ABSTRACT
Lactate Dehydrogenase (LDH) is a key enzyme in the anaerobic metabolic process. It belongs to the oxidoreductase family. In biological systems, LDH is a carbohydrate metabolism enzyme that catalyses the interconversion of lactate and pyruvate with the NAD+/NADH coenzyme system. It’s a cytoplasmic enzyme found in almost all tissues, but especially in muscle, liver, and kidney, as well as in moderate amounts in red blood cells. However, under pathological circumstances, such as cancer, the level of LDH in the blood rises, making it a possible cancer biomarker. Recently, blood LDH levels were shown to be routinely elevated in cancer patients and linked to poor clinical outcome and medication resistance. Therefore, LDH determination became a routine supportive tool in diagnosing tumours or monitoring the effects of cancer treatment. In this review paper, we have summarized an overview of about LDH enzyme, its catalytic property, functions, pathophysiology and its role as a cancer biomarker.
Keywords: Lactate dehydrogenase; Lactate; Metabolism; Biomarker; Cancer; Energy
Abbreviations: AMI: Acute Myocardial Infarction; CNS: Central Nervous System; LDH: Lactate Dehydrogenase; BC: Breast Cancer; ML-LDH: Mitochondrial LDH; NAD: Nicotinamide Adenine Dinucleotide; NADH: Nicotinamide Adenine Dinucleotide Hydrogen; NADP: Nicotinamide Adenine Dinucleotide Phosphate; Pi: Inorganic phosphate
Introduction
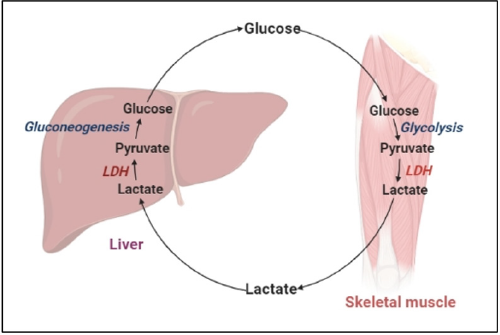
Oxidation-reduction reactions are essential to growth and survival of organisms, as the oxidation of organic molecules produces energy. The creation of essential energy molecules, such as ATP in glycolysis, can be accelerated by energy-producing reactions. As a result, dehydrogenases play an important part in metabolism. In normal circumstances, a particular amount of dehydrogenase enzyme is synthesized in each cell, but in disease situations, the amount of dehydrogenase increases and occasionally leaks out of the cells into the extracellular environment. In many biological systems, LDH is a carbohydrate metabolism enzyme that catalyses the interconversion of lactate and pyruvate in muscle tissue and in liver with the NAD+/NADH coenzyme system in anaerobic condition that allows the body to adapt to hypoxia and/ or intense exercise shown in Figure 1 [1]. The enzyme is present in a variety of organisms, including plants and animals (Robert Klein et al.). It can be found in all tissues and plays an important role in gluconeogenesis and DNA metabolism. According to a specieswide analysis, LDH has a well-preserved structure with only a few changes in amino acid sequence across species. LDH is a cytoplasmic enzyme found in almost all tissues, but especially in muscle, liver, and kidney, where it is abundant. This enzyme is also found in red blood cells in small concentrations [2]. The enzyme LDH catalysis the conversion of pyruvate to lactate and kept them in nearequilibrium. In both glycolysis and oxidative metabolism, LDH has a higher activity than any of the other enzymes [3]. As a result, lactate is generated constantly and absorbed nearly quickly. Because of its large glycogen storage, capacity to traverse concentration gradients without requiring energy input, and fast conversion to pyruvate once within a cell, it is an excellent fuel source for most tissues of the body, including the Central Nervous System (CNS). The heart, liver, skeletal muscle, kidney, and erythrocytes have high amounts of LDH activity, while the lung, smooth muscle, and brain have lower levels. Because of its wide effects in numerous physiological tissues, LDH is elevated in a variety of disorders. An increase in LDH activity can be caused by a number of reasons.
