Abstract
Background: Malnutrition is present in 25 to 54% of hospitalized patients upon admission and it has a direct association with increased morbidity, mortality, length of stay (LOS), increased readmissions, and cost of care. The high level of insulin will block the lipolysis and the usage of FAs as the primary fuel (insulin inhibits ketogenesis). Therefore, the body will shift towards gluconeogenesis and use amino acids as the main fuel. This process will lead to protein and skeletal muscle wasting and increase the risk of malnutrition and other postoperative complications. Prehabilitation includes the process of improving the patient’s overall condition before surgery to keep a higher level of the patient’s functional body capacity during surgery and also inhibit postoperative consequences including complications caused by metabolic stress. One of the best practical methods of prehabilitation is the Enhanced Recovery After Surgery (ERAS) protocol. ERAS is a multi-professional model to educate patients and improve their physical and nutritional status prior to surgery. The ERAS protocol mainly targets the inflammatory responses and hormonal changes during metabolic stress. This can alter the metabolism leading to suppression of protein-sparing resulting in a decrease in protein wasting. The ERAS program was initially started at CRMC as a pilot quality improvement project in 2017. Furthermore, there is no standardized protocol for ERAS, especially on Oral Nutrition Supplementation (ONS). To date, there is sufficient data to support the benefits of oral nutrition supplementation for patients undergoing metabolic stress. Nonetheless, there is not enough evidence regarding the effectiveness of any specific product over others for improving the patients’ nutritional status prior to surgery and the patients’ overall survival and complications post surgery.
Materials and Methods: In this review paper we sought to compare some of the most common nutritional supplements and their ingredients used for ERAS programs in the United States by focusing on the cell signaling effect they may have on metabolism, protein sparing, some elective amino acids, insulin resistance, and glycemic index. Results: The main results revealed that an optimal oral nutrition supplementation should provide an opportunity to trigger the cell signaling pathways that would increase the transcriptional level of endogenous protein synthase while other ingredients would provide further benefits. Conclusion: Despite several review articles and clinical trials and clinical outcome measurements, there is very limited metabolic research on prehabilitation biochemical mechanisms and cell signaling responses pathways. There is an absolute need for mechanistic studies that will help to select the most appropriate formulas.Keywords: Length of Stay (LOS); Enhanced Recovery After Surgery (ERAS); Oral Nutrition Supplementation (ONS)
Introduction
Major surgeries may cause several metabolic derangements in
patients’ bodies known as metabolic stress. In this situation, The
Basal metabolic Rate (BMR) will increase, and the body will go
through several hormonal changes including a higher insulin level.
Subsequently, these hormonal imbalances will trigger changes in
metabolic pathways [1,2]. In healthy individuals, when the protein
or glucose intake is high, insulin, an anabolic hormone, is secreted
by the beta cells of the pancreas. It would stimulate glycogenesis
and the uptake of glucose into muscles (for fuel) and fat cells (for
storage as triglyceride) via Glucose Transporter 4 receptor (GLU4).
However, in metabolic stress situations, cells develop insulin
resistance based on stress, which continues for up to three weeks
even after uncomplicated, moderate surgery. Likewise, common
perioperative experiences like nothing per mouth (NPO) status,
pain, and bed rest can also contribute to a reduced sensitivity to
insulin as well [3-5]. In situations such as starvation, the drop of
glucose level will cause a reduction in insulin levels and result in a
condition known as glucose repression [6].
Glucose repression includes a cascade of genes and protein
expression changes to switch to alternative fuels for different cells.
This change is due to the higher ratio of AMP/ATP, activation of the
adenosine monophosphate protein kinase (AMPK) pathway, and
consequently blockade of the Mammalian Target of Rapamycin
(mTOR) pathway. The result is Fatty acids (FA) oxidation of stored
triglycerides and the formation of ketone bodies as the primary fuel.
Please see Figure 1 for more details [7-9]. However; in metabolic
stress, as mentioned above, the insulin level is very high, and it
will block the lipolysis and beta-oxidation, resulting in decreased
usage of FA as the primary fuel (the presence of insulin inhibits
ketogenesis). Therefore the body will shift to gluconeogenesis
and will use Amino Acids as the main fuel [7,10,11]. This shift to
gluconeogenesis would initially affect the muscles by increasing
the whole body and tissue-specific protein turnover which would
consequently increase the free amino acid pool circulating in the
body. Secondly, it would decrease the uptake of amino acids into the
skeletal muscle as well [11]. Furthermore, metabolic stress also will
cause a cascade of inflammatory responses. The liver would retain
amino acids to synthesize acute phase proteins like Tumor necrosis
factor (TNF-alpha), C-Reactive Protein (CRP), and Interleukins
to send signals to the immune system, which will result in the
sparing of body proteins as well [2,12]. The scientific outcomes of
this negative protein balance can cause skeletal muscle wasting,
respiratory impairment, fatigue, higher risk of malnutrition, and
diminished mTOR signaling and muscle protein synthesis [1,2,12].
This condition would increase the risk of complications by six-folds
and severe infection by ten folds especially in major surgeries and
large burn wounds [13].
Although a well-nourished adolescent patient might not
experience a severe form of these consequences during metabolic
stress, this condition may cause serious complications known
as ‘catabolic crisis’ in malnourished or elderly patients. For this
reason, the patient’s health conditions prior to surgery including
obesity, metabolic syndrome, diabetes, and low insulin sensitivity
could impact the adverse outcomes after major surgeries [3,11,14].
Prehabilitation includes improving the patient’s overall condition
before surgery to keep a higher level of functional capacity
during and immediately after surgery. It also aims at decreasing
postoperative deleterious consequences such as complications
caused by metabolic stress and postoperative catabolism [15,11].
