Abstract
Less invasive procedures that achieve results of traditionally invasive surgical techniques are increasingly desired by patients. One technique to increase skin firmness previously attained only by more invasive excisional tucking procedures includes the use of helium-activated radiofrequency subdermal coagulation. The effectiveness of helium activated radiofrequency technology to improve redundant skin firmness over the neck, medial thigh, arms, upper and lower back using subdermal coagulation was evaluated retrospectively. To confirm increased skin firmness, we measured distraction forces intraoperatively to pull the skin off the body 0.5 inches, 1.0 inch, and 1.5 inches using a trigger force meter. Distraction force (g) measurements were performed prior to ultrasound assisted liposuction, immediately following liposuction, and following each of six passes with helium activated radiofrequency. The results demonstrate significant increase in skin firmness shown by an increase in distraction force when compared to the pre-liposuction baseline. The increase in distraction forces were appreciated following 3 to 4 passes and continued to increase through 4 to 6 passes. Helium activated radiofrequency provides an effective, minimally invasive means of decreasing skin laxity and thus increasing skin firmness intra-operatively. The efficacy of helium-activated radiofrequency to perform subdermal coagulation used synchronously following ultrasound assisted liposuction has been demonstrated.
Introduction
Precision in body sculpting and contouring has recently become popular in the aesthetic arena. While liposuction itself does not tighten any skin, the use of Ultrasonic Assisted Liposuction (UAL) technology has provided skilled surgeons the necessary tools to remove excess fat aggressively and uniformly [1]. This is because ultrasound assisted liposuction allows surgeons to not be limited to the deep fat layer only as observed in traditional liposuction or the superficial layer as observed in cryo or heat induced lipolysis [2]. With a surgeon’s ability to comprehensively remove fat, the concern of skin redundancy has become even more evident. While ultrasound-assisted technology may provide for small degrees of skin tightening, i.e. skin retraction, the need for more aggressive skin tightening measures have been established [3]. To date, effective skin tightening has only been feasible with a simultaneous wedge excision of the skin, such as brachioplasty, mini-tuck, reverse tuck, medial thigh tuck, upper body lift, or lateral thigh lift requiring an incision line and subsequent seam line. The alternative of leaving skin laxity untreated has resulted in unacceptable outcomes involving skin roll deformities and/or contour irregularities that appear operated looking and unnatural.
The advent of helium activated radiofrequency has resulted in a minimally invasive alternative to improving skin firmness while avoiding more invasive excisional surgeries [4]. This advanced energy modality combines the unique properties of cold helium plasma with the efficiency of Radiofrequency (RF) energy. This synergy allows for acute heating of and shrinking of collagen molecules in the dermis and fibroseptal network with high precision while avoiding thermal damage to any surrounding tissue. This has been termed subdermal coagulation [5]. In summary, the simultaneous application of helium activated radiofrequency above has demonstrated clinical tightening of the skin. When applied synchronously following ultrasound assisted liposuction, it has resulted in increased firmness of the skin thereby excelling efficacy of high definition liposuction contouring results with avoidance of skin laxity deformities. At our center, we have been measuring skin firmness changes following UAL and synchronous helium-activated radiofrequency used during high-definition body contouring procedures. Surgical data from prior UAL and subdermal coagulation procedures was examined retroactively to assess extent of skin tightening. We hypothesized that clinically observed skin tightness would correlate with increased skin firmness as measured by distraction forces required to displace the skin away from the body.
