falzarano D, de Wit E, Martellaro C, Callison J, Munster Vj, et al. (2013) Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci Rep 3: 1686.
- Khalili JS, Zhu H, Mak A, Yan Y, Zhu Y (2020) Novel coronavirus treatment with ribavirin: ground work for an evaluation concerning COVID-19. J Med Virol 92(7): 740-746.
- Dong L, Hu S, Gao J (2020) Discovering drugs to treat coronavirus disease 2019(COVID-19). Drug DiscovTher 14(1): 58-60.
- (2020) National Health Commission (NHC) of people's Republic of China. Diagnosis and treatment protocol for covid 19 pneumonia caused by novel coronavirus infection 7th Edn NHC.
- Barnard, D Viramidine (Ribapharm) (2002) Current Opinion in Investigational Drugs. 3: 1585-1589.
- Wu JZ, Larson G, Hong Z (2004) Dual-Action Mechanism of Viramidine Functioning as a Prodrug and as a Catabolic Inhibitor for Ribavirin. Antimicrob Agents Chemother 48 (10): 4006-4008.
- Siegel D, Hui HC, Doerffler E, Clarke MO, et al. (2017) Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f] [triazin4-amino] Adenine C-Ncleoside (GS- 5734) for the Treatment of Ebola and Emerging Viruses. Journal of medicinal chemistry 60(5): 1648-1661.
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, et al. (2018) Corona virus susceptibility to the anti-viral remdesivir is mediated by the viral polymerase and the proofreading exoribonuclease. Am Soc For Microbiology 9(2): 1-18.
- (a) Mehellou Y Rattan H Balzarin J (2018) The ProTide Prodrug Technology: From the Concept to the Clinic J Med Chem, 61(6): 2211−2226. (b) Slusarczyk M, Serpi M, Pertusati F (2018) Phosphoramidates and phosphonamidates (ProTides) with antiviral activity. Antivir Chem. Chemother; 26. (c) Curley D, et al. (1990) Synthesis and anti-HIV evaluation of some phosphoramidate derivatives of AZT: studies on the effect of chain elongation on biological activity. Antiviral Res. 14(6): 345−356. (d) McGuigan C, et al. (1993) Intracellular delivery of bioactive AZT nucleotides by aryl phosphate derivatives of AZT. J. Med. Chem.;36 (8): 1048−1052.
- (a) Murakami E (2010) Mechanism of activation of PSI-7851 and its diastereoisomer PSI- 7977. J. Biol Chem 285(45): 34337-34347. (b) Saboulard D (1999) Characterization of the activation pathway of phosphoramida tetriester prodrugs of stavudine and zidovudine. Mol Pharmacol 56(4): 693-704. (c) Tobias SC, Borch RF (2001) Synthesis and biological studies of novel nucleoside phosphoramidate prodrugs. J. Med. Chem. 44(25): 4475-4480. (d) Venkatachalam TK (2005) Protease-mediated enzymatic hydrolysis and activation of aryl phosphoramidate derivatives of stavudine. Eur. J Med Chem 40(5): 452-466.
- Warren TK, Seigel D, Hui HC (2017) Discovery and synthesis of GS-5734, a phosphoramidate prodrug for the treatment of Ebola or emerging viruses. USAMRIID Ft Detrick United States 60(5): 1648-1661.
- (a) Jordan PC, Liu C, Raynaud P, Lo MK, Spiropoulou CF, et al. (2018) Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase. PLoS Pathogens 14(2): e1006889. (b) Lo MK, Feldmann F, Gary JM, et al. (2019) Remdesivir (GS- 5734) protects African green monkeys from Nipah virus challenge. Sci Transl Med 11: e9242.
- Lo MK, Feldman F, Gary JM, et al. (2019) Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci Transl Med 11(494): e9242.
- Gordon CJ, Tchesnokov EP, Woolner, E Perry JY, Porter DP, Gotte, M (2020) Remdesivir is a direct- acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome corona virus 2 with high J Biol Chem 295(20): 6785-6797.
- Green N, Ott RD, Isaacs RJ, Fang H (2008) Cell-based Assays to Identify Inhibitors of Viral Disease. Expert Opin. Drug Discovery 3(6): 671-676.
- (2020) World Health Organisation (WH). Clinical management of covid 19. Interim guidance. Geneva, Switzerland: WHO.
- (2020) US National Institutes of Health (NIH). Interim covid 19 treatment guidelines. NIH.
- Beck BR, Shin B, Choi Y, Park S, Kang K (2020) Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS CoV-2) through a drug target interaction deep learning model. Comput Struct Biotechnol J 18: 784-790.