The use of prehabilitation is increasing in hospital settings for
high-risk patients, since evidence has shown better postoperative
outcomes, only minor infections, shorter length of stay, fewer
readmissions, and a dramatic decrease in narcotic pain medication
requirement. Several smallrandomized trials have demonstrated
that multimodal prehabilitation enhances pre- and postoperative
functional capacity in elective surgical patients. One of the best
practical methods of prehabilitation is Enhanced Recovery After
Surgery (ERAS) [11,16-18].
ERAS is a multi-professional model to educate patients and
improve their physical and nutritional status prior to surgery. ERAS
is designed to reduce complications, hospital length of stay (LOS),
and overall elective surgery setting costs. Since its introduction by
Kehlet in the 1990s, ERAS has shown several benefits in patients
undergoing elective surgeries including colorectal, gynecological
and urological surgery [19-22]. ERAS protocol mainly focuses
on the inflammatory responses and hormonal changes during
metabolic stress. This effort includes medical optimization,
psychological support, physical exercise, and nutritional support.
These interventions are provided by a multidisciplinary team
consisting of physicians, nurses, geriatricians, physiotherapists,
nutritionists, and psychologists [11,23,24]. Other than commercial
recommendations, there is no globally accepted protocol for ERAS
for oral nutrition supplements (ONS). The primary effect of ONS
in ERAS is unclear, and some of the perioperative supplements
might have limited efficiency on postoperative outcomes, if the
preoperative risk factors are not addressed properly [3,25-27].
As described by Gündoğdu, currently there are three main
categories of ONS available for ERAS. They are generally utilized
to prepare patients for major surgeries depending on the patient’s
malnutrition status and health condition [28].
1. Oral carbohydrate supplementation: It is administered for
metabolic preparation mainly via increasing insulin sensitivity.
2. High protein supplementation: It is used for severely
malnourished patients with or without metabolic stress risk to reduce the complications after surgery. This group would
benefit the ONS more than well-nourished patients.
3. Immunonutrition supplementation: It is utilized to improve
the immune system and gastrointestinal barrier.
In this review paper we sought to compare some of the most
common nutritional supplements and their ingredients used for
ERAS programs in the US by focusing on the cell signaling effect
that they may have on metabolism, protein sparing, some elective
amino acids, insulin resistance, and glycemic index.
Essential Ingredients used in the Majority of ONS
Oral Carbohydrate: Clear Carbohydrate drink is one of
the most commonly used ONS in ERAS protocols. This group
of ONS contains Maltodextrin (CF(Preop)®) or a mixture of
Corn Maltodextrin, Fructose, Sucralose, Acesulfame Potassium
(Ensure® Pre-Surgery Clear Carbohydrate). Several studies have
shown that consuming two bottles of this carbohydrate drink can
enhance insulin sensitivity and decrease a patients’ starvation
time compared to starving patients or patients consuming water
only [23,27,28]. Previous studies also have shown consumption of
these carbohydrate rich drinks could improve enterocytes function
after surgery. In addition, preoperative carbohydrate loading was
an independent predictor of positive clinical outcomes in patients
undergoing colorectal surgery [29]. Maltodextrin is a small
polysaccharide and a by-product of hydrolyzing starches. According
to the FDA, maltodextrin is a GRAS (Generally Recognized as Safe)
food additive. From a Glycemic Index (GI) standpoint, maltodextrin
is categorized as high GI, even higher than sucrose. Therefore,
maltodextrin consumption could result in a significant increase
in blood sugar levels [30]. Furthermore, all of the mentioned
sugar substitutes, including maltodextrin, fructose, sucralose, and
acesulfame potassium can alter the gut microbiome and affect the
balance of gut bacteria and cause insulin resistance in the long term
[31,32]. However, since the usage of these supplements is limited to
the day of surgery, the probability of that aforementioned problem,
in the long run, is low. Likewise, Chromium (Cr) is a trace mineral
that can improve insulin sensitivity 7 and exists in Impact Advanced
Recovery® (Nestlé) 33mcg and Ensure® Surgery (Abbott) 12mcg.
Zinc: Zinc is the essential element for the function of more than
100 metalloenzymes, including those used for protein synthesis.
It is mainly stored in muscles and bones [7]. There are several
established functions for zinc, including improving the healing
process for wounds, tissue repair and regeneration, and production
of DNA and RNA. It is also part of the enzymes and proteins that
repair skin cells and enhance their proliferation [33,34]. Metabolic
stress may cause a reduction in the serum zinc concentration,
which negatively affects its anti-inflammatory and wound-healing
properties [35]. Therefore it is recommended to incorporate
zinc into the ERAS ONS, with the cautionary note that a higher
intake of zinc (more than 40 mg/day) may suppress the immune
system [36,37]. The two examples of main ERAS ONS used in the
US are Impact Advanced Recovery® (Nestlé) and Ensure® Surgery
(Abbott). Both of these supplements contain 5mg zinc/bottle,
and the recommended intake is two bottles/day for at least five
days prior to surgery which renders both of them safe from a zinc
toxicity standpoint.
