Abstract
Aims:The relationship between intestinal inflammation and pancreatic islet endocrine dysfunction has been concerned, but the specific role remains clear. The present study aims to explore the effect of Dextran Sodium Sulfate (DSS)-induced colon inflammation on the glucose metabolism and pancreatic islets β cells.
Methods:C57Bl/6 mice (male or female) were randomly divided into normal control group and DSS model group. The mice in model group were given DSS drinking water (30g/L) for 6 consecutive days, while the mice in normal group were given ordinary clean drinking water. The morphological characteristics of stool were recorded, and HE Staining was used to identify colonic inflammation. In vivo glucose tolerance experiments were performed to detect the glucose tolerance of the two groups of mice. Immunofluorescence Staining and morphological analysis were used to detect changes in the structure and composition of islets.
Results: Stools in model mice group were wet, unformed, and bloody, and the number of stools in model mice group were significantly reduced compared with normal mice group. HE staining showed that the structure of colon in model mice group was damaged that the intestinal glands were atrophy. The glucose tolerance of model mice group was significantly impaired and the blood glucose significantly increased. The number of insulin positive islet β cells in pancreatic section was significantly reduced. Furthermore, the CD34 was expressed in islets β cells and its number was significantly increased in model mice group.
Conclusion: Our study demonstrates that DSS-induced colon inflammation can significantly reduce the number of pancreatic islets β cells and increase blood glucose levels, which may be related to the increased expression of CD34 in pancreatic islets. The present study provides new evidence for the study of the effect of intestinal inflammation on glucose metabolism.
Keywords: Intestinal Inflammation; CD34; Insulin Secretion; Islet Function; Diabetes
Abbreviations: DSS: Dextran Sodium Sulfate; TNF-α: Tumor Necrosis Factor-α; BM: Bone Marrow; GTT: Glucose Tolerant Test
Introduction
Diabetes is a chronic metabolic disease characterized by hyperglycemia, which damages the heart, brain, and kidney to affect the health of patients. Diabetes patient in China has research to 114.4 million [1]. Both type 1 diabetes and type 2 diabetes are closely related to the number and function of islet β cells. Therefore, it is important to explore the regulation of the number and function of pancreatic β cells. Intestinal inflammation is involved in the occurrence and development of diabetes. The increased expression of inflammatory cytokines including Tumor Necrosis Factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and IL-1 is closely related to the occurrence and progression of type 2 diabetes. Furthermore IL-1β and TNF-α are key pro-inflammatory factors leading to pancreatic β cell damage and insulin resistance [2]. Treatment of high-fat diet C57BL/6 mice within intestinal anti-inflammatory, 5-ASA, reversed intestinal inflammation and effectively improved blood glucose levels [3]. Current reports on the mechanism of intestinal inflammation leading to diabetes mainly include that chronic intestinal inflammation may affect the number of intestinal secretory cells, the neuroregulatory signaling pathway of the brain - intestinal axis through changing the permeability of intestinal barrier and participate in the occurrence and development of insulin resistance and type 2 diabetes [4].
As a transmembrane glycoprotein, CD34 was first identified in hematopoietic stem cells and progenitor cells [5]. Later research has demonstrated that CD34 was expressed in muscle satellite cells, corneal keratinocytes, mesenchymal cells and other nonhematopoietic cell types [6]. Studies have shown that CD34 is closely related to inflammatory response. As a peripheral lymph node transmitter, CD34 participates in the homing of lymphocytes and the recirculation of lymphocytes in the early stage, to supplement T and B lymphocytes in peripheral lymph nodes [7]. CD34 is widely expressed in vascular endothelial cells in colon tissue of patients with IBD, which is related to the increase of micro vessel density [8]. In the study of 23 patients with ulcerative colitis, 16 patients with Crohn’s disease and 18 normal controls, the expression of CD34 in the two disease groups was higher than that in the control group [9]. Stzepourginski et al have confirmed that CD34+ mesenchymal cells develop in the intestinal tract after birth, participate in the repair of intestinal inflammation and injury, and contribute to the inflammation and repair of the intestinal tract after injury [10].