LDH Structure and Its Isoform
LDH is a homo/hetero tetramer made up of four LDHA or LDHB subunits that combine to generate five different isozymes (LDH-1 to LDH-5) with tissue-specific distributions [4]. This isoform commonly found in- LDH-1 in cardiomyocytes, LDH-2 in reticuloendothelial system, LDH-3 in pneumocytes, LDH-4 in kidneys and pancreas, and LDH-5 in liver and striated muscle. Lactate is generated and released by cells via monocarboxylate transporter proteins. The LDHA homo tetramer, also known as the “muscle-type” LDH or LDH5, prefers to convert pyruvate to lactate and NAD+, and is the most abundant isozyme in muscle. In the heart, isozyme LDHB (homo tetramer) is commonly known as “heart-type” LDH or LDH1, converts lactate mostly to pyruvate and NADH and is the most abundant isozyme in the heart [5]. LDH is expressed in most tissues, including malignancies, in balanced, hetero tetrameric forms (LDH2, LDH3, and LDH4), as well as combinations of multiple isozymes. Although LDH is mostly a cytoplasmic enzyme, researches have shown that it can also be found in mitochondria. mL-LDH convert L-lactate to pyruvate in the mitochondrial matrix. Several investigations have found that the absence of an LDH isozyme prevents mitochondria from oxidising lactate [6].
LDH Mechanism Catalytic Activity
The activation of LDH enzyme takes place in a certain order. Prior to the binding of a substrate to LDH, the formation of the LDH/NADH binary complex, in which His 195 and Asp 168 residues appear to play a significant role, is required. It is followed by conformational changes in which the mobile hinged loop, which is formed by a group of surface amino acid residues (98-110) with Arg 109 hydrogen bonded to the substrate’s carbonyl, closes over through the substrate binding pocket, which is made up of residues 163–247 and 267-331, allowing cofactor-substrate interactions and facilitating on-enzyme catalysis [7]. The metabolic transition from aerobic to anaerobic respiration controls LDH activity. LDH is regulated by three types of regulators: allosteric modulation, substrate-level regulation and transcriptional regulation. The relative availability and concentration of substrates influence LDH activity. The enzyme becomes more active as the number of substrates grows during vigorous muscular activation. ADP, AMP, and Pi build when the demand for ATP exceeds the supply of aerobic ATP. The physiologic capability of pyruvate dehydrogenase and other pyruvate-metabolizing shuttle enzymes is exceeded when pyruvate is generated by glycolytic flux. In this mechanism, pyruvate and NAD+ are channelled through LDH, resulting in lactate and NADH [8].
Functions
The generation of ATP via oxidative phosphorylation is impaired when cells are subjected to anaerobic or hypoxic environments. This procedure necessitates the use of alternative metabolism by cells to generate energy. As a result, LDH is upregulated in such circumstances to meet the demand for energy production. Lactate created during anaerobic glucose conversion, on the other hand, reaches a metabolic dead end. It cannot be further processed in any tissue other than the liver. Lactate is thus released into the bloodstream and delivered to the liver, where LDH completes the Cori cycle by converting lactate to pyruvate. However, the muscles run out of oxygen, LDH catalyses the conversion of pyruvate to lactate. Pyruvate is not further processed in erythrocytes due to the lack of mitochondria and instead remains in the cytoplasm, eventually converting to lactate. NADH is oxidised to NAD+ in this process. The preparation phase of glycolysis necessitates the presence of high intracellular concentrations of NAD. Compared to oxidative phosphorylation, which produces 36 ATPs per glucose molecule, anaerobic glycolysis produces only 2 ATP per glucose molecule. The dehydrogenation of 2-hydroxybutyrate can also be catalysed by LDH, but it is a less favoured substrate for LDH than lactate [2,9].