Omega -3 Fatty Acids Supplementation: As mentioned
above Ensure® Surgery (Abbott) and Impact Advanced Recovery®
(Nestlé) are two main ONS used in pre-surgery settings, marketed
as Immunonutrition supplements. Both of these drinks contain
1100 mg/bottle of Omega-3 fatty acids as Fish oil from several fatty
fishes. Fish oils mainly contain Eicosatetraenoic acid or EPA (20 C,
and five double bonds) and Docosahexaenoic acid or DHA (22 C,
and six double bonds). Since the main omega-3 fatty acids in these
products are provided by fish oil, none of them are appropriate for
vegans. Oppositely, Ensure® Enlive (Abbott) contains Canola oil
which is rich in Alpha-linolenic acid or ALA (18C and three double
bonds) [7]. Unlike plants, vertebrates lack the enzymes needed to
incorporate a double bond beyond C # 9 in the chain. However,
given a delta 9,12 fatty acid (ALA) from the diet, additional double
bonds can be incorporated, and carbon chains can be elongated to
make more complex fatty acids, including the anti-inflammatory
markers like cyclooxygenase, lipoxygenase, prostaglandins,
leukotrienes [7]. Also, as shown by Hassman et al, Omega-3 fatty
acids can diminish inflammation by providing specialized proresolving
mediators (SPMs), which can decrease the production of
pro-inflammatory cytokines [38]. The meaningful clinical effects
of omega-3 for prehabilitation ONS in reducing mortality and a
patients’ overall outcomes have been observed by a combination of
omega-3 with high protein supplementation. These improvements
were independent of the omega-3 fatty acids type (ALA vs. EPA &
DHA) [18,39] and were not seen if offered individually, even in the
form of DHA and EPA [40,41].
Nucleotides: Purine, pyrimidine bases, ribose, and phosphoric
acid are needed to synthesize deoxynucleic acid (DNA), ribonucleic
acid (RNA), and ATP. They are required for cell growth, proliferation,
and differentiation. Therefore, they play a vital role in rapidly
dividing cells, including lymphocytes and enterocytes, and the
maintenance and restoration of the immune response [42]. Akyuz
emphasizes supplementation with nucleotide as part of an immune
nutrition supplement (including omega-3 fatty acids, arginine, and
nucleotides) that can protect the enterocytes against chemotherapy
damage [43]. Although the body can synthesize these nucleotides
during metabolic stress, their formation would be altered because
of the hormonal and metabolic changes [44]. The only commercially
available ONS in the US with nucleotides is Impact Advanced
Recovery® (Nestlé), with 430mg dietary nucleotides.
3.0.1. High Protein Supplementation: As mentioned before,
the metabolic stress of major surgeries stimulates a catabolic state
which increases gluconeogenesis and causes a higher need for
proteins in general. It has been shown that patients going through
uncomplicated elective surgery usually lose ∼2 kg total lean mass
within the first six weeks after surgery [45-47]. Although required
protein intakes for patients undergoing major surgeries are not
very well-identified, the American Society of Parenteral and Enteral
Nutrition (ASPEN) and the European Society of Clinical Nutrition
and Metabolism (ESPEN) guidelines recommend at least 1.2–2.0
gr/kg/day for during metabolic stress [48,49]. A study by Gillis
emphasizes that 30% of patients undergoing colorectal surgery are
malnourished and are not meeting their protein need via their diet
intake alone [50]. Another study conducted by Yeung compared
ERAS protocols with conventional care regarding protein intake
and showed that even though ERAS patients consume more protein
(mainly via ONS), neither ERAS nor conventional care patients
meet the required protein intake [47]. Well-nourished patients
with a functional digestive system can initiate their oral intake 24
hours after surgery to achieve most of their dietary needs. However,
any delay to restarting oral intake is connected to the higher rate
of infections and lower survival rate [29,51]. Several meta-analyses
emphasize an increased risk of vomiting and postoperative
aspiration related to early oral intake as well [29]. These results
very well establish the importance of high protein supplementation
prior to surgery to meet the patient’s requirements. There are
many different ONS in several forms (liquid, powder) to provide
different amino acids for gluconeogenesis caused by metabolic
stress and prevent the body from going through muscle wasting
and malnutrition due to metabolic stress. Several studies have
shown the effectiveness of different amino acids and high protein
supplements. However, these types of supplementations seem
to be more effective in malnourished patients undergoing major
surgeries or critically ill patients [25,47,52,53].
Single Amino Acid Supplements
Arginine: Arginine (ARG) is commonly categorized as a
nonessential amino acid, but it becomes conditionally essential
in situations like metabolic stress [54]. The main site for arginine
metabolism is the liver and kidney. The kidney can convert
citrulline to arginine, then some of this endogenous arginine would
be transported into the blood to be used by other organs. Please see
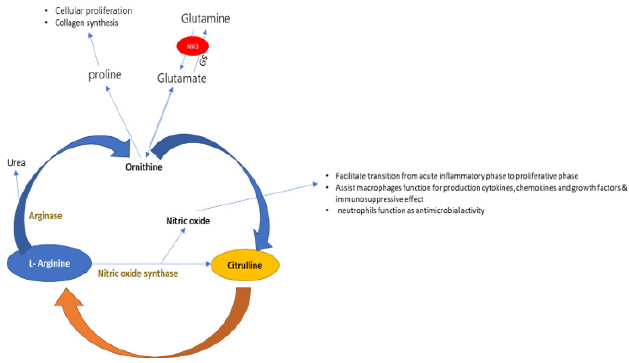
Figure 2 for more details [55]. There are several known functions
for ARG, but the most important one is the production of Nitric
Oxide (NO). Since its discovery in 1987, many biological roles have
been established for NO. It is a critical molecule in vascular dilation,
neurotransmission, acute and chronic inflammation, and the
immune system [56]. Different cells, including macrophages and
neutrophils, use ARG to make NO [57]. It also has been suggested
that the presence of NO generated from the ARG-NO pathway
facilitates the shift of a wound from the acute inflammatory
phase to the proliferative phase of wound healing [35,38]. ARG
supplementation may increase NO production in different cells,
including immune cells and endothelial cells.58 Another by-product
of the ARG-Urea pathway in Ornithine. Ornithine can be converted
to L-proline, a substrate for collagen synthesis, and polyamines,
stimulating cellular proliferation [58].
