Abstract
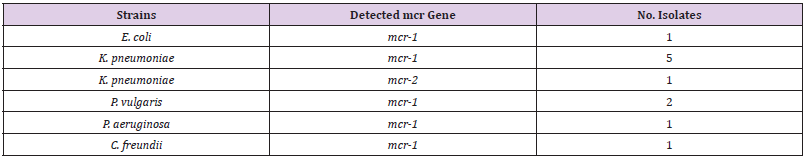
This study focuses on the identification and the distribution of Colistin resistance genes among Gram-negative bacteria isolated from clinical samples at the National Ribat Teaching hospital, in Khartoum, Sudan. A total of 165 Gram-negative isolated pathogens were as follow Klebsiella pneumoniae (73/44.2%), E. coli (53/32.1%), Pseudomonas aeruginosa (33/20%), Proteus vulgaris (6/3.6%), and Citrobacter freundii (2/1.2%), isolated between December 2019 and February 2020 from clinical samples at the National Ribat Teaching hospital in Khartoum, Sudan. Stock of all isolates were stored in 20% Glycerol-broth at -80°C for further subculture for antimicrobial susceptibility profile and resistance genes associated with Colistin resistance were identified. Colistin resistance associated genes (mcr-1, mcr-2, mcr-3, mcr-4, mcr-5) of Gram-negative isolates were determined by polymerase chain reaction (PCR) using appropriate primer sets. Out of 165; 11 isolates (7%) were resistance to Colistin antibiotic with MIC ≥ 9-10 μg/Ml. The majority 67% (4/6) of K. pneumoniae Colistin resistant strains were considered MDR. Three out of the 6 (50%) clinical Colistin-resistant K. pneumoniae isolates were resistant to carbapenems (meropenem ≤ 2 μg/ml). One of these 3 strains was resistant to all tested antibiotics. The remaining K. pneumoniae strains were susceptible to most tested antibiotics (Supplementary material Table A1). The two Colistin resistant P. vulgaris isolates were resistant to all antibiotics including the carbapenems (meropenem ≤ 2 μg/ ml) and were only susceptible to amikacin and ciprofloxacin. One Colistin resistant C. freundii isolate was only susceptible to amikacin, cotrimoxazole, and carbapenems. The Antibiotic resistant patterns were very low among P. aeruginosa isolates, as the majority of the isolates were shown more sensitive to most of the antibiotics. Only one Colistin resistant E. coli isolate was resistant to aminoglycoside and beta-lactam antibiotics. The gene detection by PCR revealed positive mcr-1 gene in 11/165 (7%) of the isolates; 6/73 (8%) were K. pneumoniae with highest mcr-1 gene among Colistin resistant K. pneumoniae 60% (6/10). 2/6 (33%) P. vulgaris, 1/53 E. coli, 1/33 (3%) P. aeruginosa, and 1/2 (50%) C. freundii. While only one (0.6%) K. pneumoniae isolate was found positive for mcr-2 (Table 4). The other Colistin resistant genes were not detected.
Keywords: Antibiotic; Colistinl Enterobacteriaceae; PCR & Resistance Genes
Introduction
Antibiotics resistance has become a major concern among Gram-negative bacterial infections because of the unavailability of alternative treatment options. The antibiotic resistance phenomenon is emerged due to antibiotics overuse and bacterial evolution [1]. Colistin is a cationic polypeptide-based antibiotic, also known as polymxin E, it’s considered the reserve antibiotics against the multidrug resistance (MDR) infections caused by Gramnegative bacterial pathogens such as Enterobacteriaceae [2,3]. The antimicrobial action of Colistin is directed towards the Gramnegative bacterial cell membrane by interacting and disrupting the lipopolysaccharide (LPS) molecules in the outer membrane which results in bacterial death [4]. In the last decade, there is an increased in the resistance of bacteria against several commonly used antibiotics and the limitation in discovery of a new ones had led the Colistin as a valuable drug of choice for many MDR strains [5-7]. MDR bacterial strains was defined MDR as acquired nonsusceptibility to at least one agent in three or more antimicrobial categories. Unfortunately, MDR, extensively drug-resistant (XDR), and pan-drug-resistant (PDR) strains of E. coli and other Enterobacteriaceae strains were detected worldwide, and they were harboring multiple resistance mechanisms [8-10].