CD34 stem cell infusion can significantly promote the increase of survival rate of mice with moderate and severe colitis induced by DSS [11]. Furthermore type 1 diabetes patients can alleviate the disease after receiving CD34+ hematopoietic stem cell treatment [12]. And some studies have confirmed that people with low CD34 levels have a higher risk of diabetes, and the occurrence of diabetes is related to the decrease in the number of CD34+ cells and functional defects [13]. It has also been documented that diabetes impair the Bone Marrow (BM) microenvironment, thereby reducing the number of circulating CD34 (+) cells [14]. The above reports show that CD34 is closely related to intestinal inflammation and diabetes. However, it is not clear what role CD34 molecules play in the relationship between colitis inflammation and insulin secretion. The present study aims to explore the relationship between intestinal inflammation and islet function and the change of CD34 ,34, which will provide a new theoretical basis for the study of the effect of intestinal inflammation on diabetes.
Material and Methods
Experimental Animal
C57BL/6 mice (18-20g) were purchased from Nanjing Qinglongshan Animal Breeding Farm. All animals are raised in a standard animal laboratory, with free eating and drinking water, the animal room is well ventilated. All experiments in this study have been approved by the Animal Experiment Ethics Committee of Wannan Medical College and meet the requirements of Anhui Province Animal Experiment Management Regulations. Twenty mice were divided into normal group and model group. Mice in the model group were given DSS mixed solution (30g/L in water) for 6 consecutive days, followed by normal drinking water for 4 days. Mice in the normal group were given normal drinking water for 10 consecutive days. The weight and diet were measured from the beginning of model building. The free diet blood glucose and glucose tolerance tests were performed before and after modeling. The liver, colon and pancreas tissues were collected immediately after the mice were killed by dislocation.
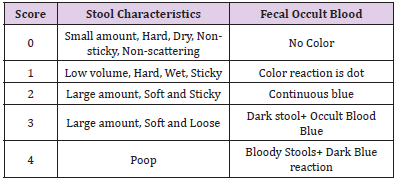
Observation and Scoring of Feces of Laboratory Animals
On the 4th day of modeling, the fecal morphology of the normal group and the model group was observed and scored according to the stool scoring standard table (Table 1). The superposition of the score of fecal morphology and the score of blood stool is the result of fecal score. After the establishment of the model, the mice were continued to be fed for 4 days, and the changes of behavior, body weight and diet of mice were observed.
Glucose Tolerant Test (GTT)
All food and bedding were removed at 5 pm the day before the experiment, fasting for 16 hours, normal drinking water was given during fasting, and glucose tolerance test began at 9 am the next morning. After weighing, blood samples were taken from the tip of the tail to measure blood glucose, which was recorded as 0. Glucose (1.5g/kg body weight) was then injected intraperitoneally and timing began when the first mouse was injected. The blood glucose values measured by 0 min, 15 min, 30 min, 45 min, 60 min, 90 min, 120 min were recorded, respectively. The software prism is used for data processing and statistics. P < 0.05 was recorded as significant difference.
Hematoxylin-eosin (HE) Staining
Mouse liver, colon, and pancreas tissues fixed with 4% paraformaldehyde for 24 hours, dehydrated with 15% and 30% sucrose solution for 24 hours, embedded in OTC, and sliced using a cryostat [15]. The sections immersed in Phosphate-Tween Buffer (PBST) for 5 minutes were stained with hematoxylin solution for 10 minutes, then stained with tap water was stopped, the differentiation solution was washed twice, the nuclei were stained with eosin solution for 1 minute and rinsed under tap water for 2 minutes. After mounting the slides, the results were taken under a microscope (OLYMPUS, BX-53), and the colon histology score was performed according to the colon histopathology score sheet.
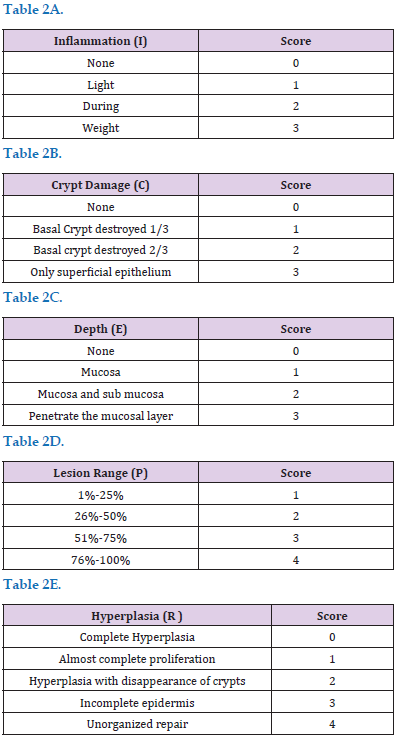
Colon Tissue Inflammation Score
The colon is scored according to the HE Staining results of colon tissue sections, mainly based on the following scoring table (Tables 2A-2E). The final score is the average score of the five assessments.