Materials and Methods
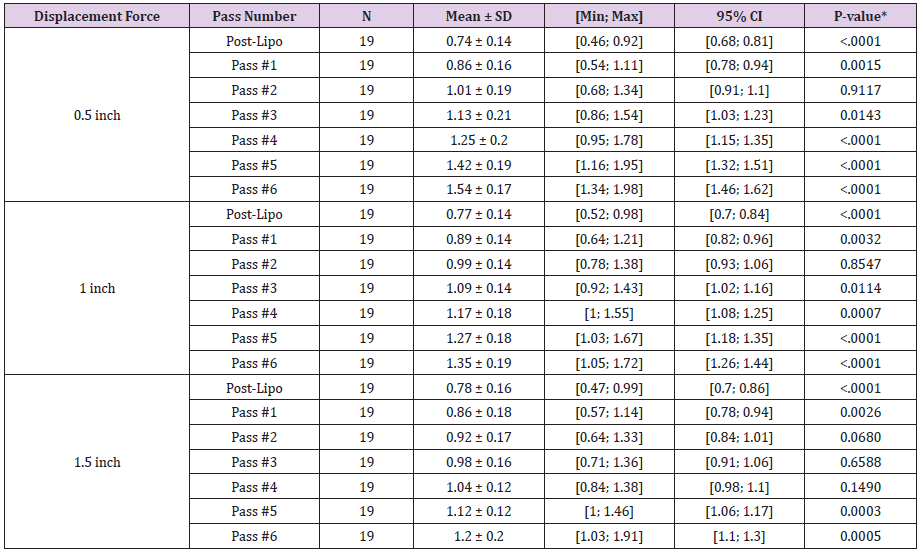
Ultrasonic Assisted Liposuction (Vaser®) was performed on six different patients. Six passes of helium activated radiofrequency (Renuvion®) were then delivered over 9 anatomical locations: left/ right arm, left/right lower back, left/right upper back, left/right medial thigh, and neck (n=19). Helium activated radiofrequency was delivered at 80% power and a Helium flow rate of 3.0 L/min. The above energy was administered using previously created liposuction port sites using a long delivery probe and with application of six separate passes. The energy was delivered to the underlying dermis and fibroseptal network, termed subdermal coagulation, of above areas when skin laxity was observed. Change in skin firmness was documented intraoperatively using a trigger force meter at the center of the anatomical location of maximum skin laxity. Displacement force (grams) was measured at skin displacements of 0.5 inches, 1.0 inch, and 1.5 inches away from the body for the pre-lipo, post-lipo, and immediately following each pass of activated helium radiofrequency. Patients were evaluated for the change in skin firmness from pre- and post- Vaser liposuction through 6 passes of Renuvion Helium Plasma at nine (9) anatomical locations, including 1) left/right arm, 2) left/right lower back, 3) left/right upper back, 4) left/right medial thigh, and 5) neck. Given the limited sample size (n=19), all gross anatomical locations were consolidated with regards to the change in skin firmness, via displacement force (grams). Consolidation requires evaluating the magnitude of change (i.e. the ratio) and the % change from preliposuction to post treatments. Above values were statistically compared utilizing a repeated measuring model. The threshold of determining statistical significance was set at a p-value < 0.05.
Results
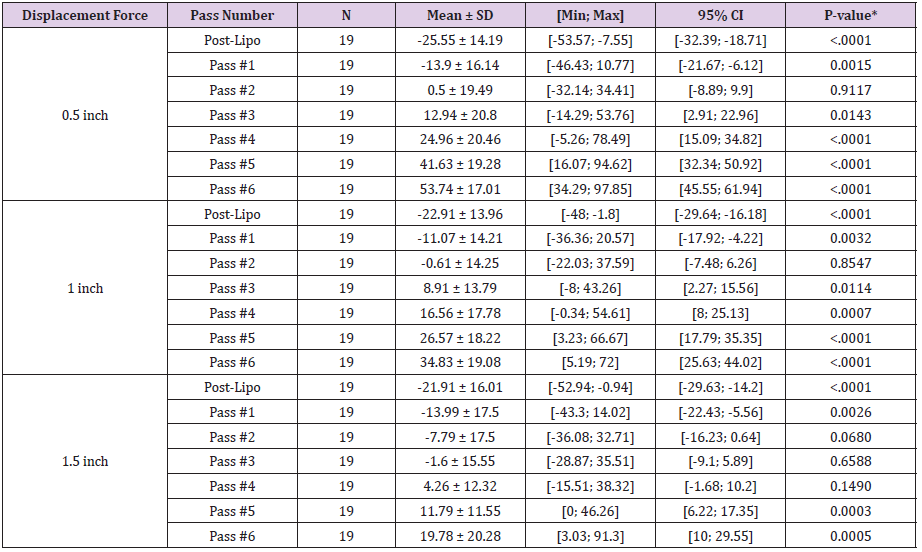
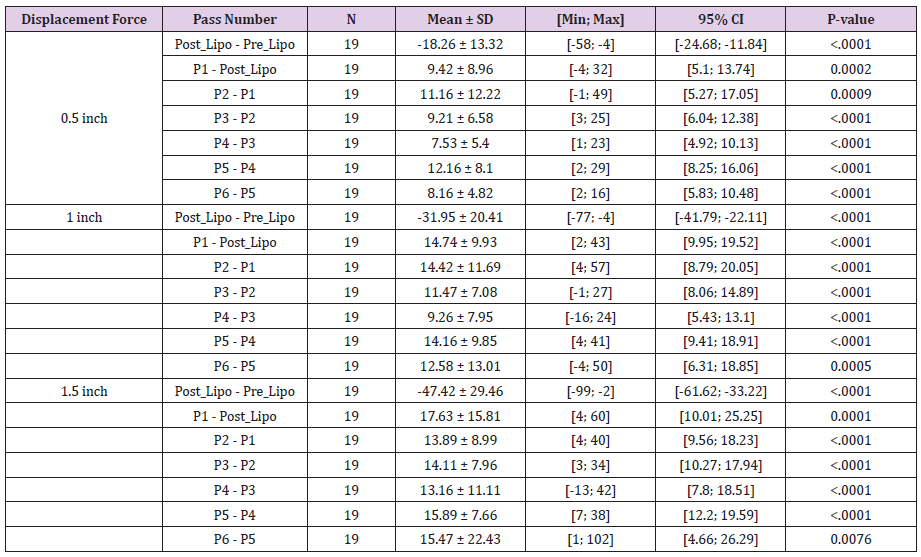
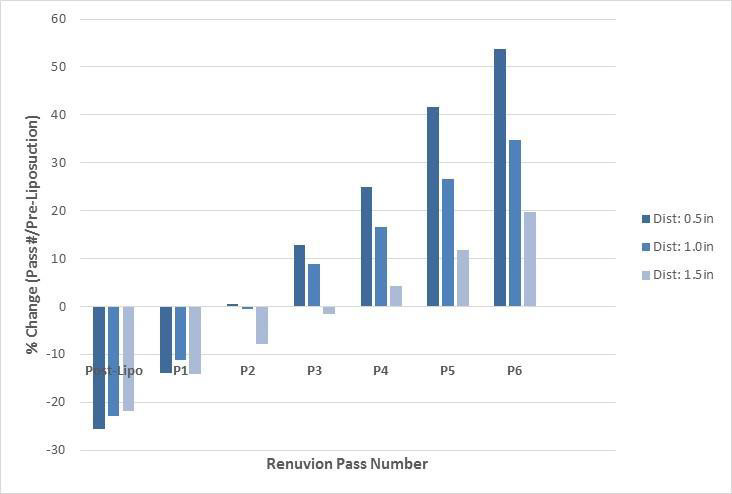