Interestingly, supplementation with L-citrulline increases
levels of circulating L-arginine more than supplementing with ARG
itself [59,60] In a normal situation, around half of the consumed
ARG would be entered into the portal vein and the other half will
be directly used by enterocytes or will be degraded [35]. ARG
supplementation is generally safe when the consumed amount
is 20 grams or less per day, but it could trigger gastrointestinal
symptoms at quantities as low as 5 grams per day [61]. L-citrulline
is claimed to be one of the ingredients in CF(Preop)® but there is
no information about the quantity in the nutrition facts about this
ingredient. Studies have shown that major surgical procedures
can diminish circulating ARG due to more ARG breakdown for NO
synthases and less endogenous ARG production [62]. The two main
ERAS ONS used in the US are Impact Advanced Recovery® (Nestlé)
and Ensure®® Surgery (Abbott). Both of these supplements contain
4.2 g ARG/bottle. The other ONS Ensure® Enlive, which is mainly
recommended for postoperative care, does not have any additional
ARG.
Glutamine: Glutamine (GLN) is another nonessential amino
acid. The majority of cells and tissues can synthesize glutamine
from glutamate and ammonia, in a process catalyzed by the enzyme
glutamine synthetase (GS). GLN is also a precursor of glutathione, an
important ingredient of glutathione peroxidase (a major antioxidant
enzyme). During metabolic stress, GLN is the main fuel for rapidly
dividing cells, including gastrointestinal cells, epithelial cells, and
immune cells as well as it protects the digestive barrier against
infection [63,64]. Also, by blocking the activity of NFκB and STATE
proteins GLN acts as an anti-inflammatory marker [65-66]. Several
studies also have confirmed the effect of GLN on cell differentiation
regulation, mucin formation, and nucleotide synthesis stimulation
[67-69]. However, the best effect of GLN supplementation happens
when it has been combined with other amino acids, especially
BCAA and/or HMB [70]. For example, a clinical trial has compared
two different ONS containing GLN (Free GLN vs. GLN-Alanine) and
showed both of these ONS had the same effect on promoting neovascularization
and improving skin flap survival in rats [71]. The
Most common ONS in the US for ERAS protocol with GLN is Impact
Advanced Recovery® (Nestlé) with 2.8g dietary GLN. Neither
Ensure® Enlive nor Ensure® surgery contains GLN. Additionally,
Selenium is required for synthesizing glutathione peroxidase [7]
and it exists in Impact Advanced Recovery® (Nestlé) 16mcg and
Ensure® Surgery (Abbott) 19mcg.
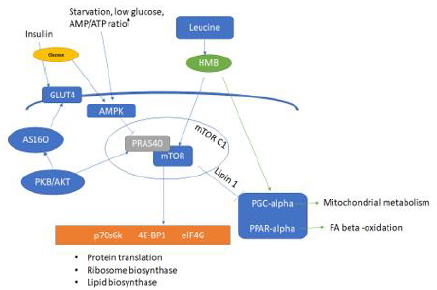
Figure 1: Activation of the mammalian target of rapamycin (mTOR) pathway in the muscle protein synthesis by β-hydroxy
β-methylbutyrate (HMB) and anabolic factors.
Note: Adapted with permission from “Leucine stimulates mTOR and muscle protein synthesis in both animal and human, GD
Pimentel, JCS Zemdegs - efdeportes.com”
PKB/Akt: protein kinase B, AS160: Akt substrate of 160 kDa, PRAS40:proline-rich Akt substrate of 40 kDa, AMPK: adenosine
monophosphate protein kinase, mTOR: mammalian target of rapamycin, p70S6K: ribosomal protein S6 kinase, 4E-BP1:
eukaryotic initiation factor 4E binding protein 1, eIF4G: eukaryotic initiation factor 4G. PGC-alpha: Peroxisome proliferatoractivated
receptor-gamma coactivator (PGC)-1alpha, PPAR-alpha: Peroxisome proliferator-activated receptor-alpha, lipin-1:
Phosphatidate phosphatase-1.
Figure 2: Biosynthesis of Nitric oxide. adapted with permission from “The Nitric Oxide Pathway in Pulmonary Vascular Disease” James R. Klinger, Philip J. Kadowitz, American Journal of Cardiology Volume 120 Issue 8 Pages S71-S79 (October 2017).
Alanine: Alanine is a nonessential amino acid. During
metabolic stress, when the body shifts to gluconeogenesis for
energy production, alanine is the primary fuel. This would increase
the importance of alanine supplementation during metabolic
stress to provide the amino acid precursor for gluconeogenesis and
protect the body’s protein and skeletal muscles. This can decrease
muscle wasting and protein malnutrition [52,72]. The main concern
with using alanine supplementation is that it would cause a slight
increase in circulating alanine aminotransferase concentration
which may interfere with liver function tests [73]. Neither Ensure®
Enlive, Ensure® Surgery (Abbott), nor Impact Advanced Recovery®
(Nestlé) contain alanine. β-hydroxy β-methyl butyrate (HMB)
Branch Chain Amino Acids (BCAA), especially Leucine and its
metabolite β-hydroxy β-methyl butyrate (HMB), play an important
role in maintaining skeletal muscle. Approximately 70% of BCAAs
are metabolized in skeletal muscles [7]. Studies have also shown
that HMB can interact with the mTOR complex-1 pathway to
promote muscle protein synthesis [7,74-75]. Please see Figure 1
for more details. Supplementation with HMB has been studied in
elderly patients and athletes. HMB has been used in several overthe-
counter products and supplements to improve muscle function
and increase lean body mass [76-78]. He X and Stancliffe R, in two
different studies, stated that the effect of HMB is by increasing
gene expression of peroxisome proliferator-activated receptorgamma
coactivator 1-alpha (PGC-1α), which is a master regulator
of mitochondrial metabolism 74 and mTOR complex-1 pathway,
which control the protein biogenesis [7,79-80].