Some bacterial strains have a natural Colistin resistance ability, such as S. marcescens, Proteus spp., B. cepacia, M. morganii, Providencia spp., and Vibrio cholera [11]. However, recently, several studies have reported Colistin-resistant isolates that have acquired Colistin resistance via chromosomal genes or plasmids worldwide [12,13]. Resistance to Colistin was associated with mutation in two components regulatory system that mediated by chromosomal genes [14,15]. The gene mcr-1 is gene of the phosphoethanolamine transferase enzyme family, which reported for the first time from China in 2016 as the first plasmid-mediated Colistin resistance gene among the Escherichia coli (E. coli) strains obtained from patients, animals, and food [16-19]. Ever since, mcr-1 positive strains have been detected in Enterobacteriaceae worldwide [20-24]. This first plasmid-mediated gene was followed by reports of variants mcr-1 genes (mcr-1.2, mcr-1.3…) and also the description of new other 7 mcr genes (mcr-2 - mcr-8) [25-29]. The emergence of colistin resistance genes have the ability to cause a major therapeutic challenge in the treatment of Enterobacteriaceae infections, which led to new recommendations for clinicians and laboratory diagnosis [30-32]. The Colistin-resistant Gram-negative bacteria impact on the clinical outcomes is clear. Thus, an appropriate and concrete actions against this strain is urgently required. This study focuses on the identification and the distribution of Colistin resistance genes among Gram-negative bacteria isolated from clinical samples at the National Ribat Teaching hospital, in Khartoum, Sudan.
Materials And Methods
Samples Collection and Processing, Growth Conditions and Chemicals
To investigate the occurrence of Colistin resistant Gramnegative bacteria, a total of 165 different clinical samples obtained from the Department of Medical Microbiology at the National Ribat University Teaching Hospital in Khartoum, Sudan. All samples were transported to the laboratory in an insulated cool box with ice packs. The isolation of Gram-negative strains from all samples were performed using Cysteine Lactose Electrolyte Deficient (CLED) agar medium (HiMedia, India). The inoculated plates were incubated aerobically at 37ºC overnight. Bright yellow lactose fermenting and pale green non-lactose fermenting colonies were selected for further analysis. The isolate were identified based on biochemical standard phenotypic analysis according to Cowan and Steel, 1985. All the samples were collected and processed after approval of the ethical committee at the National Ribat University.
Bacterial Strains
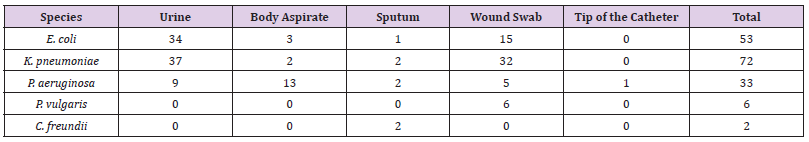
A total of 165 Gram-negative strains, consist of Klebsiella pneumoniae (73/44.2%), E. coli (53/32.1%), Pseudomonas aeruginosa (33/20%), Proteus vulgaris (6/3.6%), and Citrobacter freundii (2/1.2%), were isolated between December 2019 and February 2020 from 165 different clinical samples at the National Ribat Teaching hospital in Khartoum, Sudan. Stock of all isolates were stored in 20% Glycerol-broth at -80°C for further subculture for antimicrobial susceptibility profile and resistance genes associated with Colistin resistance were identified.
Determination of Minimal Inhibitory Concentration (In Vitro Susceptibility Testing)
Antibiotic susceptibility testing was performed by disc diffusion assay according to guidelines from the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2012). Our first approach to screen Colistin resistance among bacterial isolates, a 10 μg disc of Colistin was used and the test was performed on Mueller Hinton agar (HiMedia, India) and included a panel of 14 antibiotics belonging to different classes (Table 3). Isolates displaying an inhibition zone ≤ 10 mm (n = 61) were selected for further testing of Colistin resistance (mcr-1, mcr-2, mcr-3, mcr-4, mcr-5) genes.