- Sacramen CQ, Fintelman-Rodrigues (2020) The in vitro antiviral activity of the anti- hepatitis C virus (HCV) drugs daclatasvir and sofosbuvir against SARS CoV- 2.BioRxiv.
- AA Elfiky (2020) Anti-HCV, nucleotide inhibitors, repurposing against COVID-19, Life Sci 248: 117477.
- Ustianowski A, Arends JE. (2015) Tenofovir: What We Have Learnt After 7.5 Million Person- Years of Use”. Infectious Diseases and Therapy 4(2): 145-57.
- Irvin E. Liener MLK (2012) Toxic Constituents of Plant Foodstuffs.
- Dipti Mothay KV, Ramesh (2020) Binding site analysis of potential protease inhibitors COVID-19 using AutoDock. Indian Virol Soc VirusDis 31(2): 194-199.
- Kaletra O (2019) (lopinavir and ritonavir) tablets and oral solution. North Chicago, IL: AbbVie Inc.
- (a) Sanders JM, Monogue, Jodlowski TZ Cutrel JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID 19): a review. JAMA. 323(18): 1824-1836. (b) Cvetkovic RS, Goa KL (2003) Lopinavir/ritonavir: a review of its use in the management of HIV. infection Drugs 63(8): 769-802.
- Aljofan M, Gaipov A (2020) COVID-19 treatment: the race against time. Electron J Gen Med 17(6): e227.
- Baden LR, Rubin EJ (2020) Covid-19 - The search for effective therapy. The New England Journal of 382(19): 1851-1852.
- Bin Cao MD, Yeming Wang MD, Danning Wen MD, Wen Liu MS, Jingli Wang MD, et al. (2020) A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. The New England Journal of Medicine 1-13. 382: 1787-1799.
- (2020) National Health Commission (NHC) of people's Republic of China. Diagnosis and treatment protocol for covid 19 pneumonia caused by novel coronavirus infection 7th edn NHC
- (2020) US National Institutes of Health (NIH). Interim covid 19 treatment guidelines. NIH.
- Frederik BM, Chiam H, Kvetoslava V, Margit W, Florian R (2020) Pyrazines: Synthesis and Industrial Application of these Valuable Flavor and Fragrance Compounds. Biotechnol J 15(11).
- Pankaj B Miniyar, Prashant R Murumkar, Pallavi S Patil, Mahesh A Barmade, Kailash G Bothara (2013) Unequivocal Role of Pyrazine Ring in Medicinally Important Compounds: A Review. Mini reviews in Med Chem13(11): 1607-1625.
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (2013) Favipiravir (T- 705), a novel viral RNA polymerase inhibitor. Antivir Res 100(2): 446-454.
- El-Nahas A, Hirao K (1999) A theoretical study on 2-hydroxypyrazine and 2,3- dihydroxypyrazine: tautomerism, intramolecular hydrogen bond, solvent effects. J Mol Struct 459(1): 229–237.
- (a) Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, (2005) Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 49(3): 981-986. (b) Sangawa H, Komeno T, Nishikawa H, Yoshida A, Takahashi K, et al. (2013) Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase Antimicrob Agents Chemother. 57(11): 5202-5208. (c) Naesens L, Guddat LW, Keough DT, van Kuilenburg AB, Meijer J, et al. (2013) Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir). Mol Pharmacol. 84(4): 615–629.
- (a) Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, et al. (2009) T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antivir Res. 82(3): 95-102. (b) Jin Z, Tucker K, Lin X, Kao CC, Shaw K, et al. (2015) Biochemical evaluation of the inhibition properties of favipiravir and 2'-C-methyl-cytidine triphosphates against human and mouse norovirus RNA polymerases. Antimicrob Agents Chemother. 59(12): 7504-7516.
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 323(18): 1824-1836.
- Cecil, Russell La Fayette, Lee Goldman, Andrew I Schafer (2012) Goldman’s Cecil Medicine, Expert Consult Premium Edition—Enhanced Online Features and Print p. 24
- Zahra Sahraei, MinooshShabani, Shervin Shokouhi, Ali Saffaei (2020) Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents 55(4): 105945.
- Plantone D, Koudriavtseva T (2018) Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini- Review. Clin Drug Investig 38(8):653-671.
- Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R (2003) Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis 3(11): 722-727.
- (a) Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone (2005) A Lancet Infect Dis 2006; 6: 67-69. (b) Yan, Y et al. Cell Res. 23: 300-302 (2013). 10. Vincent MJ et al. Virol J. 2:
- Sahraei Z, Shabani M, Shokouhi S, Saffaei A (2020) Aminoquinolines against coronavirus disease 2019 (COVID 19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents 55(4): 105945.