Although Tokunaga has shown that Leucine can promote p70α
phosphorylation via the mTOR complex-1 pathway by serving as a
mitochondrial fuel 9, further studies have clarified that this effect
is not directly via Leucine and is mainly through its metabolite
HMB [80]. As shown in Figure 1, HMB’s effect on reducing the
muscle protein degradation and improving the muscle mass is
independent of ARG and GLN [70]. Hsieh has further explained
that HMB supplementation might have anti-inflammatory effects
and improve pulmonary function in COPD patients in an intensive
care unit setting. The evidence indicates a lower level of CRP and
ventilator modes improvement [81]. It can also decrease apoptosis
and increase cell proliferation and has been used safely in patients
with malnutrition, cancer, chronic disease, sepsis, and HIV [82].
In healthy individuals, only 5% of Leucine would be converted
to HMB. Therefore the increased requirements for HMB due to
metabolic stress cannot be met by a regular diet, Leucine, or
high protein supplementations [83]. Despite all of these essential
functions on protein synthase and mitochondrial biogenesis, HMB
is not one of the ingredients in the majority of ERAS ONS. Only
Ensure® Enlive (Abbott) contains HMB, while this supplement is
only recommended for postoperative care by the company.
The above facts suggest that the main focus of current available
ERAS ONS recommendations is boosting the Immunonutrition,
improvement, and maintenance of the intestinal barrier and
providing the fuel for rapidly dividing cells rather than enhancing
the endogenous protein synthase or mitochondrial biogenesis. We
have found a study that compared the effect of Immunonutrition
boosting vs. regular ERAS ONS (with or without ARG, RNA, and
omega-3 fatty acids) on patients undergoing colorectal surgeries. It
showed that the median length of postoperative hospital stay was five
days with no differences between the groups. The only statistically
significant change in this study was a lower rate of wound infection
in the Immunonutrition group [25]. Since in normal conditions,
the main path for HMB would be a conversion to HMG-CoA, HMB
supplementation might increase the cholesterol biogenesis [7,83].
The advantage of combining HMB with a high protein nutrition
supplement (Ensure® Enlive) could be hypothesized that HMB
exerts its effect by increasing the transcriptional level of protein
synthase, while other high protein supplements or amino acid
containing products like Glutamine [63,64], or Arginine [84] only
provide protein/amino acids to the pool for patients. It is worth
mentioning that the body’s preferred fuel during metabolic stress
is an endogenous protein, rather than an exogenous diet [63,64,85].
Conclusion
Despite the increasing interest in the usage of ONS as part of prehabilitation programs, many areas remain unclear. While several review articles and clinical trials have shown improvements in clinical outcomes for patients adhering to perioperative ERAS protocols as mentioned above, other studies beg to differ [29]. Multiple publications have emphasized that currently there is no clear evidence for the sole use of specific amino acids, Omega-3 FAs, or antioxidants vs. standard oral nutritional supplements (ONS) in the preoperative period unless patients suffer from severe malnutrition prior to surgery [40,52,86]. The current recommendation from ESPEN mentions that prehabilitation with a specific formula enriched with arginine, omega-3-fatty acids, and nucleotides should only be offered to malnourished patients undergoing major surgeries [52]. Furthermore, there is very limited metabolic research on biochemical mechanisms and cell signaling pathways for different ONS interventions. These mechanistic studies can help us to better understand the body’s response to the ONS ingredients which in turn results in more effective formulations and proper dosage [11,39,52].
Summary
In this paper, we sought to introduce the most commonly used oral nutrition supplements in the prehabilitation protocols in the US that are commercially available. Additionally, we have tried to further analyze supplements and explain their major ingredients Table 1. summarizes the findings. The biochemical characteristics of these active ingredients and their effect at the molecular and cellular level are explained in detail as well. To our knowledge, this is the first review paper that gathers and collectively compares different types of ONS. Based on this review, we hypothesize that an optimal oral nutrition supplementation should provide an opportunity to trigger the cell signaling pathways that would increase the transcriptional level of endogenous protein synthase while other ingredients would provide further benefits. We hope that this detailed understanding of these ONS and their ingredients will help the providers in determining the proper supplementation for their patients, based on their individualized and complex needs. Also, we hope that this paper could pique some interest in designing human trials to better understand the mechanisms of function, proper dosage, and optimal formulations for future types of nutrition supplementation.