During hyperlactatemia and physical exertion lactate absorption occur which is responsible for 60% of brain metabolism. When compared to normal cells, the function of LDH, specifically LDHA, is altered in cancer cells. Even in the presence of oxygen, cancer cells use LDH to boost their aerobic metabolism (glycolysis, ATP synthesis, and lactate production). The Warburg effect is the name given to this phenomenon [10]. By avoiding the formation of oxidative stress by the Electron Transport Chain (ETC), aberrant cancer cells profit from switching to anaerobic metabolic state. Furthermore, cancer cells have access to the metabolic intermediates of the tricarboxylic acid cycle (TCA cycle), which are produced by glucose and pyruvate, in order to synthesis lipids and nucleic acid for rapid cell multiplication.
Pathophysiology Associated with LDH
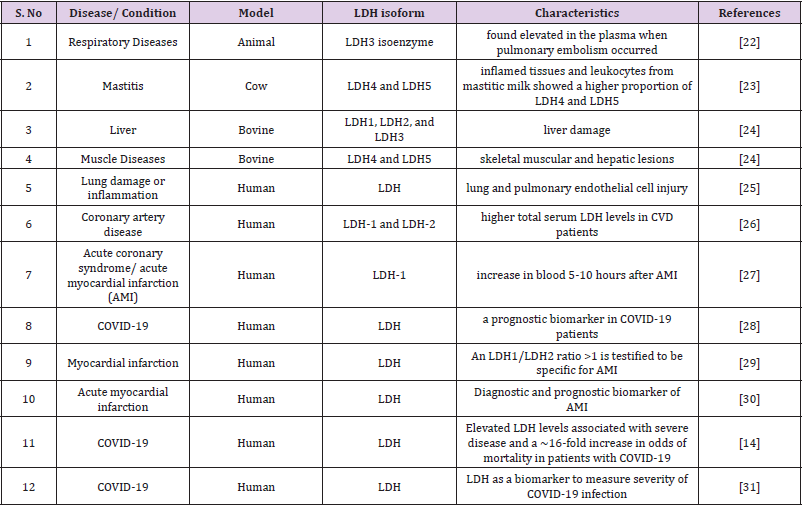
LDH measurement is important in clinical practise because serum LDH isozyme concentrations reflect tissue-specific disease states. As a result of its isozyme form and widespread prevalence, LDH can be employed as a marker for a variety of tissue damage. When cells are damaged, they release LDH into the bloodstream. The enzyme can stay high in the bloodstream for up to 7 days depending on the type of tissue injury. The increased LDH in serum as a result of organ destruction is caused by substantial cell death and cytoplasm loss [2]. Acute Myocardial Infarction (AMI), anaemia, hepatitis, and renal failure are among diseases that can damage tissue. LDH is a useful marker for determining the severity of an illness, assessing prognosis or therapy response, and evaluating body fluids other than blood. A decrease in LDH levels throughout treatment indicates a better prognosis and/or outstanding response to treatment in situations such as AMI or liver injury. From the second to the fourth day after an acute myocardial infarction, the LDH-1 isozyme stays elevated. In cases of liver injury, LDH-5 levels are elevated. A significant increase in LDH-5 above LDH-4 is seen in hepatocellular injuries such as hepatitis or cirrhosis [2]. LDH levels rise in serious bodily fluids including pericardial and peritoneal fluids after effusion. As a result, it is used to describe effusion. LDH levels in the cerebrospinal fluid rise in bacterial meningitis, but remain normal in viral meningitis. The ratio of fluid LDH to the upper limit of normal serum LDH (>0.6) shows that there is an inflammatory process going on, and thus exudate.
During cerebral bleeding, LDH levels rise dramatically. In central nervous system lymphoma, leukaemia, and metastatic carcinoma, there is a more than 40 U/L increase above normal values. Increased levels of more than one isoenzyme could indicate multiple causes of tissue injury, such as in cases where pneumonia is also linked to a heart attack. The presence of high amounts of LDH appears to be linked to severe illness or multiple organ failure. The only serum biomarker that can be used to assess metastatic melanomas is LDH. Because the growth of tumour cells requires more oxygen than the supply, hypoxia is typical in malignancy. To meet the demand for rapid cellular expansion, developing tumours use LDH-mediated energy production. As a result, LDH is a wellestablished indicator of metastases, particularly in the liver. It’s also a critical single prognostic marker, as patients with high LDH have a lower chance of surviving. LDH levels can also be used to predict the occurrence of metastasis in melanoma. In tumours, LDH has a strong relationship with tyrosine kinase expression [11,12]. The metabolic switch (Warburg effect) from oxidative phosphorylation to greater anaerobic glycolysis occurs in most aggressive tumours. Upregulation of the LDH-5 (also known as LDH-A) isoform, which is generally found in muscles and the liver, causes this transition. As a result, inhibiting LDH-5 can target the site of tumour growth and invasiveness directly [13].