Evaluation of distraction forces demonstrates significant changes in distraction forces when pre-liposuction forces were compared to post-liposuction and six subsequent subdermal coagulation passes. For the 0.5 inches distraction distances significant changes included: a decrease in skin firmness following liposuction and after a single pass, followed by increase in skin firmness after the 3rd, 4th, 5th and 6th passes (Tables 1 and 2). Skin firmness reached pre-liposuction levels after the 3rd pass. In addition, statistically significant increase in skin firmness was observed for each subsequent pass comparing the 3rd to the 4th pass, the 4th to the 5th pass, and the 5th to the 6th pass (Table 3). The maximum % increase in distraction force, i.e. skin firmness, was observed following the 6th pass at 54% firmer than pre-liposuction state. For the 1.0-inch distraction distances, significant changes included: a decrease in skin firmness following liposuction and following a single pass, followed by increase in skin firmness after the 3rd, 4th, 5th and 6th passes. Skin firmness reached pre-liposuction levels after the 3rd pass. In addition, statistically significant increase in skin firmness was observed for each subsequent pass comparing the 3rd to the 4th pass, the 4th to the 5th pass, and the 5th to the 6th pass. The maximum % increase in distraction force, i.e. skin firmness, was observed following the 6th pass at 35% firmer than preliposuction state. For the 1.5 inches distraction distances significant changes included: a decrease in skin firmness following liposuction and after a single pass, followed by increase in skin firmness after the 5th and 6th passes. Skin firmness did not reach pre-liposuction levels until the 4th pass. In addition, statistically significant increase in skin firmness was observed for each subsequent pass comparing the 4th to 5th and 5th to the 6th pass. The maximum % increase in distraction force, i.e. skin firmness, was observed following the 6th pass at 20% stronger than preliposuction state (Table 2). Finally, no adverse effects were identified in any of the treated areas.
Table 1: Summary statistics of ratio (magnitude of change) of renuvion pass number versus pre-liposuction.
P-value from one sample t-test (two-sided) comparing to Ratio = 1.
Table 2: Summary of % Change of Renuvion Pass Number versus Pre-Liposuction.
P-value from one sample t-test (two-sided) comparing to % Change = 0.