Molecular Characterization
Bacterial Genomic DNA Isolation: Bacterial genomic DNA was extracted manually by phenol chloroform extraction method from Colistin resistance isolates. Extracted genomic DNA were stored at -20ºC for further analysis. Nano-Drop from Analytik Jena Thermal Cycler (Jena, Germany) was used to determine the DNA concentration and purity of the extracts.
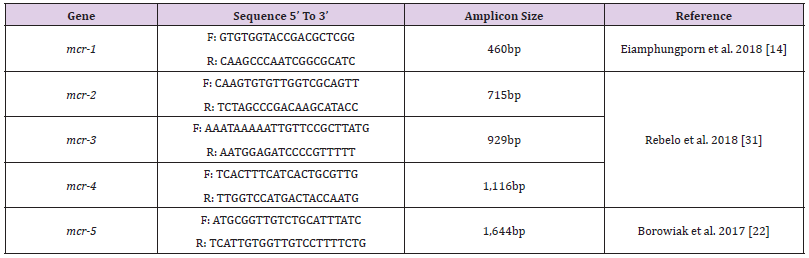
Detection Of Colistin Resistance mcr Genes: Colistin resistance associated genes (mcr-1, mcr-2, mcr-3, mcr-4, mcr-5) of Gram-negative isolates were determined by polymerase chain reaction (PCR) using appropriate primer sets. The primer sequences and corresponding annealing temperatures used in all PCR reactions in this study are listed in (Table 1). Amplifications were carried out using Analytik Jena Thermal Cycler (Jena, Germany) for 35 cycles in 25μl in 2X PCR Master Mix solution (i-TaqiTM) (iNtRON Biotechnology, South Korea) using 50 ng/μl of each of DNA template. For detection of mcr-1 gene, initial denaturation at 94°C for 3 mins, followed by 35 cycles of denaturation at 94°C for 20 s, annealing at 50°C for 20 s, and extension at 72°C for 30 s, followed by a final 5 min extension at 72°C. The PCR products were separated by electrophoresis in a 1% agarose gel and visualized by Ethidium bromide staining and visualized by UV-transilluminator. For detection of other mcr genes, initial denaturation at 94°C for 15 mins, followed by 25 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 90 s, and extension at 72°C for 60 s, followed by a final 10 mins extension at 72°C. A negative control, a reaction mixture without DNA was included in the experiment. The PCR products were separated by electrophoresis in a 1.5% agarose gel and visualized by Ethidium bromide staining and visualized by UV transilluminator.
Results
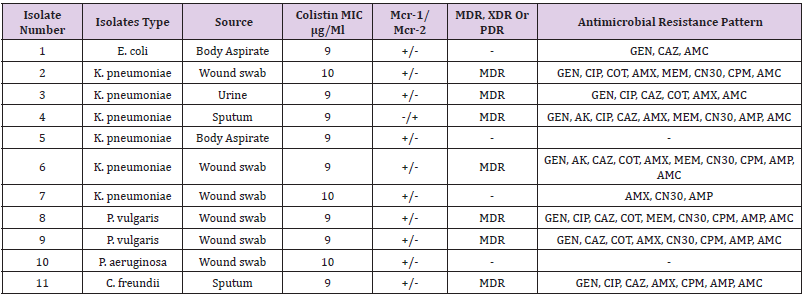
Out of 165 different clinical samples collected from patients suffering from different infections, 165 Gram-negative bacterial strains were obtained as following, E. coli (53/32.1%), K. pneumoniae (73/44.2%), P. aeruginosa (33/20%), Colistin-resistant (6/3.6%), and C. freundii (2/1.2%), (Table 2). In the present study, the prevalence of E. coli strains was relatively resistant to (25/47%) ciprofloxacin, (26/49%) ceftazidime, (29/55%) cotrimoxazole, (33/62%) amoxicillin, (30/57%) gentamicin CN30, (25/47%) cefepime, (36/68%) ampicillin, and (28/52%) amoxicillin/ clavulanate. K. pneumoniae isolates were highly resistant to amoxicillin (58/79%), gentamicin CN30 and ampicillin (52/71%). Antibiotic resistant patterns were very low among P. aeruginosa isolates, as the majority of the isolates were shown more sensitive to most of the antibiotics. Only one Colistin-resistant E. coli isolate was resistant to aminoglycoside and beta-lactam antibiotics. It was found that out of 165 Gram-negative isolates, 11 isolates (7%) were resistance to Colistin antibiotic with MIC ≥ 9-10 μg/Ml. The majority 67% (4/6) of K. pneumoniae Colistin-resistant strains were considered MDR. Although, the two P. vulgaris strains and one C. freundii strain were also considered as an MDR strains.