LDH is one such biomarker of relevance, particularly as elevated LDH levels have previously been linked to poorer outcomes in patients with other viral infections. Early evidence in COVID-19 patients suggests that there are significant disparities in LDH levels between patients with severe illness and those who do not [14]. In a study by Erika Poggiali et al. (2020) they were observed that, in CoVID-19 individuals, LDH and CRP may be linked to respiratory function (PaO2/FiO2) and may be a prediction of respiratory failure. To avoid a bad prognosis, LDH and CRP should be regarded a valuable test for identifying individuals who require closer respiratory monitoring and more aggressive supportive therapy early on [15]. Yi Han et al. study also found that LDH can be used as a powerful predictor of lung injury in severe COVID-19 patients [16]. The effects of cell growth inhibition and/or cell death have been studied extensively in biological study. Two of the most often used approaches for this purpose are LDH-based cytotoxicity tests [17]. When the plasma membrane is disrupted, LDH is rapidly released into the cell culture supernatant, which is a fundamental aspect of cells suffering apoptosis, necrosis, and other forms of cellular destruction. It could be a potent biomarker for cell viability and cell number in vitro [18,19]. The quantification of LDH release is a commonly used technique for determining cell viability and latestage apoptosis [20]. In the presence of certain bacterial species, bacterial interference with LDH activity led to an underestimate of cytotoxicity, according to a study (Table 1) [21].
LDH as a Diagnostic and Prognostic Marker for Cancer
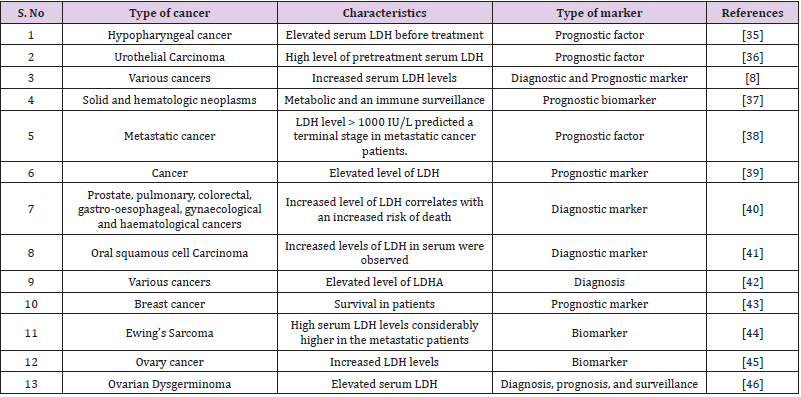
Due to its widespread presence in cells, isoenzymatic tissue specificity, and changes in the expression of individual subunits of this enzyme, which are linked to changes in tissue metabolic function, LDH has become a significant diagnostic parameter in myocardial infarction, liver diseases with hepatic cell damage such as acute liver failure (ALF), hemolytic anaemia, and other pathological conditions. LDH, on the other hand, is now known to be a non-specific cancer diagnostic indication, according to more recent study. Although LDH activity in urine has been recommended as a marker for bladder and kidney neoplasms, it has also been found to be elevated in cases of upper urinary tract infections. When attempting to distinguish benign and malignant tumours based on total LDH activity, similar issues exist. In situations of blood or breast cancer, however, total LDH activity should be measured as a predictive factor. Total LDH activity appears to be a valuable marker in making therapeutic management options for testicular cancer patients [8]. Most human cancer tissues show alterations in the ratio of lactate dehydrogenase isotypes, which tend to enhance the expression of forms with a majority of the A subunit, in addition to increased overall LDH activity. Many cancers, including those of the thyroid, colon, uterus, and ovaries, stomach, kidneys, central nervous system, and lungs, have shown significant alterations in favour of LDH-A isoenzymes. Significant variations in the number of LDH-B isoforms are seen less commonly. LDH-B is overexpressed in lung and breast cancers, and it has been proposed as a potential therapeutic target [32].