Immunofluorescence Staining
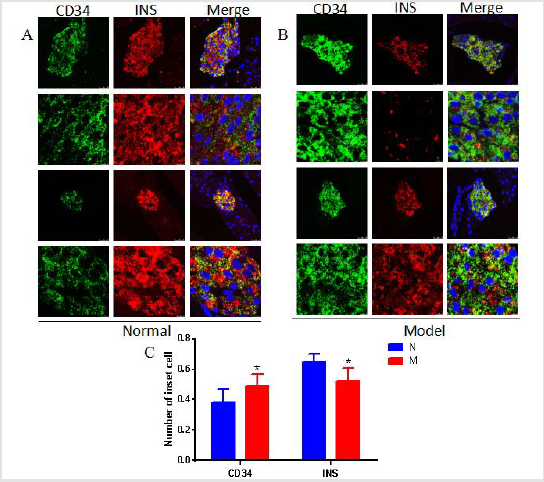
The pancreas sections were immersed in PBST solution for 3 times, 10 min each time. After blocking with 5% horse serum for 30 minutes, add the primary antibody (CD34: INS: PBST=1:5:1000), and incubate overnight at 4 ℃ in the refrigerator. The next day, they were immersed in PBST solution 3 times, 10 min each time. Drop the secondary antibody mixture (donkey anti-rabbit 488 fluorescent secondary antibodies: donkey anti-mouse 594 fluorescent secondary antibody: DAPI: PBST=1:1:1:1000). After incubating for 2hours at room temperature, PBST soaked 3 times. After mounting the slides in glycerol, photographs were taken with a laser confocal fluorescence microscope, and the expression of CD34 and insulin in the islets was calculated.
Data Analysis
The samples used for data analysis were randomly selected from the normal, model group. Data were compared between the normal or model group using analysis of variance (ANOVA). Statistical analyses were performed using GraphPad Prism 6.0 software. P< 0.05 was considered statistically significant.
Results
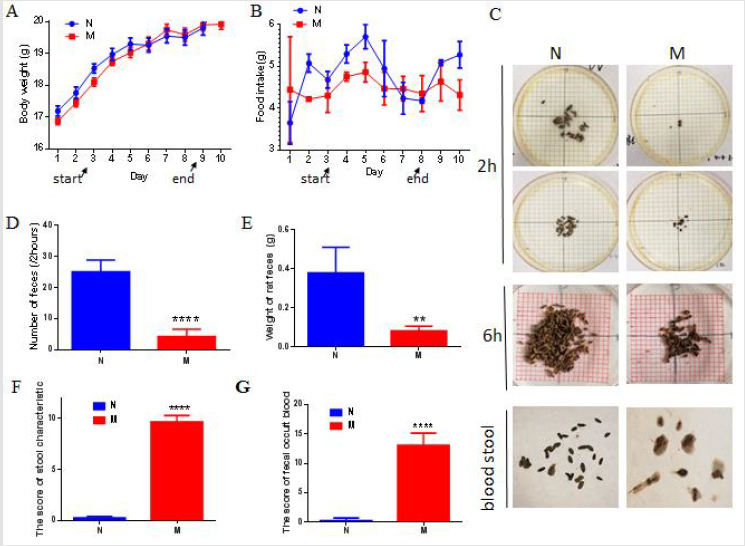
Body Weight, Food Intake and Stool Status in DSS Model Mouse Group
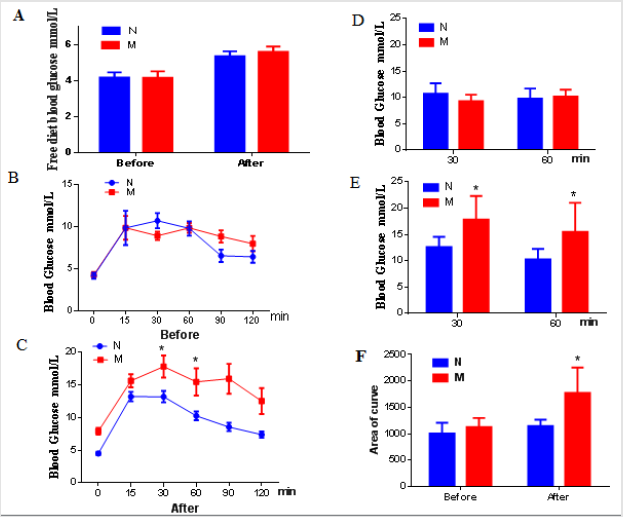
No significant difference in body weight between the DSSinduced model group and the normal group (Figure 1A). Compared with the normal group, the food intake of the model group was reduced slightly, but no significant difference was show (Figure 1B). The number of stools in the model group within 2 hours and 6 hours was significantly decreased compared normal group. Furthermore, the stools were moist and with blood (Figures 1C & 1D, P<0.001). Similarly, the weight of feces in the model group was significantly lower than that in the normal group (P<0.01). The amount of feces excreted within 4 hours of each normal group mouse was 0.3800 ± 0.05831 g, while that of each model group mice within 4 hours The stool weight is 0.0820 ± 0.01114 g (Figure 1E). Stool score results showed that the model group mice had a high colon inflammation score (P<0.0001, Figure 1F) and severe fecal blood (Figure 1G).