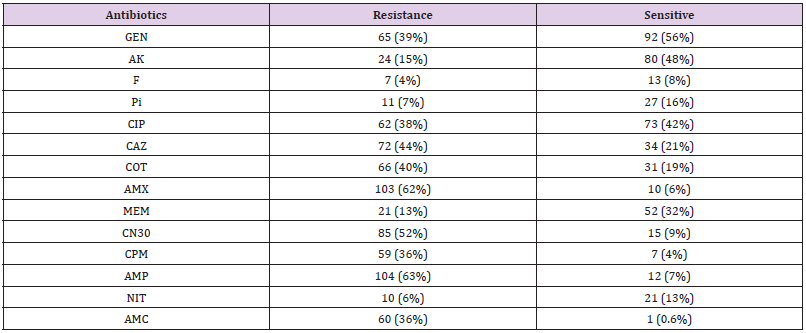
Three out of the 6 (50%) clinical Colistin-resistant K. pneumoniae isolates were resistant to carbapenems (meropenem ≤ 2 μg/ml). One of these 3 strains was resistant to all tested antibiotics. The remaining K. pneumoniae strains were susceptible to most tested antibiotics (Supplementary material Table. A1). The two Colistin-resistant P. vulgaris isolates were resistant to all antibiotics including the carbapenems (meropenem ≤ 2 μg/ml) and were only susceptible to amikacin and ciprofloxacin. Colistin resistant C. freundii isolate was only susceptible to amikacin, cotrimoxazole, and carbapenems. Antimicrobial susceptibility testing revealed that the isolated Colistin-resistant E. coli strain was resistant to aminoglycoside and beta lactam antibiotics. No isolate was classified as XDR or PDR (Table 2). High resistance was observed against both amoxicillin (62%) and ampicillin (63%). Moderate resistance was observed against ceftazidime (44%) and gentamicin N30 (52%). Furthermore, low resistance was shown against florfenicol (4%), nitrofurantoin (6%), piperacillin (7%), and meropenem (13%). The majority 55 % (6/11) of Colistin-resistant strains were isolated from wound infection samples, (Table 3).
GEN: Gentamycin, AK: Amikacin, F: Florfenicol, Pi: Piperacillin, CIP: Ciprofloxacin, CAZ: Ceftazidime, COT: Cotrimoxazole, AMX: Amoxicillin, MEM: Meropenem, CN30: Gentamicin, CPM: Cefepime, AMP: Ampicillin, NIT: Nitrofurantoin, AMC: Amoxicillin/ Clavulanate
Determination of Colistin-Resistant Genes
The genetic detection of mcr (1-5) genes using conventional PCR revealed that 11/165 (7%) of our isolates; 6/73 (8%) K. pneumoniae, 1/53 (2%), 2/6 (33%) P. vulgaris, E. coli, 1/33 (3%) P. aeruginosa, and 1/2 (50%) C. freundii were positive for mcr-1. In addition, only one (0.6%) K. pneumoniae isolate was positive for mcr-2 (Table 4). The other Colistin-resistant genes were not detected. The majority 67% (4/6) of K. pneumoniae Colistinresistant strains were considered MDR. Although, the two P. vulgaris strains and one C. freundii 11 isolates (7%) were resistance to Colistin antibiotic with MIC ≥ 9-10 μg/Ml. mcr-1 gene was higher among K. pneumoniae 60% (6/10), K. pneumoniae was the major Colistin-resistant strains 67% (4/6), Figure 1 (Table 4 and Table 5).