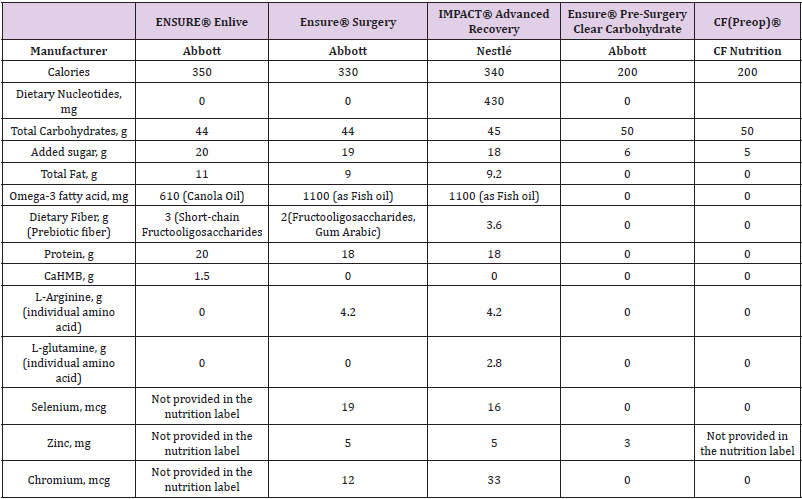
Table 1: Most common nutritional supplements and their ingredients used for ERAS programs in the United States.
Conflict of Interest
There is no conflict of interest.
References
- Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN (2013) The surgically induced stress response. JPEN J parenter Enteral Nutr 37(5): 21S-29S.
- Parlato M, Cavaillon JM (2015) Host response biomarkers in the diagnosis of sepsis: a general overview. Methods Mol Biol 1237: 149-211.
- Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramirez B, Andrada P, et al. (2015) Expression of S6K1 in human visceral adipose tissue is upregulated in obesity and related to insulin resistance and inflammation 52(2): 257-266.
- Blixt C, Larsson M, Isaksson B, Ljungqvist O, Rooyackers O (2021) The effect of glucose control in liver surgery on glucose kinetics and insulin resistance. Clinical Nutrition 40(7): 4526-4534.
- Schram A (2017) Strategies for Minimizing Bed Rest in Post-Operative Colorectal Cancer Patients: Exercising to ERAS. McGill University (Canada).
- Steinhauser ML, Olenchock BA, O'Keefe J, Lun M, Pierce KA, et al. (2018) The circulating metabolome of human starvation. JCI Insight 3(16): e121434.
- Gropper SS, Smith JL (2012) Advanced nutrition and human metabolism. Cengage Learning.
- Pimentel GD, Zemdegs JCS (2019) Leucine stimulates mTOR and muscle protein synthesis in both animal and human.
- Tokunaga C, Yoshino K-i, Yonezawa K (2004) mTOR integrates amino acid-and energy-sensing pathways. Biochemical and biophysical research communications 313(2): 443-446.
- Laidlaw KM, Bisinski DD, Shashkova S, Paine KM, Veillon MA, et al. (2021) A glucose-starvation response governs endocytic trafficking and eisosomal retention of surface cargoes in budding yeast. Journal of cell science 134(2): jcs257733.
- Chabot K, Gillis C, Carli F (2020) Prehabilitation: metabolic considerations. Cur Opin Clin Nutr Metab Care 23(4): 271-276.
- Nicholson A, Lowe M, Parker J, Lewis S, Alderson P, et al. (2014) Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. Centre for Reviews and Dissemination (UK) 101(3): 172-188.
- Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN (2013) The surgically induced stress response. Journal of parenteral and enteral nutrition 37: 21S-29S.
- Lieffers J, Bathe O, Fassbender K, Winget M, Baracos VE (2012) Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. BR J Cancer 107(6): 931-936.
- Banugo P, Amoako D (2017) Prehabilitation. 17(12): 401-405.
- Minnella EM, Awasthi R, Loiselle S-E, Agnihotram RV, Ferri LE, et al. (2018) Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial 153(12): 1081-1089.
- Ciacio O, Voron T, Pittau G, Lewin M, Vibert E, et al. (2014) Interest of preoperative immunonutrition in liver resection for cancer: study protocol of the PROPILS trial, a multicenter randomized controlled phase IV trial. BMC Cancer 14(1): 980.
- Hendry P, Van Dam R, Bukkems S, McKeown DW, Parks RW, et al. (2010) Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg 97(8): 1198-1206.
- Bardram L, Funch-Jensen P, Jensen P, Kehlet H, Crawford M (1995) Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilization. Lancet 345(8952): 763-764.
- Kehlet H, Joshi GP (2017) Enhanced recovery after surgery: current controversies and concerns Anesth Analg 125(6): 2154-2155.
- Kehlet H (2018) ERAS implementation-time to move forward. Ann Surg 267(6): 998-999.
- Kehlet H (1997) Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 78(5): 606-617.
- Kahokehr A, Sammour T, Zargar-Shoshtari K, Thompson L, Hill A (2009) Implementation of ERAS and how to overcome the barriers. Int J Surg 7(1): 16-19.
- Thiele R, Friedman J (2020) Enhanced Recovery After Surgery (ERAS) and Immunonutrition: An Evidence-Based Approach.
- Moya P, Soriano-Irigaray L, Ramirez JM, Garcea A, Blasco O, et al. (2016) Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol: a multicenter randomized clinical trial (SONVI study). Medicine 95(21): e3704.
- Peng H, Zhang Q, Qian J, Ruan F, Mai H, et al. (2020) Electrolyte disorders are ERAS-associated in patients undergoing hepato-pancreato-biliary surgery. Langenbecks Arch Surg 405(5): 603-611.
- Rossoni C, Magro DO, Santos ZC, Cambri MPC, Patias L, et al. (2020) Enhanced Recovery After Surgery (ERAS) protocol in bariatric and metabolic surgery (BMS)-analysis of practices in nutritional aspects from five continents. Obes Surg 30(11): 4510-4518.
- Gündoğdu RH (2019) Current approach to perioperative nutrition in the ERAS age. Clin Sci Nutr 1: 1-10.
- Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, et al. (2011) Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Archives of surgery 146(5): 571-577.