NOTE: % Change from Baseline (either pre- or post-liposuction) = 100*(pass (i) – baseline)/baseline
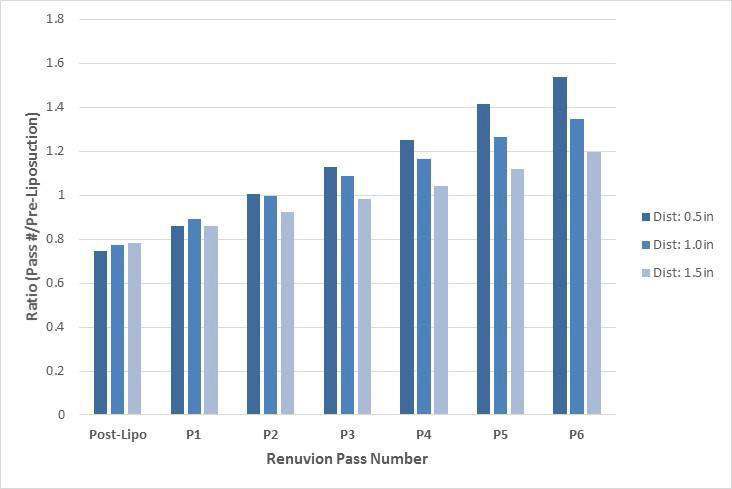
Figure 1 demonstrates the mean ratio (magnitude of change) of forces for post-liposuction and six subsequent subdermal coagulation passes when compared to pre-liposuction forces. An increasing linear trend in change in displacement force ratio from pre-liposuction is evident following a drop in force following liposuction for all three 0.5, 1.0, and 1.5 inch displacement distances. Figure 2 demonstrates the % change of displacement forces from pre-liposuction to post-liposuction and six subsequent subdermal coagulation passes. An increase in linear trend in % change of displacement forces is observed across 0.5, 1.0, and 1.5 inch displacement distances, following initial drop in force following liposuction.
Figure 1: Ratio (magnitude of change) of displacement forces compared to pre-liposuction forces at various displacement distances.
Figure 2: Percent change of displacement forces compared to pre-liposuction forces at various displacement distances.
Discussion
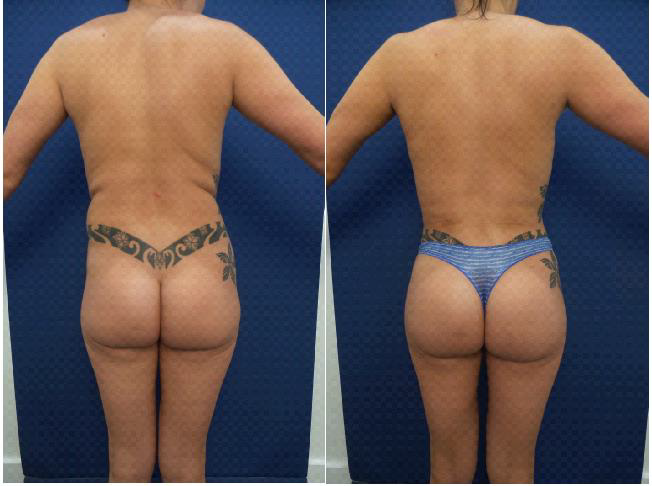
Body contouring has undergone several significant advances over the last few years. Traditionally, body contouring has required evaluation of excess fat as well as skin redundancy. When considering removal of excess fat, multiple liposuction modalities have been introduced following traditional liposuction techniques and they include power assisted, tickle liposuction, laser liposuction, ultrasound assisted liposuction as well as non-surgical modalities including cryo, heat, and injection lipolysis [6-8]. The advent of ultrasound assisted liposuction has been revolutionary since it has provided the ability to perform high definition liposuction by removing fat comprehensively and uniformly while maintaining viability of the fat cells for purposes of fat transfer [2]. When considering elimination of skin redundancy, until recently, plastic surgeons were limited to more invasive excisional procedures. While various advanced liposuction modalities such as laser-assisted liposuction claim to also correct skin firmness, these results are minimal and insignificant, evident by the 1 month postoperative skin firmness measurements previously published9. For patients with minimal to moderate skin redundancy (Figure 3), options for skin tightening included wedge excisions such as a brachioplasty, medial thigh tuck, upper body lift, lateral thigh tuck, mini tummy tuck, and reverse tummy tuck. Unfortunately, these options are more invasive and requiring excisional surgeries with subsequent surgical incision lines and prolonged healing times.