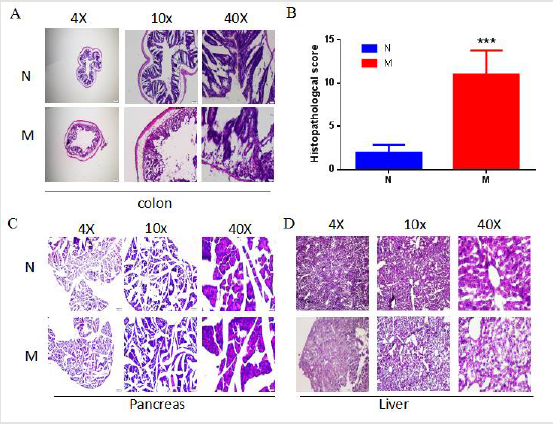
Evaluation of Colon Inflammation in DSS Model Group Mouse
HE Staining results showed that the structure of the colon of the model group mice was significantly damaged, mucosal epithelial cells were shed, intestinal glands mucosa was missing and glands were destroyed (Figure 2A). The results of colon histopathology score showed that the pathology score of the model group was significantly higher than that of the normal group (11.00±0.8312; Figure 2B). The pancreatic tissue structure is intact and has not been significantly damaged, indicating that the overall structure of the pancreatic tissue in the model group has not been affected (Figure 2C). Simultaneously, the staining of liver sections showed that the histological morphology of the liver was consistent with the normal group (Figure 2D).
Changes of Glucose Metabolism in DSS Model Mouse Group
The model group showed no difference in free diet blood glucose compared with the normal group (Figure 3A). GTT result showed that no difference in glucose induced blood glucose between the normal group and the model group before modeling (Figures 3B & 3C). Interestingly, after DSS induced colon inflammation, the curve of GTT the model group was significantly move up (Figure 3D). Furthermore, the levels of blood glucose at 30 min and 60 min (Figure 3E, P <0.05), and the area under the curve were all significantly increased (Figure 3F). Those results demonstrate that the blood glucose tolerance of the model group mice was significantly impaired.
Changes in the Structure of Pancreatic Islets and the Number of β Cells in DSS Model Mouse Group
The results of Iiimmunofluorescence Staining showed that CD34 molecules were coexist with insulin-secreting β cells in pancreatic tissue of normal mouse (group (Figure 4A). Compared with the normal group, the number of β cells expressing insulin in DSS model mouse group was reduced (Figures 4A & 4B). The statistical results of morphological analysis showed that the number of pancreatic β cells was significantly reduced (Figure 4C). At the same time, the positive expression of CD34 in the pancreas of the model group increased compared with the normal group (Figure 4C). The results showed that mouse colon inflammation significantly damage the glucose metabolism in model mouse group.