- Stevenson EJ, Watson A, Theis S, Holz A, Harper LD, et al. (2017) A comparison of isomaltulose versus maltodextrin ingestion during soccer-specific exercise. European journal of applied physiology 117(11): 2321-2333.
- Abbott A (2014) Sugar substitutes linked to obesity. Nature News 513(7518): 290.
- Tandel KR (2011) Sugar substitutes: Health controversy over perceived benefits. Journal of pharmacology & pharmacotherapeutics 2(4) :236-243.
- Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Ågren MS (2007) Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound repair and regeneration 15(1): 2-16.
- Schwartz JR, Marsh RG, Draelos ZD (2005) Zinc and skin health: overview of physiology and pharmacology. Dermatologic surgery 31: 837-847.
- Palmieri B, Vadalà M, Laurino C (2019) Nutrition in wound healing: investigation of the molecular mechanisms, a narrative review. Journal of wound care 28(10): 683-693.
- Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. International journal of environmental research and public health 7(4): 1342-1365.
- Stefanidou M, Maravelias C, Dona A, Spiliopoulou C (2006) Zinc: a multipurpose trace element. Archives of toxicology 80(1): 1-9.
- Hassman L, Pollock S, Phipps RP (2016) Specialized pro-resolving mediators (SPMs) promote resolution of inflammation in human retinal pigment epithelial cells. Investigative Ophthalmology & Visual Science 57(12): 1860-1860.
- Torgersen Z, Balters M (2015) Perioperative nutrition. Surgical Clinics 95(2): 255-267.
- Sorensen LS, Thorlacius-Ussing O, Schmidt EB, Rasmussen HH, Lundbye-Christensen L, et al. (2014) Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br J Surg 101(2): 33-42.
- Chen W, Jiang H, Zhou Z-Y, Tao Y-X, Cai B, et al. (2014) Is omega-3 fatty acids enriched nutrition support safe for critical ill patients? A systematic review and meta-analysis. Nutrients 6(6): 2148-2164.
- Pizzini RP, Kumar S, Kulkarni AD, Rudolph FB, Van Buren CT (1990) Dietary Nucleotides Reverse Malnutrition and Starvation-Induced Immunosuppression. Archives of Surgery 125(1): 86-89.
- Akyuz EY, Akyüz C, Kiziltan G, Sehirli A, Çetinel S (2018) The effects of oral nutritional formula enriched with arginine, omega 3 fatty acids and nucleotides on methotrexate-induced experimental intestinal mucositis. Progress in Nutrition 20: 248-256.
- Pollock GR, Van Way III CW (2012) Immune-Enhancing Nutrition in Surgical and Critical Care. Missouri medicine 109(5): 388-392.
- Phillips BE, Smith K, Liptrot S, Atherton PJ, Varadhan K, et al. (2013) Effect of colon cancer and surgical resection on skeletal muscle mitochondrial enzyme activity in colon cancer patients: a pilot study. Journal of cachexia, sarcopenia and muscle 4(1): 71-77.
- Gillis C, Fenton TR, Sajobi TT, Minnella EM, Awasthi R, et al. (2019) Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: A pooled analysis of randomized controlled trials. Clinical Nutrition 38(3): 1053-1060.
- Yeung SE, Hilkewich L, Gillis C, Heine JA, Fenton TR (2017) Protein intakes are associated with reduced length of stay: a comparison between Enhanced Recovery After Surgery (ERAS) and conventional care after elective colorectal surgery. The American journal of clinical nutrition 106(1): 44-51.
- Gillis C, Wischmeyer P (2019) Pre‐operative nutrition and the elective surgical patient: why, how and what? Anaesthesia 74: 27-35.
- Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, et al. (2017) ESPEN guideline: clinical nutrition in surgery. Clinical nutrition 36(3): 623-650.
- Gillis C, Nguyen TH, Liberman AS, Carli F (2015) Nutrition adequacy in enhanced recovery after surgery: a single academic center experience. Nutrition in Clinical Practice 30(3): 414-419.
- Andersen HK, Lewis SJ, Thomas S (2006) Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database of Systematic Reviews 4: CD004080.
- Weimann A, Braga M, Carli F, Waitzberg D, Bischoff SC, et al. (2021) ESPEN practical guideline: Clinical nutrition in surgery. Clinical Nutrition 40(7): 4745-4761.
- Shin HD, Rodriguez AM, Abraham JT, Cargile JC, Brown CN, et al. (2021) Does ERAS benefit higher BMI patients? A single institutional review. Journal of Plastic, Reconstructive & Aesthetic Surgery 74(3): 475-479.
- Stechmiller JK, Childress B, Cowan L (2005) Arginine supplementation and wound healing. Nutrition in Clinical Practice 20(1): 52-61.
- Klinger JR, Kadowitz PJ (2017) The nitric oxide pathway in pulmonary vascular disease. The American journal of cardiology 120(8): S71-S79.
- Tripathi P, Tripathi P, Kashyap L, Singh V (2007) The role of nitric oxide in inflammatory reactions. FEMS Immunology & Medical Microbiology 51(3): 443-452.
- Debats IB, Wolfs TG, Gotoh T, Cleutjens JP, Peutz-Kootstra CJ, et al. (2009) Role of arginine in superficial wound healing in man. Nitric Oxide 21(3-4): 175-183.
- Curran JN, Winter DC, Bouchier-Hayes D (2006) Biological fate and clinical implications of arginine metabolism in tissue healing. Wound Repair Regen 14(4): 376-386.
- Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, et al. (1993) Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol 265(4 Pt 1): E532-E539.