MDR: Multiple drug resistance, XDR: extensively drug resistant, PDR: pandrug-resistant, GEN: gentamycin, AK: amikacin, CIP: ciprofloxacin, CAZ: ceftazidime, COT: Cotrimoxazole, AMX: amoxicillin, MEM: meropenem, CN30: Gentamicin, CPM: cefepime, AMP: Ampicillin, AMC: amoxicillin/clavulanate. +: positive result; −: negative result.
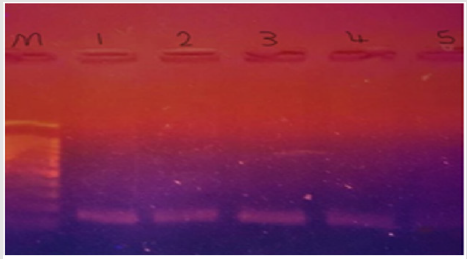
Figure 1:mrc-1 gene detected in colistin resistant klebsiella pneumonae; lane m = DNA marker; 1, 2, 3 & 4 = strong +ve; lane 5 = weak +ve.
Discussion
In this study, the phenotypic and genomic characterized Gram-negative isolates with Colistin-resistant genes in different clinical samples obtained from the National Ribat hospital. One hundred sixty-five Gram-negative bacterial strains were obtained as following, E. coli (53/32.1%), K. pneumoniae (73/44.2%), P. aeruginosa (33/20%), P. vulgaris (6/3.6%), and C. freundii (2/1.2%). Knowing the fact that Colistin antibiotics are considered the last-resort antibiotics in many areas with MDR Gram-negative strains has led the occurrence of Colistin-resistant bacteria to increases around the world. For this reason, the prevalence of Colsitin-resistant (mcr) gene among Gram-negative strains isolated from clinical samples in Khartoum, Sudan was evaluated; The prevalence of Colistin-resistant Enterobacteriaceae strains was low in many studies around the world [33]. In this study, although the mcr-1 detection rate was not particularly high among Gramnegative isolates (7%). K. pneumoniae was the major Colistinresistant strains 67% (4/6), this was consistent with previous study conducted by Sherry and Howden in 2018 that showed K. pneumoniae strains were more resistance to Colistin than E. coli strains. Moreover, K. pneumoniae Colistin-resistant strains were considered MDR and this is in contrast with the recent report by Abd El-Baky et al. 2020, who found that P. aeruginosa represented the majority of MDR strains.
In the present study, the prevalence of mcr-1 gene was higher among K. pneumoniae 60% (6/10), and this is in contrast with those reported by Nahed Adam and Hisham N Altayb, 2017, they found that the majority of mcr-1 gene was detected among E. coli strains. This difference can be explained by the fact that they majority of their isolates were E. coli compared to this study; it was K. pneumoniae. Surprisingly, in this study the prevalence of Colistin resistance among P. aeruginosa isolates was considered low which was similar to the reported rates from previous study [34]. Additionally, the MDR P. vulgaris and C. freundii isolates were both have maintained a very low Colistin resistance rate (Table 4). So far, a high mortality rates have been immensely associated with the colsitin-resistant Gram-negative infection [35-39]. Thus, an intense investigation is required to increase the therapeutic choices for such pathogens. This study showed that overall characteristics of Gram-negative isolates were susceptible to florfenicol (4%), nitrofurantoin (6%), piperacillin (7%), and meropenem (13%) (Table 3). Interestingly, 9 (82%) out of 11 Colistin-resistsnt Gram-negative isolates were susceptible to amikacin. Thus, aminoglycoside (amikacin) might be options for treating Colistin-resistant Gram-negative bacteria, and this finding is consistent with previous reports showing that Colistin-resistant strains were susceptible to amikacin [40].
It seems in Gram-negative pathogens isolated from different clinical samples have acquired Colistin-resistant gene, either through mutation of the genes or acquisition of mcr gene from other bacteria. However, the relatively large number of isolates and the low prevalence of mcr gene carrying strains obtained from the patients suggests that the evolution of Colistin resistance is currently of major concern in hospital, this was the first report of mcr-1&2 genes detected among Gram-negative strain in Sudan at the National Ribat hospital in Khartoum.