Figure 3: A 29-year old female 3 months before and after Ultrasound assisted liposuction and Helium-activated radiofrequency subdermal coagulation.
Most recently, helium activated radiofrequency subdermal coagulation has proven clinically to possess the capacity to effectively reduce skin laxity intraoperatively. Subdermal coagulation is believed to increase skin firmness by acting on collagen molecules present in the skin dermis and underlying fibroseptal network. Skin tightening through the shortening of collagen molecules via heat has previously shown to be a viable non-invasive modality [9,10]. When a region of skin laxity is treated by subdermal coagulation, the skin is observed to shrivel up much like a shrink wrap. We hypothesized whether this observation of increased skin firmness would be confirmed by data collected intraoperatively using a non-invasive trigger meter maneuver to document increased skin tightness. When we evaluated cases of UAL combined with heliumactivated radiofrequency subdermal coagulation retrospectively, we found statistically significant displacement forces, i.e. increased skin firmness, for all distraction distances occurring at 3rd pass (0.5 and 1.0 inch distraction) and 5th pass (1.5 inch distraction). Furthermore, this study has demonstrated continued significant increase in distraction force, i.e. skin firmness, at 6th pass over the 5th pass. The conclusion from this finding is that we may not have reached maximal skin firmness capacity. Since no adverse effects of skin compromise were observed following six passes, it is conceivable that even firmer skin may be achieved with more passes. This conclusion will require future studies to determine a maximal tightening effect while ensuring that skin compromise does not occur.
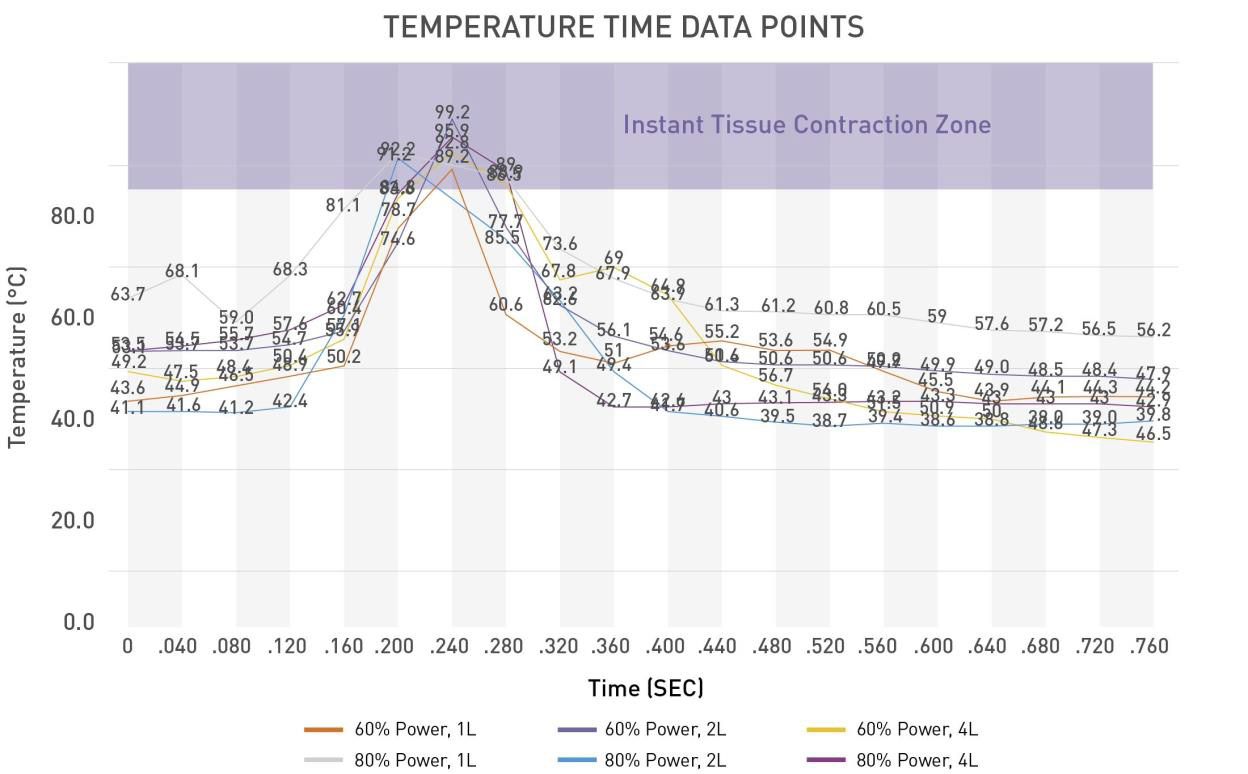
More recently, minimally invasive skin tightening procedures called Bodytite have been exhausted. However, unlike the present study, no objective measurements in terms of skin firmness have been presented introperatively [11-13]. There is no doubt Bodytite may provide an effective ancillary service in the office setting. This is because the length of procedure time required for this modality forbids its use in the operating room; treatment times have ranged from 40 to 45 minutes per area in comparison to helium activated radiofrequency procedure times of 5-10 minutes per area [12]. Longer procedure times are due to the low power wattage of Bodytite that takes longer to heat up the tissues. In contrast, Renuvion® heats the dermis and fibroseptal network to 85 °C within 0.04 to 0.08 seconds which speeds up the shrinking of collagen (occurs at 62-65 °C) while also keeping the epidermis well below 45 °C [14]. As such, injury to the epidermis is prevented. Another limitation of Bodytite involves the fact that this technology must be administered prior to liposuction. This is because the tumescent solution is required to ensure that overheating of the skin layer does not occur. However, as demonstrated in the findings above, skin firmness is significantly reduced following liposuction. As such, it is difficult to gauge degree of skin firmness required prior to having completed the liposuction stage. This would result in a theoretical under treatment of skin tightening. The final limitation of Bodytite