Discussion
In this study, it was found for the first time that there was no abnormal blood glucose in mice with colonic inflammation induced by DSS, but the blood glucose increased significantly after glucose injection, that is, abnormal glucose tolerance. Colonic inflammation induced by DSS results in a decrease in the number of islets β-cells and a decrease in insulin secretion, which is related to the increase of CD34 molecules expressed on islet β-cells. In recent years, more and more studies have shown that there is a correlation between intestinal inflammation and islet function. In this study, colonic inflammation was induced by administration of 3%DSS solution in mice. The results showed that the number of feces decreased and hematochezia appeared in the model group. HE Staining showed abnormal colon morphology and significant differences in histopathological scores. During the detection of glucose metabolism, it was found that there was no significant change in blood glucose in DSS mice model group with free diet, but the results of glucose tolerance test showed that the glucose tolerance curve of DSS mice model group and the value of blood glucose significantly increased.
Through further morphological examination, we found that the number and proportion of β cells in islets of DSS mice model group decreased. At the same time, the expression of CD34 on β cells is significantly up-regulated. As an adhesion molecule, CD34 participates in intercellular adhesion and plays an important role in the process of migration and cell maturation. In vitro studies have shown that the decrease of CD34 secretion is involved in the normal differentiation of mast cells [16] and the occurrence of diabetes. And CD34 is widely expressed on vascular endothelial cells in colonic tissue of patients with IBD [17] Clinical studies have reported that patients with intestinal inflammation have a higher risk of insulindependent diabetes and higher insulin levels than healthy people [18]. This change may be related to the specific histocompatibility site antigen type. It may also be related to the activation of isletspecific T cells in intestinal mucosa.
Conclusion
We demonstrate that the decrease of islet function caused by abnormal glucose metabolism in mice with colonic inflammation induced by DSS is related to the increased expression of CD34, and its specific mechanism needs to be further studied.
References
- (2017) International Diabetes Federation. IDF Diabetes Atlas. Brussels: International Diabetes Federation (8th).
- Kahn SE, Cooper ME, Del Prato S (2014) Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383(9922): 1068-1083.
- Knudsen SH, Pedersen BK (2015) Targeting Inflammation Through a Physical Active Lifestyle and Pharmaceuticals for the Treatment of Type 2 Diabetes. Curr Diab Rep 15(10): 82.
- Donath MY (2014) Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 13(6): 465-476.
- Luck H, Tsai S, Chung J, Clemente Casares X, Ghazarian M, et al. (2015) Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab 21(4): 527-542.
- Iyer SS, Gensollen T, Gandhi A, Oh SF, Neves JF, et al. (2018) Dietary and Microbial Oxazoles Induce Intestinal Inflammation by Modulating Aryl Hydrocarbon Receptor Responses. Cell 173(5): 1123-1134.
- Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A (2014) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 32(6): 1380-1389.
- Suzawa K, Kobayashi M, Sakai Y, Hoshino H, Watanabe M (2007) Preferential induction of peripheral lymph node addressin on high endothelial venule-like vessels in the active phase of ulcerative colitis. Am J Gastroenterol 102(7): 1499-1509.
- Middleton J, Americh L, Gayon R, Julien D, Mansat M, et al. (2005) A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol 206(3): 260-268.
- Burgio VL, Fais S, Boirivant M, Perrone A, Pallone F (1995) Peripheral monocyte and naive T-cell recruitment and activation in Crohn's disease. Gastroenterology 109(4): 1029-1038.
- Seidelin JB, Vainer B, Horn T, Nielsen OH (1998) Circulating L-selectin levels and endothelial CD34 expression in inflammatory bowel disease. Am J Gastroenterol 93(10): 1854-1859.
- Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ (2017) CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci USA 114(4): E506-E513.
- Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S (2007) Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology 132(3): 944-954.
- El Badawy A, El Badri N (2016) Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus: A Meta-Analysis. PLoS One 11(4): e0151938.
- Zafar Nagma, Krishnasamy Sathya S, Shah Jasmit, Rai Shesh N, Riggs Daniel W (2014) Bhatnagar Aruni O’‘ Toole Timothy E.PLOS One 13(10).
- Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, et al. (2014) Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes 63(4): 1353-1365.
- Walczak Drzewiecka A, Salkowska A, Ratajewski M, Dastych J (2013) Epigenetic regulation of CD34 and HIF1A expression during the differentiation of human mast cells. Immunogenetics 65(6): 429-438.
- Korkmaz H, Sahin F, Ipekci SH, Temel T, Kebapcilar L (2014) Increased pulse wave velocity and relationship with inflammation, insulin, and insulin resistance in inflammatory bowel disease. Eur J Gastroenterol Hepatol 26(7): 725-732.

 Research Article
Research Article