Conclusion
In the past few years, the prevalence of Colistin-resistant pathogens has been emerged worldwide. In addition, Colistin resistant pathogens are considered a critical issue to deal with nowadays. The study findings confirmed the presence of Colistin resistant Gram-negative bacteria in Khartoum, Sudan. The highest rate of Colistin resistant pathogens was found among K. pneumoniae isolates. As a last resort, amikacin showed the best in vitro activity against Colistin resistant Gram-negative bacteria. Further wide scale studies are required to investigate the prevalence the Colistin resistant pathogens in Sudan that can identify the exact role involved in the emergence of Colistin resistance. Due to the increasing carriage of Colistin resistant pathogens among patients in the clinical settings worldwide, urgent and concrete actions must be taken to reduce and prevent the spreading of such pathogens. This study was the first nationwide surveillance report the detection of mcr-2 genes among Gram-negative isolates from clinical samples in Sudan.
Disclosure
Authors declare that they have no conflicts of interest in this work.
References
- Nation RL, Li J, Turnidge JD, Milne RW, Coulthard K, et al. (2006) Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis 6(9): 589-601.
- (2018) World Health Organization (WHO). Global Antimicrobial Resistance Surveil- lance System (GLASS) report: early implementation 2016–2017. Geneva, Switzerland: WHO, 2018.
- Tang SS, Apisarnthanarak A, Hsu LY (2014) Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv Drug Deliv Rev 78: 3-13.
- Falagas ME, Kasiakou SK, Saravolatz LD (2005) Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis 40(9): 1333-1341.
- Nation RL, Li J (2009) Colistin in the 21st century. Curr Opin Infect Dis 22(6): 535-543.
- Gregoire N, Aranzana-Climent V, Magreault S, Marchand S, Couet W, et al. (2017) Clinical pharmacokinetics and pharmacodynamics of Colistin. Clin Pharmacokinet 56(12):1441-1460.
- Kanazawa K, Sato Y, Ohki K, Okimura K, Uchida Y, et al. (2009) Contribution of each amino acid residue in polymyxin B (3) to antimicrobial and lipopolysaccharide binding activity. Chem. Pharm. Bull. (Tokyo) 57(3): 240-244.
- Kempf I, Fleury MA, Drider D, Bruneau M, Sanders P, et al. (2013) What do we know about resistance to Colistin in Enterobacteriaceae in avian and pig production in Europe? Int J Antimicrob Agents 42(5): 379-383.
- Gunn JS (2008) The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol 6(6): 284-290.
- Cannatelli A, D’Andrea MM, Giani T, Di Pilato V, Arena F, et al. (2013) In vivo emergence of Colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother 57(11): 5521-5526.
- Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, et al. (2016) Emergence of plasmid-mediated Colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16(2): 161-168.
- Zurfuh K, Poirel L, Nordmann P, Nüesch-Inderbinen M, Hächler H, et al. (2016) Occurrence of the plasmid-borne mcr-1 Colistin resistance gene in extended-spectrum β-lactamase-producing Enterobacteriaceae in river water and imported vegetable samples in Switzerland. Antimicrob Agents Chemother 60(4): 2594-2595.
- Malhotra-Kumar S, Xavier BB, Das AJ, Lammens C, Hoang HT, et al. (2016) Colistin-resistant Escherichia coli harbouring mcr-1 isolated from food animals in Hanoi, Vietnam. Lancet Infect Dis 16(3): 286 - 287.
- Falgenhauer L, Waezsada SE, Yao Y, Imirzalioglu C, Käsbohrer A, et al. (2016) RESET Consortium. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis 16(3): 282-283.
- Sherry N, Howden B (2018) Emerging Gram-negative resistance to last-line antimicrobial agents fosfomycin, Colistin and ceftazidime-avibactam-epidemiology, laboratory detection and treatment implications. Expert Rev Anti Infect Ther 16(4): 289-306.
- Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, et al. (2006) Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious disease’s society of America. Clin Infect Dis 42 (5): 657-668.
- Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, et al. (2016) Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance. 21(27): 30280.
- Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, et al. (2016) mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrobial agents and chemotherapy 60(9): 5612-2615.