is that the fat from the treated areas cannot be used for fat transfer as the fat gets injured. Since most 360° body contouring cases require fat transfer (i.e. Brazilian Buttock Lift), this modality is not considered a viable alternative. High-definition liposuction has changed the way surgeons contour the human body (Figure 4). With the ability to remove both superficial and deep fat comprehensively, surgeons are now going beyond traditional outcomes and sculpting patients’ muscular anatomy [8]. With this degree of fat removal and contouring ability, skin laxity and redundancy has become an even greater concern. Ability to increase skin firmness now plays a key role in a surgeon’s armamentarium. The present study demonstrates the effectiveness of coupling ultrasound assisted liposuction with a novel helium-activated radiofrequency modality to provide superior outcomes when performing high-definition liposuction body contouring.
Figure 4: For 60% and 80% power, the internal tissue is heated above 85 °C for between 0.040 seconds and 0.080 seconds – adequate time period for maximum collagen contraction to occur [14].
Conclusion
This study has demonstrated the effectiveness of increasing skin firmness intraoperatively with a minimally invasive subdermal coagulation modality using helium activated radiofrequency. Across all distraction distances, the trend of increasing displacement forces has been documented with no adverse effects. Based on the observations that maximal skin firmness had not peaked following six passes, future studies will be required to determine the limits of increasing skin firmness against potential side effects and safety risks.
References
- James R, Kesturu G, Balian G, Chhabra AB (2008) Tendon: Biology, Biomechanics, Repair, Growth Factors, and Evolving Treatment Options. Journal of Hand Surgery 33(1): 102-112.
- Khan KM, Cook JL, Bonar F, Harcourt P, Åstrom M (1997) Histopathology of Common Tendinopathies. Sports Medicine 27(6): 393-408.
- Maffulli N, Barrass V, Ewen S (2000) Light Microscopic Histology of Achilles Tendon Ruptures: A Comparison with Unruptured Tendons. The American journal of sports medicine 28: 857-863.
- Aicale R, Tarantino D, Maffulli N (2017) Basic Science of Tendons. In: Gobbi A, Espregueira Mendes J, Lane JG, Karahan M (Eds.)., Bio-orthopaedics: A New Approach. Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 249-273.
- Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, et al. (2021) Tendinopathy. Nature Reviews Disease Primers 7(1): 1.
- Jackson C, Whitmont K, Tritton S, March L, Sambrook P, et al. (2008) New therapeutic applications for the anticoagulant, activated protein C. Expert Opinion on Biological Therapy 8(8): 1109-1122.
- Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68(2): 320-344.
- Jackson MT, Moradi B, Zaki S, Smith MM, McCracken S, et al. (2014) Depletion of Protease-Activated Receptor 2 but Not Protease-Activated Receptor 1 May Confer Protection Against Osteoarthritis in Mice Through Extra cartilaginous Mechanisms. Arthritis & Rheumatology 66(12): 3337-3348.
- Xue M, Chan YKA, Shen K, Dervish S, March L, et al. (2012) Protease-activated receptor 2, rather than protease-activated receptor 1, contributes to the aggressive properties of synovial fibroblasts in rheumatoid arthritis. Arthritis & Rheumatism 64(1): 88-98.
- Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, et al. (2005) Activation of Protease-Activated Receptor-1 Triggers Astrogliosis after Brain Injury. The Journal of Neuroscience 25(17): 4319-4329.
- Wang J, Yang L, Rezaie AR, Li J (2011) Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. Journal of thrombosis and haemostasis: JTH 9 (7): 1308-1317.
- Schuepbach RA, Feistritzer C, Fernández JA, Griffin JH, Riewald M (2009) Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1. Thromb Haemost 101(4): 724-733.
- McMahon DB, Workman AD, Kohanski MA, Carey RM, Freund JR, et al. (2018) Protease-activated receptor 2 activates airway apical membrane chloride permeability and increases ciliary beating. FASEB J 32(1): 155-167.
- Xue M, Campbell D, Sambrook PN, Fukudome K, Jackson CJ (2005) Endothelial Protein C Receptor and Protease-Activated Receptor-1 Mediate Induction of a Wound-Healing Phenotype in Human Keratinocytes by Activated Protein C. Journal of Investigative Dermatology 125(6): 1279-1285.
- Duitman JW, Ruela-de-Sousa RR, Shi K, De Boer OJ, Borensztajn KS, et al. (2014) Protease Activated Receptor-1 Deficiency Diminishes Bleomycin-Induced Skin Fibrosis. Molecular Medicine 20(1): 410-416.
- Dömötör E, Benzakour O, Griffin JH, Yule D, Fukudome K, et al. (2003) Activated protein C alters cytosolic calcium flux in human brain endothelium via binding to endothelial protein C receptor and activation of protease activated receptor-1. Blood 101(12): 4797-4801.
- Marshall JC (2003) Such stuff as dreams are made on: Mediator-directed therapy in sepsis. Nature Reviews Drug Discovery 2(5): 391-405.
- Frateschi S, Camerer E, Crisante G, Rieser S, Membrez M, et al. (2011) PAR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin. Nature Communications 2: 161.
- Tsujii K, Andoh T, Ui H, Lee JB, Kuraishi Y (2009) Involvement of Tryptase and Proteinase-Activated Receptor-2 in Spontaneous Itch-Associated Response in Mice With Atopy-like Dermatitis. Journal of Pharmacological Sciences 109(3): 388-395.
- Shpacovitch V, Feld M, Bunnett NW, Steinhoff M (2007) Protease-activated receptors: Novel PARtners in innate immunity. Trends in Immunology 28(12): 541-550.
- Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, et al. (2008) Acute Modulations in Permeability Barrier Function Regulate Epidermal Cornification: Role of Caspase-14 and the Protease-Activated Receptor Type 2. The American Journal of Pathology 172(1): 86-97.
- Lee SE, Jeong SK, Lee SH (2010) Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei medical journal 51(6): 808-822.
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, et al. (2007) Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4-ion channel to cause mechanical hyperalgesia in mice. The Journal of Physiology 578(3): 715-733.
- Rattenholl A, Steinhoff M (2008) Proteinase-activated receptor-2 in the skin: Receptor expression, activation and function during health and disease. Drug News Perspect 21(7): 369-381.
- Xue M, Smith MM, Little CB, Sambrook P, March L, et al. (2009) Activated protein C mediates a healing phenotype in cultured tenocytes. J Cell Mol Med 13(4): 749-757.
- Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z (1986) Secretion of metalloproteinase by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. Journal of Biological Chemistry 261(6): 2814-2818.
- (2021) USNLM: Primer-BLAST: A tool for finding specific primers. In: Medicine USNLo (Edt.)., National Center for Biotechnology Information.
- Xue M, Thompson P, Kelso I, Jackson C (2004) Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp Cell Res 299(1): 119-127.
- Xue M, Dervish S, Chan B, Jackson CJ (2017) The endothelial protein C receptor is a potential stem cell marker for epidermal keratinocytes. Stem Cells 35(7): 1786-1798.
- Peiris TH (2016) The Akt signaling pathway is required for tissue maintenance and regeneration in planarians. BMC Developmental Biology.
- Xiao W, Tang H, Wu M, Liao Y, Li K, et al. (2017) Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Bioscience Reports 37(6): BSR20170658.
- Karrasch T, Spaeth T, Allard B, Jobin C (2011) PI3K-Dependent GSK3ß(Ser9)-Phosphorylation Is Implicated in the Intestinal Epithelial Cell Wound-Healing Response. PloS one 6(10): e26340.