In haematological malignancies such as leukaemia, serum LDH is frequently increased. Hodgkin’s Lymphoma (HL) and Non- Lymphoma Hodgkin’s (NHL) are two types of Lymphoma (NHL). Surgical excision of the original tumour resulted in a substantial drop in serum LDH levels during the first week after surgery, according to Colgan et al. LDH levels can rise as a result of tumour spreading, suggesting that LDH could be used as a cancer diagnostic sign. The lack of consistency between LDHA expression in cancer tissues and serum LDH levels could indicate that tumour LDHA expression and serum LDH levels are two separate predictors of cancers [33]. In the case of ovarian cancer, serum LDH may be a useful diagnostic for distinguishing between ovarian carcinoma and benign ovarian tumours (Table 2) [34-46].
Table 2: List of various research studies indicates LDH as a potent biomarker in various types of cancers.
Discussion
Anaerobic metabolism is the principal pathway used during high-intensity exercise to make ATP, and the enzyme activity of LDH is a necessary component for rapid creation of NAD+ from NADH in order to sustain energy production [47]. The LDHA homotetramer, commonly known as “muscle-type” LDH or LDH5, preferentially converts pyruvate to lactate and NAD+ and is the most abundant isozyme in muscle. LDHB homotetramer, commonly known as “heart-type” LDH or LDH1, reduces lactate to pyruvate and NADH and is the most abundant isozyme in the heart. Lactate is a vital oncometabolite that facilitates continuous glycolysis, serves as an energy fuel for oxidative metabolism, and serves as a valuable precursor for biosynthesis, according to recent research. LDH is well known biomarker for tissue injury, inflammation, haemolysis, myocardial infarction and cancer. Various research have also shown that LDH takes role in immunosuppression, and LDH can be regarded as an immune surveillance prognostic marker and its aberrant elevation was antecedent of negative outcomes in cancer patients. The prognostic relevance of pretreatment serum LDH in numerous malignancies is well established, such as lung cancer, breast cancer, gastric cancer, colorectal cancer, and also in others [35]. A research study has shown the importance of timing in LDH measurement in addition to the positive association between prediagnostic LDH and death after cancer diagnosis. Lactate dehydrogenase levels evaluated within 12 months of a cancer diagnosis were found to be strongly linked to general and cancer-specific death, showing that LDH and tumour growth or severity are linked [40]. Various studies have shown that LDH is a prognostic factor for various kinds of cancers (Table 2).
In conclusion, changes in LDH enzyme activity and/or LDH isoform composition are widely recognised as indicators of enhanced glycolysis in quickly developing tumour cells. However, these changes in LDH activity have a limited specificity and sensitivity, therefore there is need to develop a sensitive assay to detect LDH level with picogram sensitivity. A sensitive and specific detection of LDH have potential to be used as diagnostic biomarker for cancer detection.
Acknowledgements
All authors thankful to HOD, Department of Biochemistry & Genetics, Barkatullah University, Bhopal for providing facilities.
Conflicts of Interests
The authors disclose that they don’t have any conflicts of interests.
References
- Goto T, Sugawara K, Nakamura S, Kidokoro SI, Wakui H, et al. (2016) Enzymatic and thermodynamic profiles of a heterotetramer lactate dehydrogenase isozyme in swine. Biochem Biophys Res Commun 479(4): 860-867.
- Farhana A, Lappin SL (2021) Biochemistry, lactate dehydrogenase.
- Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB (2015) Lactate is always the end product of glycolysis. Front Neurosci 9: 22.