- Chou AC, CD Fitch (1992) Heme polymerase. Modulation by chloroquine treatment of a rodent malaria. Life Sci. 51(26): 2073-2078.
- Song Y, Zhang M, Yin L, Wang K, Zhou Y, et al. (2020) COVID-19 treatment: close to a cure? a rapid review of pharmacotherapies for the novel coronavirus. International journal of antimicrobial agents. 56(2): 106080.
- Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID 19: results of an open-label non-randomized clinical trial. Int Antimicrob Agents 56(1): 105949.
- Yao X, Ye F, Zhang M, Cui C, Huang B, et al. (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of SARS CoV-2. Clin Infect Dis 71(15): 732-739.
- Scheim D (2020) Antimalarials for COVID-19 treatment: rapid reversal of oxygen status decline with the Nobel Prize-honored macrocyclic lactone ivermectin. Available at SSRN
- https://www.merck.com/product/usa/pi_circulars/s/stromectol/stromectol_pi.pd.
- Yavuz SS, Unal S (2020) Antiviral treatment of COVID-19 Turk J Med Sci 50(Sl-1): 611-619.
- Tu YF, Chien CS, Yarmishyn AA, Lin YY, Luo YH, (2020) A review of SARS CoV-2 and the ongoing clinical trials Int J Mol Sci 21(7):2657.
- Turner MJ, Schaeffer JM (1989) Mode of Action of I Vermectin. W.C. Campbell (ed.), Ivermectin Abamectin 73-88.
- Caly L. Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved Drug Ivermectin inhibits the replication of SARS C0V-2 in vitro. Antivir Res 178: 104787.
- Kumar R, Takkar P (2021) Repositioning of Isatin hybrids as novel anti-tubercular agents overcoming pre-existing antibiotics resistance. Med Chem Res 30(4): 847-876.
- Deswal N, Shrivastava A, Hossain MD, Gahlyan DP, Bawa R, et al. (2021) Design, Synthesis, Evaluation and Molecular Docking Studies of Novel Triazole Linked 1,4-Dihydropyridine- Isatin Scaffols as Potent Anticancer agents. Chem Sel 6(4): 717-725.
- Zhang J, Zhou L, Yang Y, Pang W, Wang W, et al. (2020) Therapeutic and triage strategies for 2019 novel corona virus disease in fever clinics. Lancet Respir Med 8(3): 11-12.
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 323(18): 1824-1836.
- Proskurnina EV, Izmailov DY, Sozarukova MM, Zhuravleva TA, Leneva IA, et al. (2020) Antioxidant potential of antiviral drug umifenovir. Molecules 25(7): 1577.
- Maag H. (2007) Prodrugs of Carboxylic Acids. Prodrugs pp 703-729.
- McCreary EK, Pogue JM (2020) Corona virus disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis 7(4):105.
- C Offord (2020) Flu and HIV drugs show efficacy against coronavirus. The Scientist.
- Zhang D, Li J, Wang F, Hu J, Wang S, et al. (2014) 2-Deoxy-D-Glucose Targeting of Glucose Metabolism in Cancer Cells as a Potential Therapy. Cancer Lett 355(2): 176-183.
- Karangula J, Boggula N, Kappari V (2002) 2-DEOXY-D-GLUCOSE : AN UPDATE REVIEW. J Innov Dev Pharm Tech Sci 4(5): 68-78.
- Aft RL, Zhang FW, Gius D (2002) Evaluation of 2-Deoxy-D-Glucose as a Chemotherapeutic Agent: Mechanism of Cell Death. Br J Cancer 87(7): 805-812.
- Xi H, Kurtoglu M, Lampidis TJ (2014) The Wonders of 2-Deoxy-d-Glucose. IUBMB Life 66(2): 110-121.
- Anant Narayan Bhatt, Abhishek Kumar, Yogesh Rai, Neeraj Kumari, Dhiviya Vedagiri, et al. (2021) Glycolytic Inhibitor 2-Deoxy-D-Glucose Attenuates SARS-CoV-2 Multiplication in Host Cells and Weakens the Infective Potential of Progeny Virions. bioRxiv.
- Kailash Chandra Samal (2021) Anti-Covid Drug: 2-Deoxy-D-Glucose and Its Mechanism of Action. Biot Res Today 3 (5): 345-347.
- Malgotra V, Sharma V (2021) 2-Deoxy-d-Glucose Inhibits Replication of Novel Coronavirus ( SARS-CoV-2 ) with Adverse Effects on Host Cell Metabolism. pp. 1–19.

 Review Article
Review Article