- Bedi A, Kovacevic D, Hettrich C, Gulotta LV, Ehteshami JR, et al. (2010) The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. Journal of shoulder and elbow surgery 19(3): 384-391.
- Creemers LB, Jansen IDC, Docherty AJP, Reynolds JJ, Beertsen W, et al. (1998) Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biology 17(1): 35-46.
- Foda HD, Zucker S (2001) Matrix metalloproteinase in cancer invasion, metastasis and angiogenesis. Drug Discov Today 6(9): 478-482.
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, et al. (2001) Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. New England Journal of Medicine 344(10): 699-709.
- Montes R, Puy C, Molina Buey E, Hermida J (2012) Is EPCR a multi-ligand receptor? Pros and cons, pp. 815-826.
- Jackson CJ, Xue M (2008) Activated protein C--an anticoagulant that does more than stop clots. Int J Biochem Cell Biol 40(12): 2692-2697.
- Whitmont K, McKelvey KJ, Fulcher G, Reid I, March L, et al. (2015) Treatment of chronic diabetic lower leg ulcers with activated protein C: A randomised placebo-controlled, double-blind pilot clinical trial. International Wound Journal 12(4): 422-427.
- Whitmont K, Reid I, Tritton S, March L, Xue M, et al. (2008) Treatment of Chronic Leg Ulcers With Topical Activated Protein C. JAMA Dermatology 144(11): 1479-1483.
- Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, Jackson CJ (2011) Combination of Activated Protein C and Topical Negative Pressure Rapidly Regenerates Granulation Tissue Over Exposed Bone to Heal Recalcitrant Orthopedic Wounds. The International Journal of Lower Extremity Wounds 10(3): 146-151.
- Bouwens EAM, Stavenuiter F, Mosnier LO (2013) Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost 11 Suppl 1(01): 242-253.
- Julovi SM, Xue M, Dervish S, Sambrook PN, March L, et al. (2011) Protease activated receptor-2 mediates activated protein C-induced cutaneous wound healing via inhibition of p38. The American journal of pathology 179(5): 2233-2242.
- Shen K, Murphy CM, Chan B, Kolind M, Cheng TL, et al. (2014) Activated protein C (APC) can increase bone anabolism via a protease-activated receptor (PAR)1/2 dependent mechanism. Journal of Orthopaedic Research 32(12): 1549-1556.
- Gur Cohen S, Itkin T, Chakrabarty S, Graf C, Kollet O, et al. (2015) PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nature Medicine 21: 1307-1317.
- Yoshida K, Akita N, Okamoto T, Asanuma K, Uchida A, et al. (2018) Activated protein C suppresses osteoclast differentiation via endothelial protein C receptor, protease-activated receptor-1, sphingosine 1-phosphate receptor, and apolipoprotein E receptor 2. Thrombosis Research 163: 30-40.
- Zhao P, Metcalf M, Bunnett NW (2014) Biased signaling of protease-activated receptors. Front Endocrinol (Lausanne) 5: 67-67.
- Sun HB, Andarawis Puri N, Li Y, Fung DT, Lee JY, et al. (2010) Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J Orthop Res 28(10): 1380-1386.
- Garofalo R, Cesari E, Vinci E, Castagna A (2011) Role of Metalloproteinase in Rotator Cuff Tear. Sports Medicine and Arthroscopy Review 19(3): 207-212.
- Choi HR, Kondo S, Hirose K, Ishiguro N, Hasegawa Y, et al. (2002) Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. Journal of Orthopaedic Research 20(5): 927-933.
- Oshiro W, Lou J, Xing X, Tu Y, Manske PR (2003) Flexor tendon healing in the rat: a histologic and gene expression study. Journal of Hand Surgery 28(5): 814-823.
- Jackson MT, Moradi B, Smith MM, Jackson CJ, Little CB (2014) Activation of matrix metalloproteinase 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol 66(6): 1525-1536.
- Sharma P, Maffulli N (2005) Tendon Injury and Tendinopathy: Healing and Repair. JBJS 87(1): 187-202.
- Jakob F, Ebert R, Rudert M, Nöth U, Walles H, et al. (2012) In situ guided tissue regeneration in musculoskeletal diseases and aging. Cell and Tissue Research 347(3): 725-735.
- Yang G, Rothrauff BB, Tuan RS (2013) Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Research Part C: Embryo Today: Reviews 99(3): 203-222.

 Research Article
Research Article