- Friberg A, Rehwinkel H, Nguyen D, Pütter V, Quanz M, et al. (2020) Structural evidence for isoform-selective allosteric inhibition of lactate dehydrogenase A. ACS Omega 5(22): 13034-13041.
- Duka T, Anderson SM, Collins Z, Raghanti MA, Ely JJ, et al. (2014) Synaptosomal lactate dehydrogenase isoenzyme composition is shifted toward aerobic forms in primate brain evolution. Brain Behav Evol 83(3): 216-230.
- Young A, Oldford C, Mailloux RJ (2020) Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol 28: 101339.
- Gulotta M, Deng H, Deng H, Dyer RB, Callender RH (2002) Toward an understanding of the role of dynamics on enzymatic catalysis in lactate dehydrogenase. Biochemistry 41(10): 3353-3363.
- Forkasiewicz A, Dorociak M, Stach K, Szelachowski P, Tabola R, et al. (2020) The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol Biol Lett 25: 35.
- Melkonian EA, Schury MP (2019) Anaerobic glycolysis. Biochemistry.
- Goodwin ML, Gladden LB, Nijsten MW (2020) Lactate-Protected Hypoglycemia (LPH). Front Neurosci 14: 920.
- Palmer SR, Erickson LA, Ichetovkin I, Knauer DJ, Markovic SN (2011) Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin Proc 86(10): 981-990.
- Jurisic V, Radenkovic S, Konjevic G (2015) The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv Exp Med Biol 267: 115-124.
- Mishra D, Banerjee D (2019) Lactate dehydrogenases as metabolic links between tumor and stroma in the tumor microenvironment. Cancers (Basel) 11(6): 750.
- Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, et al. (2020) Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med 38(9): 1722-1726.
- Poggiali E, Zaino D, Immovilli P, Rovero L, Losi G, et al. (2020) Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clinica Chim Acta 509: 135-138.
- Han Y, Zhang H, Mu S, Wei W, Jin C, et al. (2020) Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY) 12(12): 11245-11258.
- Smith SM, Wunder MB, Norris DA, Shellman YG (2011) A simple protocol for using a LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PloS One 6(11): e26908.
- Kumar P, Nagarajan A, Uchil PD (2018) Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb Protoc 2018(6): pdb-rot095497.
- Allen M, Millett P, Dawes E, Rushton N (1994) Lactate dehydrogenase activity as a rapid and sensitive test for the quantification of cell numbers in vitro. Clin Mater 16(4): 189-194.
- Kaja S, Payne AJ, Singh T, Ghuman JK, Sieck EG, et al. (2015) An optimized lactate dehydrogenase release assay for screening of drug candidates in neuroscience. J Pharmacol Toxicol Methods 73: 1-6.
- Van den Bossche S, Vandeplassche E, Ostyn L, Coenye T, Crabbé A (2020) Bacterial interference with lactate dehydrogenase assay leads to an underestimation of cytotoxicity. Front Cell Infect Microbiol 10: 494.
- Hagadorn JE, Bloor CM, Yang MS (1971) Elevated plasma activity of lactate dehydrogenase isoenzyme-3 (LDH3) in experimentally induced immunologic lung injury. Am J Pathol 64(3): 575-581.
- Bogin E, Ziv G, Avidar J, Rivetz B, Gordin S, Saran A. Distribution of lactate dehydrogenase isoenzymes in normal and inflamed bovine udders and milk. Res Vet Sci 22(2): 198-200.
- Keller P (1974) Lactate dehydrogenase isoenzymes in normal bovine serum and during experimental liver and muscle damage. Res Vet Sci 17(1): 49-58.
- Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M (1996) Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 9(8): 1736-1742.
- Danese E, Montagnana M (2016) An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med 4(10): 194.
- Li P, Wu W, Zhang T, Wang Z, Li J, et al. (2021) Implications of cardiac markers in risk-stratification and management for COVID-19 patients. Crit Care 25(1): 158.
- Aydin S, Ugur K, Aydin S, Sahin İ, Yardim M (2019) Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag 15: 1-10.