- Engelen M, Klimberg VS, Allasia A, Deutz NEP (2018) Major surgery diminishes systemic arginine availability and suppresses nitric oxide response to feeding in patients with early stage breast cancer. Clin Nutr 37(5): 1645-1653.
- Collier SR, Casey DP, Kanaley JA (2005) Growth hormone responses to varying doses of oral arginine. Growth Horm IGF Res 15(2): 136-139.
- Engelen MP, Klimberg VS, Allasia A, Deutz NE (2018) Major surgery diminishes systemic arginine availability and suppresses nitric oxide response to feeding in patients with early stage breast cancer. Clinical Nutrition 37(5): 1645-1653.
- Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M (2013) A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr 32(2): 213-223.
- Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, et al. (2013) A randomized trial of glutamine and antioxidants in critically ill patients. New England Journal of Medicine 368(16): 1489-1497.
- Kim M-H, Kim H (2017) The roles of glutamine in the intestine and its implication in intestinal diseases. International journal of molecular sciences 18(5): 1051.
- Kim H (2011) Glutamine as an immunonutrient. Yonsei medical journal 52(6): 892-897.
- Grimble RF (2005) Immunonutrition. Current opinion in gastroenterology 21(2): 216-222.
- Wischmeyer PE (2007) Glutamine: mode of action in critical illness. Critical care medicine 35(9): S541-S544.
- Rhoads JM, Argenzio R, Chen W, Rippe RA, Westwick JK, et al. (1997) L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. American Journal of Physiology-Gastrointestinal and Liver Physiology 272(5): G943-G953.
- Maykish A, Sikalidis AK (2020) Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia-Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. Journal of Personalized Medicine 10(1): 19.
- Karimipour M, Hassanzadeh M, Zirak Javanmard M, Farjah G (2018) Oral administration of alanyl-glutamine and glutamine improve random pattern dorsal skin flap survival in rats. Iran J Basic Med Sci 21(8): 842-847.
- Liu Y, Liu Z, Zhang Y, Cui Y, Pei L, et al. (2021) The Protocol For The Prehabilitation For Thoracic Surgery Study: A Randomized Pragmatic Trial Comparing A Short Home-Based Multimodal Program To Aerobic Training In Patients Undergoing Video-Assisted Thoracoscopic Surgery Lobectomy.
- Dolan E, Swinton PA, Painelli VdS, Hemingway BS, Mazzolani B, et al. (2019) A Systematic Risk Assessment and Meta-Analysis on the Use of Oral β-Alanine Supplementation. Advances in Nutrition 10(3): 452-463.
- He X, Duan Y, Yao K, Li F, Hou Y, et al. (2016) β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino acids 48(3): 653-664.
- Brown A (2020) Impact of Leucine and HMB on Acute Muscle Damage: Focus on Repair and Regeneration in vivo and in vitro. Liverpool John Moores University.
- Peng LN, Cheng YC, Yu PC, Lee WJ, Lin MH, et al. (2021) Oral Nutritional Supplement with β-hydroxy-β-methylbutyrate (HMB) Improves Nutrition, Physical Performance and Ameliorates Intramuscular Adiposity in Pre-Frail Older Adults: A Randomized Controlled Trial. J Nutr Health Aging 25(6): 767-773.
- Inaba M, Terai H, Nakajima Y, Fukui N, Suwa Y, et al. (2016) Usefulness of oral administration of the specialized amino acid supplement consisting of β-hydroxy-β-methylbutyrate, L-arginine and L-glutamine (Abound™) for chronic soft tissue diseases in the mouth. SDRP Journal of Food Science & Technology 1(4): 123-127.
- Lowery RP, Joy JM, Rathmacher JA, baier SM, Fuller JC, et al. (2016) Interaction of beta-hydroxy-beta-methylbutyrate free acid and adenosine triphosphate on muscle mass, strength, and power in resistance trained individuals. Journal of strength and conditioning research 30(7): 1843-1854.
- Landi F, Calvani R, Picca A, Marzetti E (2019) Beta-hydroxy-beta-methylbutyrate and sarcopenia: from biological plausibility to clinical evidence. Curr Opin Clin Nutr Metab Care 22(1): 37-43.
- Stancliffe RA (2012) Role of beta-hydroxy-beta-methylbutyrate (HMB) in leucine stimulation of mitochondrial biogenesis and fatty acid oxidation.
- Hsieh LC, Chien SL, Huang MS, Tseng HF, Chang CK (2006) Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr 15(4): 544-550.
- Sipahi S, Gungor O, Gunduz M, Cilci M, Demirci MC, et al. (2013) The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: a retrospective analysis of diabetic haemodialysis patients. BMC nephrology 14(1): 1-6.
- Zanchi NE, Gerlinger-Romero F, Guimaraes-Ferreira L, Filho MAD S, Felitti V, et al. (2011) HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino acids 40(4): 1015-1025.
- Gade J, Levring T, Hillingsø J, Hansen CP, Andersen JR (2016) The Effect of Preoperative Oral Immunonutrition on Complications and Length of Hospital Stay After Elective Surgery for Pancreatic Cancer--A Randomized Controlled Trial. Nutr Cancer 68(2): 225-233.
- Bozkırlı BO, Gündoğdu RH, Ersoy E, Lortlar N, Yildirim Z, et al. (2015) Pilot Experimental Study on the Effect of Arginine, Glutamine, and β‐Hydroxy β‐Methylbutyrate on Secondary Wound Healing. Journal of Parenteral and Enteral Nutrition 39(5): 591-597.
- Kuthiah N (2021) Perioperative prehabilitation. Singapore Med J 1: 15.

 Review Article
Review Article