- Yang YQ, Li YX, Song T, Yang YX, Jiang W, et al. (2017) Colistin resistance gene mcr-1 and its variant in Escherichia coli isolates from chickens in China. Antimicrobial agents and chemotherapy 24;61(5): e01204-e01205.
- Yin W, Li H, Shen Y, Liu Z, Wang S, et al. (2017) Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio. 278(3): e00543-e005417.
- Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, et al. (2017) Aug. Novel plasmid-mediated colistin resistance mcr-4 g ene in Salmonella and Escherichia coli, 2013. Spain and Belgium, 2015 to 2016,” Eurosurveillance, 22(31): 30589.
- M Borowiak, J Fischer, J A Hammerl, R S Hendriksen, I Szabo, et al. (2017) Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring Colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. Journal of Antimicrobial Chemotherapya 72(12): 3317-3324.
- AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, et al. (2017) mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. Journal of Antimicrobial Chemotherapy 72(10): 2745-2749.
- Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN, et al. (2018) Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. Journal of Antimicrobial Chemotherapy 1;73(7): 1791-1795.
- Wang X, Wang Y, Zhou Y, Li J, Yin W, et al. (2018) Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerging microbes & infections 1;7(1): 1-9.
- Fournier S (2014) Control of bacteria highly resistant to emerging antibiotics (BHRe). Journal of Anti-infectives 1;16(2): 80-88.
- Cowan ST (1985) Cowan and Steel’s manual for identification of medical bacteria, 2nd edn., Cambridge University Press, Cambridge, London, pp: 138-139.
- Srinivas P, Rivard K (2017) Polymyxin resistance in Gramnegative pathogens. Curr Infect Dis Rep. 19 (11): 38.
- (2012) linical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. Wayne, PA: CLSI; 2012. CLSI document M100-S22.
- Eiamphungporn W, Yainoy S, Jumderm C, Tan-Arsuwongkul R, Tiengrim S, et al. (2018) Prevalence of the colistin resistance gene mcr-1 in colistin-resistant Escherichia coli and Klebsiella pneumoniae isolated from humans in Thailand. Journal of global antimicrobial resistance 1;15: 32-35.
- Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, et al. (2018) Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillance 8;23(6): 17-00672.
- Olaitan AO, Morand S, Rolain JM (2016) Emergence of Colistin-resistant bacteria in humans without Colistin usage: a new worry and cause for vigilance. Int J Antimicrob Agents 47(1): 1-3.
- Jeannot K, Bolard A, Plésiat P (2017) Resistance to polymyxins in Gram-negative or- ganisms. Int J Antimicrob Agents 49(7): 526-535.
- Nahed Adam, Hisham N Altayb (2017) First report of Colistin Resistance (MCR-1) Gene in Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Specimens in Khartoum, Sudan. J Proteomics Bioinform 10:11.
- Abd El-Baky RM, Masoud SM, Mohamed DS, Waly NGFM, Shafik EA, et al. (2020) Prevalence and Some Possible Mechanisms of Colistin Resistance Among Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa. Infect Drug Resist 3: 323-332.
- Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, et al. (2013) High rate of Colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 19(1): E23-E30.
- Lertsrisatit Y, Santimaleeworagun W, Thunyaharn S, Traipattanakul J (2017) In vitro activity of Colistin mono- and combination therapy against Colistin-resistant Acinetobacter baumannii, mechanism of resistance, and clinical outcomes of patients infected with Colistin-resistant A. baumannii at a Thai university hospital. Infect Drug Resist 10: 437-443.
- Arjun R, Gopalakrishnan R, Nambi PS, Kumar DS, Madhumitha R, et al. (2017) A study of 24 patients with Colistin-resistant Gram-negative isolates in a tertiary care hospital in South India. Indian J Crit Care Med, 21(5): 317-321.
- Quan J, Li X, Chen Y, Jiang Y, Zhou Z, et al. (2017) Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. The Lancet Infectious Diseases 17(4): 400-410.
- Falagas ME, Rafailidis PI, Matthaiou DK (2010) Resistance to polymyxins: mechanisms, frequency and treatment options. Drug resistance updates 13(4-5): 132-138.

 Research Article
Research Article