- Chen Y, Tao Y, Zhang L, Xu W, Zhou X (2019) Diagnostic and prognostic value of biomarkers in acute myocardial infarction. Postgraduate Med J 95(1122): 210-216.
- Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A (2020) The role of biomarkers in diagnosis of COVID-19-A systematic review. Life Sci 254: 117788.
- Papaneophytou C, Zervou ME, Theofanous A (2020) Optimization of a colorimetric assay to determine lactate dehydrogenase b activity using design of experiments. SLAS DISCOVERY: Advancing the Science of Drug Discovery 26(3): 383-399.
- Feng Y, Xiong Y, Qiao T, Li X, Jia L, et al. (2018) Lactate dehydrogenase A: a key player in carcinogenesis and potential target in cancer therapy. Cancer Med 7(12): 6124-6136.
- Ikeda A, Yamaguchi K, Yamakage H, Abiko K, Satoh-Asahara N, et al. (2020) Serum lactate dehydrogenase is a possible predictor of platinum resistance in ovarian cancer. Obstet Gynecol Sci 63(6): 709-718.
- Wu J, You K, Chen C, Zhong H, Jiang Y, et al. (2021) High Pretreatment LDH Predicts Poor Prognosis in Hypopharyngeal Cancer. Front Oncol 11: 641682.
- Wu M, Lin P, Xu L, Yu Z, Chen Q, et al. (2020) Prognostic role of serum lactate dehydrogenase in patients with urothelial carcinoma: a systematic review and meta-analysis. Front Oncol 10: 677.
- Ding J, Karp JE, Emadi A (2017) Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark 19(4): 353-363.
- Liu R, Cao J, Gao X, Zhang J, Wang L, et al. (2016) Overall survival of cancer patients with serum lactate dehydrogenase greater than 1000 IU/L. Tumor Biol 37(10): 14083-14088.
- Lianidou ES, Markou A, Strati A (2015) The role of CTCs as tumor biomarkers. Adv Exp Med Biol 867: 341-367.
- Wulaningsih W, Holmberg L, Garmo H, Malmstrom H, Lambe M, et al. (2015) Serum lactate dehydrogenase and survival following cancer diagnosis. Br J Cancer 113(9): 1389-1396.
- Pereira T, Shetty S, Pereira S (2015) Estimation of serum lactate dehydrogenase level in patients with oral premalignant lesions/conditions and oral squamous cell carcinoma: a clinicopathological study. J Cancer Res Ther 11(1): 78-82.
- Miao P, Sheng S, Sun X, Liu J, Huang G (2013) Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Llife 65(11): 904-910.
- Brown JE, Cook RJ, Lipton A, Coleman RE (2012) Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate-treated patients. Clin Cancer Res 18(22): 6348-6355.
- Bacci G, Avella M, McDonald D, Toni A, Orlandi M, et al. (1988) Serum lactate dehydrogenase (LDH) as a tumor marker in Ewing's sarcoma. Tumori J 74(6): 649-655.
- Patel PS, Sharma VM, Raval GN, Rawal RM, Patel MM, et al. (1996) Serum lactate dehydrogenase levels in malignant germ cell tumors of ovary. Int J Gynecol Cancer 6(4): 328-332.
- Pode D, Kopolovic S, Gimmon Z (1984) Serum lactic dehydrogenase: A tumor marker of ovarian dysgerminoma in a female pseudohermaphrodite. Gynecol Oncol 19(1): 110-113.
- Lee SH, Pekas EJ, Lee S, Headid III RJ, Park SY (2020) The impact of aspirin intake on lactate dehydrogenase, arterial stiffness, and oxidative stress during high‐intensity exercise: a pilot study. J Hum Kinet 72: 101-113.
- Kalezic A, Udicki M, Srdic Galic B, Aleksic M, Korac A, et al. (2020) Lactate metabolism in breast cancer microenvironment: contribution focused on associated adipose tissue and obesity. Int J Mol Sci 21(24): 9676.

 Research Article
Research Article